The Use of Nanomedicine for Targeted Therapy against Bacterial Infections
Abstract
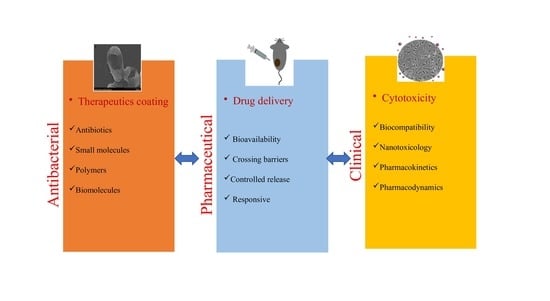
:1. Introduction
2. Therapeutic Efficacy of Nanoparticles
2.1. Antibiotics Capped Nanoparticles
2.2. Small Molecules Conjugated Nanoparticles
2.3. Polymer Stabilized Nanomaterials
2.4. Biomolecules Functionalized Nanoparticles
3. Nanoparticles as Drug Delivery Systems
4. Nanoparticles Cytotoxicity
5. Potential Future Strategies against Bacterial Infections
6. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Nii-trebi, N.I. Emerging and Neglected Infectious Diseases: Insights, Advances, and Challenges. BioMed Res. Int. 2017, 6, 1–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ali, S.M.; Siddiqui, R.; Ong, S.K.; Shah, M.R.; Anwar, A.; Heard, P.J.; Khan, N.A. Identification and characterization of antibacterial compound(s) of cockroaches (Periplaneta americana). J. Appl. Microbiol. Biotechnol. 2017, 101, 253–286. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ganewatta, M.S.; Tang, C. Controlling macromolecular structures towards effective antimicrobial polymers. J. Polym. 2015, 63, A1–A29. [Google Scholar] [CrossRef]
- Duijkeren, E.V.; Schink, A.K.; Roberts, M.C.; Wang, Y.; Schwarz, S. Mechanisms of Bacterial Resistance to Antimicrobial Agents. Microbiol. Spectr. 2018, 6, 51–81. [Google Scholar] [CrossRef]
- Duin, D.V.; Paterson, D.L. Multidrug-Resistant Bacteria in the Community Trends and Lessons Learned. Infect. Dis. Clin. N. Am. 2016, 30, 377–390. [Google Scholar] [CrossRef] [Green Version]
- Pageni, P.; Yang, P.; Chen, Y.P.; Huang, Y.; Bam, M.; Zhu, T.; Nagarkatti, M.; Benicewicz, B.C.; Decho, A.W.; Tang, C. Charged Metallopolymer-Grafted Silica Nanoparticles for Antimicrobial Applications. Biomacromolecules 2018, 19, 417–425. [Google Scholar] [CrossRef]
- Poulikakos, P.; Falagas, M.E. Aminoglycoside therapy in infectious diseases. Expert Opin. Pharmacother. 2013, 14, 1–13. [Google Scholar] [CrossRef]
- Gounani, Z.; Asadollahi, M.A.; Meyer, R.L.; Arpanaei, A. Loading of polymyxin B onto anionic mesoporous silica nanoparticles retains antibacterial activity and enhances biocompatibility. Int. J. Pharm. 2018, 537, 148–161. [Google Scholar] [CrossRef]
- Akbar, N.; Siddiqui, R.; Iqbal, M.; Sagathevan, K.; Khan, N.A. Gut bacteria of cockroaches are a potential source of antibacterial compound(s). Lett. Appl. Microbiol. 2018, 66, 416–426. [Google Scholar] [CrossRef]
- Bhatia, S. Nanoparticles Types, Classifi cation, Characterization, Fabrication Methods and Drug Delivery Applications. In Natural Polymer Drug Delivery Systems; Springer: Cham, Switzerland, 2016; pp. 33–93. [Google Scholar]
- Gupta, A.; Mumtaz, S.; Li, C.H.; Hussain, I.; Rotello, V.M. Combatting antibiotic-resistant bacteria using nanomaterials. Chem. Soc. Rev. 2018, 48, 415–427. [Google Scholar] [CrossRef]
- Jones, M.G.; Blonder, R.; Gardner, G.E.; Albe, V.; Falvo, M.; Chevrier, J. Nanotechnology and Nanoscale Science: Educational challenges. Int. J. Sci. Educ. 2013, 34, 1490–1512. [Google Scholar] [CrossRef]
