Conjugation of Penicillin-G with Silver(I) Ions Expands Its Antimicrobial Activity against Gram Negative Bacteria
Abstract
1. Introduction
2. Results
2.1. General Aspects