- Zhu, X.; Radovic-Moreno, A.F.; Wu, J.; Langer, R.; Shi, J. Nanomedicine in the management of microbial infection–overview and perspectives. Nano Today 2014, 4, 478–498. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Pornpattananangkul, D.; Hu, C.M.J.; Huang, C.M. Development of Nanoparticles for Antimicrobial Drug Delivery. Curr. Med. Chem. 2010, 17, 585–594. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grace, N.A.; Pandian, K. Antibacterial efficacy of aminoglycosidic antibiotics protected gold nanoparticles—A brief study. Colloids Surf. A Physicochem. Eng. Asp. 2007, 297, 63–70. [Google Scholar] [CrossRef]
- Christenaa, L.R.; Mangalagowria, V.; Pradheebaa, P.; Ahmed, K.B.A.; Shalinia, B.I.S.; Vidyalakshmia, M.; Anbazhagana, V.; Sai Subramaniana, N. Copper Nanoparticles as Efflux Pump Inhibitor to Tackle Drug Resistant Bacteria. RSC Adv. 2013, 5, 1–15. [Google Scholar] [CrossRef]
- Shaker, M.A.; Shaaban, M.I. Formulation of carbapenems loaded gold nanoparticles to combat multi-antibiotic bacterial resistance: In vitro antibacterial study. Int. J. Pharm. 2017, 525, 71–84. [Google Scholar] [CrossRef]
- Ahmed, D.; Anwar, A.; Khan, A.K.; Ahmed, A.; Shah, M.R.; Khan, N.A. Size selectivity in antibiofilm activity of 3-(diphenylphosphino) propanoic acid coated gold nanomaterials against Gram-positive Staphylococcus aureus and Streptococcus mutans. AMB Express 2017, 7, 210. [Google Scholar] [CrossRef] [Green Version]
- Penders, J.; Stolzoff, M.; Hickey, D.J.; Andersson, M.; Webster, T.J. Shape-dependent antibacterial effects of non-cytotoxic gold nanoparticles. Int. J. Nanomed. 2017, 12, 2457. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Hu, C.; Shoa, L. The antimicrobial activity of nanoparticles: Present situation and prospects for the future. Int. J. Nanomed. 2017, 12, 1227–1249. [Google Scholar] [CrossRef] [Green Version]
- Sambhy, V.; Peterson, B.R.; Sen, A. Antibacterial and Hemolytic Activities of Pyridinium Polymers as a Function of the Spatial Relationship between the Positive Charge and the Pendant Alkyl Tail. Angew. Chem. Int. Ed. 2008, 120, 1270–1274. [Google Scholar] [CrossRef]
- Berry, V.; Gole, A.; Kundu, S.; Murphy, C.J.; Saraf, R.F. Deposition of CTAB-Terminated Nanorods on Bacteria to Form Highly Conducting Hybrid Systems. J. ACS 2005, 127, 17600–17601. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.C.; Yeh, Y.C.; Yang, C.Y.; Chen, C.L.; Chen, G.F.; Chen, C.C.; Wu, Y.C. Selective Binding of Mannose-Encapsulated Gold Nanoparticles to Type 1 Pili in Escherichia coli. J. ACS 2002, 124, 3508–3509. [Google Scholar]
- Prabhu, S.; Poulose, E.K. Silver nanoparticles: Mechanism of antimicrobial action, synthesis, medical applications, and toxicity effects. Int. Nano Lett. 2012, 2, 32. [Google Scholar] [CrossRef] [Green Version]
- Bayraktar, H.; You, C.C.; Rotello, V.M.; Knapp, M.J. Facial Control of Nanoparticle Binding to Cytochromec. J. ACS 2007, 129, 2732–2733. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.; Thamphiwatana, S.; Angsantikul, P.; Zhang, L. Nanoparticle approaches against bacterial infections. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2014, 6, 532–547. [Google Scholar] [CrossRef] [PubMed]
- Laverman, P.; Dams, E.T.; Storm, G.; Hafmans, T.G.; Croes, H.J.; Oyen, W.J.; Corstens, F.H.; Boerman, O.C. Microscopic localization of PEG-liposomes in a rat model of focal infection. J. Control. Release 2001, 75, 347–355. [Google Scholar] [CrossRef]
- Dakal, T.C.; Kumar, A.; Majumdar, R.S.; Yadav, V. Mechanistic Basis of Antimicrobial Actions of Silver Nanoparticles. Front. Microbiol. 2016, 7, 1–17. [Google Scholar] [CrossRef] [Green Version]
- Chattopadhyay, P.; Gupta, R.B. Production of Antibiotic Nanoparticles Using Supercritical CO2 as Antisolvent with Enhanced Mass Transfer. Ind. Eng. Chem. Res. 2001, 40, 3530–3539. [Google Scholar] [CrossRef]
- Shah, M.; Badwaik, V.; Kherde, Y.; Waghwani, H.K.; Modi, T.; Aguilar, Z.P.; Rodgers, H.; Hamilton, W.; Marutharaj, T.; Webb, C.; et al. Gold nanoparticles: Various methods of synthesis and antibacterial applications. Front. Biosci. 2014, 19, 1320–1344. [Google Scholar] [CrossRef] [Green Version]
- Rai, A.; Prabhune, A.; Perry, C.C. Antibiotic mediated synthesis of gold nanoparticles with potent antimicrobial activity and their application in antimicrobial coatings. J. Mater. Chem. 2010, 20, 6789–6798. [Google Scholar] [CrossRef] [Green Version]
- Sangaonkar, G.M.; Pawar, K.D. Garcinia indica mediated biogenic synthesis of silver nanoparticles with antibacterial and antioxidant activities. Colloids Surf. B Biointerfaces 2018, 164, 210–217. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Saleh, N.M.; Das, R.; Landis, R.F.; Bigdeli, A.; Motamedchaboki, K.; Campos, A.R.; Pomeroy, K.; Mahmoudi, M.; Rotello, V.M. Synergistic antimicrobial therapy using nanoparticles and antibiotics for the treatment of multidrug-resistant bacterial infection. Nano Future 2017, 1, 015004. [Google Scholar] [CrossRef]
- Banoee, M.; Seif, S.; Nazari, Z.E.; Fesharaki, P.J.; Shahverdi, H.R.; Moballegh, A.; Moghaddam, K.M.; Shahverdi, A.R. ZnO nanoparticles enhanced antibacterial activity of ciprofloxacin against Staphylococcus aureus and Escherichia coli. J. Biomed. Mater. Res. B Appl. Biomater. 2010, 93B, 557–561. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kooti, M.; Sedeh, A.N.; Motamedi, H.; Rezatofighi, S.E. Magnetic graphene oxide inlaid with silver nanoparticles as antibacterial and drug delivery composite. Appl. Microb. Biotechnol. 2018, 102, 3607–3621. [Google Scholar] [CrossRef] [PubMed]
- Masri, A.; Anwar, A.; Ahmed, D.; Siddiqui, R.; Shah, M.R.; Khan, N. Antibacterial activty of cephradine and vildagliptin conjugated silver nanoparticles. Antibiotics 2018, 7, 100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weissleder, R.; Kelly, K.; Sun, E.Y.; Shtatland, T.; Josephson, L. Cell-specific targeting of nanoparticles by multivalent attachment of small molecules. Nat. Biotechnol. 2005, 23, 1418–1423. [Google Scholar] [CrossRef]
- Zhao, Y.; Tian, Y.; Cui, Y.; Liu, W.; Ma, W.; Jiang, X. Small Molecule-Capped Gold Nanoparticles as Potent Antibacterial Agents That Target Gram-Negative Bacteria. J. ACS 2010, 132, 12349–12356. [Google Scholar] [CrossRef]
- Bresee, J.; Bond, C.M.; Worthington, R.J.; Smith, C.A.; Gifford, J.C.; Simpson, C.A.; Carter, C.J.; Wang, G.; Hartman, J.; Osbaugh, N.A.; et al. Nanoscale Structure−Activity Relationships, Mode of Action, and Biocompatibility of Gold Nanoparticle Antibiotics. J. ACS 2014, 14, 5295–5300. [Google Scholar] [CrossRef]
- Anwar, A.; Khalid, S.; Perveen, S.; Ahmed, S.; Siddiqui, R.; Khan, N.A.; Shah, M.R. Synthesis of 4-(dimethylamino)pyridine propylthioacetate coated gold nanoparticles and their antibacterial and photophysical activity. J. Nanobiotechnol. 2018, 16, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Mizutani, M.; Palermo, E.F.; Thoma, M.; Satoh, K.; Kamigaito, M.; Kuroda, K. Design and Synthesis of Self-Degradable Antibacterial Polymers by Simultaneous Chain- and Step-Growth Radical Copolymerization. Biomacromolecules 2012, 13, 1554–1563. [Google Scholar] [CrossRef]
- Tew, G.N.; Scott, R.W.; Klein, M.L.; Degrado, W.F. De Novo Design of Antimicrobial Polymers, Foldamers, and Small Molecules: From Discovery to Practical Applications. Acc. Chem. Res. 2010, 43, 30–39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adiseshaiah, P.P.; Hall, J.B.; Mcneil, S.E. Nanomaterial standards for efficacy and toxicity assessment. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2009, 2, 99–112. [Google Scholar] [CrossRef] [PubMed]
- Bing, W.; Sun, H.; Wang, F.; Song, Y.; Ren, J. Hydrogen-producing Hyperthermophilic Bacteria Synthesized Size- controllable Fine Gold Nanoparticles with Excellence for Eradicating Biofilm and Antibacterial Applications. J. Mater. Chem. 2018, 6, 4602–4609. [Google Scholar] [CrossRef]
- Basu, A.; Ray, S.; Chowdhury, S.; Sarkar, A.; Mandal, D.P.; Bhattacharjee, S.; Kundu, S. Evaluating the antimicrobial, apoptotic, and cancer cell gene delivery properties of protein-capped gold nanoparticles synthesized from the edible mycorrhizal fungus Tricholoma crassum. Nanoscale Res. Lett. 2018, 13, 1–16. [Google Scholar] [CrossRef]
- Anwar, A.; Masri, A.; Rao, K.; Rajendran, K.; Khan, N.A.; Shah, M.R.; Siddiqui, R. Antimicrobial activities of green synthesized gums-stabilized nanoparticles loaded with flavonoids. Sci. Rep. 2019, 9, 1–12. [Google Scholar] [CrossRef]
- Futyra, A.R.; Liskiewicz, M.K.; Sebastian, V.; Irusta, S.; Arruebo, M.; Stochel, G. Development of non-cytotoxic chitosan-gold nanocomposites as efficient antibacterial materials. ACS Appl. Mater. Interfaces 2015, 8, 176–189. [Google Scholar]
- Shah, S.T.; Yehya, W.A.; Saad, O.; Simarani, K.; Chowdhury, Z.A.; Alhadi, A.; Al-Ani, L. Surface Functionalization of Iron Oxide Nanoparticles with Gallic Acid as Potential Antioxidant and Antimicrobial Agents. Nanomaterials 2017, 7, 306. [Google Scholar] [CrossRef]
- Huma, Z.; Gupta, A.; Javed, I.; Das, R.; Hussain, S.Z.; Mumtaz, S.; Hussain, I.; Rotello, V.M. Cationic Silver Nanoclusters as Potent Antimicrobials against Multidrug-Resistant Bacteria. ACS Omega 2018, 3, 16721–16727. [Google Scholar] [CrossRef] [Green Version]
- D’Agostino, A.; Taglietti, A.; Grisoli, P.; Dacarro, G.; Cucca, L.; Patrin, M.; Pallavicini, P. Seed mediated growth of silver nanoplates on glass: Exploiting the bimodal antibacterial effect by near IR photo-thermal action and Ag+ release. RSC Adv. 2016, 74, 70414–70423. [Google Scholar] [CrossRef] [Green Version]
- D’Agostino, A.; Taglietti, A.; Desando, R.; Bini, M.; Patrini, M.; Dacarro, G.; Cucca, L.; Pallavicini, P.; Grisoli, P. Bulk surfaces coated with triangular silver nanoplates: Antibacterial action based on silver release and photo-thermal effect. Nanomaterials 2017, 1, 7. [Google Scholar] [CrossRef]
- Aoki, W.; Ueda, M. Characterization of Antimicrobial Peptides toward the Development of Novel Antibiotics. Pharm 2013, 6, 1055–1081. [Google Scholar] [CrossRef] [Green Version]
- Cappiello, F.; Grazia, A.D.; Li-av, S.Z.; Scal, S.; Ferrera, L.; Galietta, L.; Pini, A.; Shai, Y.; Di, P.; Mangoni, M.L. Esculentin-1a-derived peptides promote clearance of P. aeruginosa internalized in cystic fibrosis bronchial cells as well as lung cells migration: Biochemical properties and a plausible mode of action. Antimicrob. Agents Chemother. 2016, 60, 7252–7262. [Google Scholar]
- Chowdhury, R.; Ilyas, H.; Ghosh, A.; Ali, H.; Ghorai, A.; Midya, A.; Jana, N.R.; Das, S.; Bhunia, A. Multivalent Gold nanoparticle—Peptide Conjugates for Targeting Intracellular Bacterial Infections. Nanoscale 2017, 9, 14074–14093. [Google Scholar] [CrossRef]
- Khan, S.A.; Singh, A.K.; Senapati, D.; Fan, Z.; Ray, P.C. Bio-conjugated popcorn shaped gold nanoparticles for targeted photothermal killing of multiple drug resistant Salmonella DT104. J. Mater. Chem. 2011, 44, 17705–17709. [Google Scholar] [CrossRef]
- Huang, J.; Zhou, J.; Zhuang, J.; Gao, H.; Huang, D.; Wang, L.; Wu, W.; Li, Q.; Yang, D.P.; Han, M.Y. Strong near-infrared absorbing and biocompatible CuS nanoparticles for rapid and efficient photothermal ablation of gram-positive and-negative bacteria. ACS Appl. Mater. Interfaces 2017, 42, 36606–36614. [Google Scholar] [CrossRef]
- Tong, C.; Zou, W.; Ning, W.; Fan, J.; Li, L.; Liu, B.; Liu, X. Synthesis of DNA-guided silver nanoparticles on a graphene oxide surface: Enhancing the antibacterial effect and the wound healing activity. RSC Adv. 2018, 8, 28238–28248. [Google Scholar] [CrossRef] [Green Version]
- Javani, S.; Lorca, R.; Latorre, A.; Flors, C.; Cortajarena, A.L.; Somoza, Á. Antibacterial Activity of DNA-Stabilized Silver Nanoclusters Tuned by Oligonucleotide Sequence. ACS Appl. Mater. Interfaces 2016, 8, 10147–10154. [Google Scholar] [CrossRef]
- Thiyagarajan, K.; Bharti, V.K.; Tyagi, S.; Tyagi, P.K.; Ahuja, A.; Kumar, K.; Raja, T.; Kumarc, B. Synthesis of non-toxic, biocompatible, and colloidal stable silver nanoparticle using egg-white protein as capping and reducing agents for sustainable antibacterial application. RSC Adv. 2018, 8, 23213–23229. [Google Scholar] [CrossRef] [Green Version]
- Kubik, T.; Kubik, B.K.; Sugisaka, M. Nanotechnology on Duty in Medical Applications. Curr. Pharm. Biotechnol. 2005, 6, 17–33. [Google Scholar] [CrossRef]
- Mody, V.V.; Siwale, R.; Singh, A.; Mody, H.R. Introduction to metallic nanoparticles. J. Pharm. Bioallied Sci. 2010, 2, 282. [Google Scholar] [CrossRef]
- Saini, R.K.; Bagri, L.P.; Bajpai, A.K.; Mishra, A. Responsive polymer nanoparticles for drug delivery applications. Polym. Nanocarriers Drug Deliv. Appl. 2018, 1, 289–320. [Google Scholar]
- El-Boubbou, K. Magnetic iron oxide nanoparticles as drug carriers: Clinical relevance. Nanomed 2018, 13, 953–971. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.K.; Wells, S. Surface-Modified Superparamagnetic Nanoparticles for Drug Delivery: Preparation, Characterization, and Cytotoxicity Studies. Nanobioscience 2004, 3, 66–73. [Google Scholar] [CrossRef] [PubMed]
- Torchilin, V.P. Multifunctional, stimuli-sensitive nanoparticulate systems for drug delivery. Nat. Rev. Drug Discov. 2014, 13, 813–827. [Google Scholar] [CrossRef] [Green Version]
- Imbuluzqueta, E.; Gamazo, C.; Ariza, J.; Blanco-Prieto, M.J. Drug delivery systems for potential treatment of intracellular bacterial infections. Front. Biosci. 2010, 15, 397. [Google Scholar] [CrossRef]
- Pornpattananangkul, D.; Olson, S.; Aryal, S.; Sartor, M.; Huang, C.; Vecchio, K.; Zhang, L. Stimuli-Responsive Liposome Fusion Mediated by Gold Nanoparticles. ACS 2010, 4, 1935–1942. [Google Scholar] [CrossRef] [Green Version]
- Ji, H.; Dong, K.; Yan, Z.; Ding, C.; Chen, Z.; Ren, J.; Qu, X. Bacterial Hyaluronidase Self-Triggered Prodrug Release for Chemo-Photothermal Synergistic Treatment of Bacterial Infection. Small 2016, 12, 6200–6206. [Google Scholar] [CrossRef]
- Thamphiwatana, S.; Fu, V.; Zhu, J.; Lu, D.; Gao, W.; Zhang, L. Nanoparticle-Stabilized Liposomes for pH-Responsive Gastric Drug Delivery. Langmuir 2013, 29, 12228–12233. [Google Scholar] [CrossRef]
- Xiong, M.H.; Bao, Y.; Yang, X.; Wang, Y.; Sun, B.; Wang, J. Lipase-Sensitive Polymeric Triple-Layered Nanogel for “On-Demand” Drug Delivery. J. ACS 2012, 134, 4355–4362. [Google Scholar] [CrossRef]
- Fu, P.P.; Xia, Q.; Hwang, H.M.; Ray, P.C. Mechanisms of nanotoxicity: Generation of reactive oxygen species. J. Food Drug Anal. 2014, 22, 64–75. [Google Scholar] [CrossRef] [Green Version]
- Manso-Ríos, M.; García, P.B.C.; Agarwal, V.; Lee, S. Citotoxicidad de nanopartículas metálicas en cultivos de células cerebrales. Arch. Neurocien 2017, 22, 13–23. [Google Scholar]
- Rigo, C.; Ferroni, L.; Tocco, I.; Roman, M.; Munivrana, I.; Gardin, C.; Cairns, W.R.L.; Vindigni, V.; Azzena, B.; Barbante, C.; et al. Active Silver Nanoparticles for Wound Healing. Int. J. Mol. Sci. 2013, 14, 4817–4840. [Google Scholar] [CrossRef] [Green Version]
- Maity, P.; Bepari, M.; Pradhan, A.; Baral, R.; Roy, S.; Choudhury, M.S. Synthesis and characterization of biogenic metal nanoparticles and its cytotoxicity and anti-neoplasticity through the induction of oxidative stress, mitochondrial dysfunction and apoptosis. Colloids Surf. B Biointerfaces 2018, 161, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Jeong, J.; Kim, S.H.; Lee, S.; Lee, D.K.; Han, Y.; Jeon, S.; Cho, W.S. Differential Contribution of Constituent Metal Ions to the Cytotoxic Effects of Fast-Dissolving Metal-Oxide Nanoparticles. Front. Pharm. 2018, 9, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rajendran, K.; Anwar, A.; Khan, N.A.; Siddiqui, R. Brain-Eating Amoebae: Silver Nanoparticle Conjugation Enhanced Efficacy of Anti-Amoebic Drugs against Naegleria fowleri. ACS Chem. Neurosci. 2017, 8, 2626–2630. [Google Scholar] [CrossRef] [PubMed]
- Bobo, D.; Robinson, K.J.; Islam, J.; Thurecht, K.J.; Corrie, S.R. Nanoparticle-Based Medicines: A Review of FDA-Approved Materials and Clinical Trials to Date. Pharm. Res. 2016, 33, 2373–2387. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Hussain, A.; Shakeel, F.; Ahsan, M.J.; Alshehri, S.; Webster, T.J.; Lal, U.R. Recent insights on nanomedicine for augmented infection control. Int. J. Nanomed. 2019, 14, 2301. [Google Scholar] [CrossRef] [Green Version]
- Muzammil, S.; Hayat, S.; Alam, M.F.; Aslam, B.; Siddique, M.H.; Nisar, M.A.; Saqalein, M.; Atif Sarwar, A.; Khurshid, A.; Amin, N.; et al. Nanoantibiotics: Future nanotechnologies to combat antibiotic resistance. Front. Biosci. 2018, 10, 352–374. [Google Scholar]
- Katva, S.; Das, S.; Moti, H.S.; Jyoti, A.; Kaushik, S. Antibacterial Synergy of Silver Nanoparticles with Gentamicin and Chloramphenicol against Enterococcus faecalis Sagar. Pharm. Mag. 2017, 13, 828–833. [Google Scholar]
- Díez-martínez, R.; Fernández, E.G.; Manzano, M.; Martínez, Á.; Domenech, M.; Vallet-Regí, M.; Garcia, P. Auranofin-loaded nanoparticles as a new therapeutic tool to fight streptococcal infections. Sci. Rep. 2016, 6, 19525. [Google Scholar] [CrossRef] [Green Version]
- Kim, D.; Shin, K.; Kwon, S.G.; Hyeon, T. Synthesis and Biomedical Applications of Multifunctional Nanoparticles. Acc. Chem. Res. 2018, 42, 1097–1107. [Google Scholar] [CrossRef] [PubMed]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Masri, A.; Anwar, A.; Khan, N.A.; Siddiqui, R. The Use of Nanomedicine for Targeted Therapy against Bacterial Infections. Antibiotics 2019, 8, 260. https://doi.org/10.3390/antibiotics8040260
Masri A, Anwar A, Khan NA, Siddiqui R. The Use of Nanomedicine for Targeted Therapy against Bacterial Infections. Antibiotics. 2019; 8(4):260. https://doi.org/10.3390/antibiotics8040260
Chicago/Turabian StyleMasri, Abdulkader, Ayaz Anwar, Naveed Ahmed Khan, and Ruqaiyyah Siddiqui. 2019. "The Use of Nanomedicine for Targeted Therapy against Bacterial Infections" Antibiotics 8, no. 4: 260. https://doi.org/10.3390/antibiotics8040260
APA StyleMasri, A., Anwar, A., Khan, N. A., & Siddiqui, R. (2019). The Use of Nanomedicine for Targeted Therapy against Bacterial Infections. Antibiotics, 8(4), 260. https://doi.org/10.3390/antibiotics8040260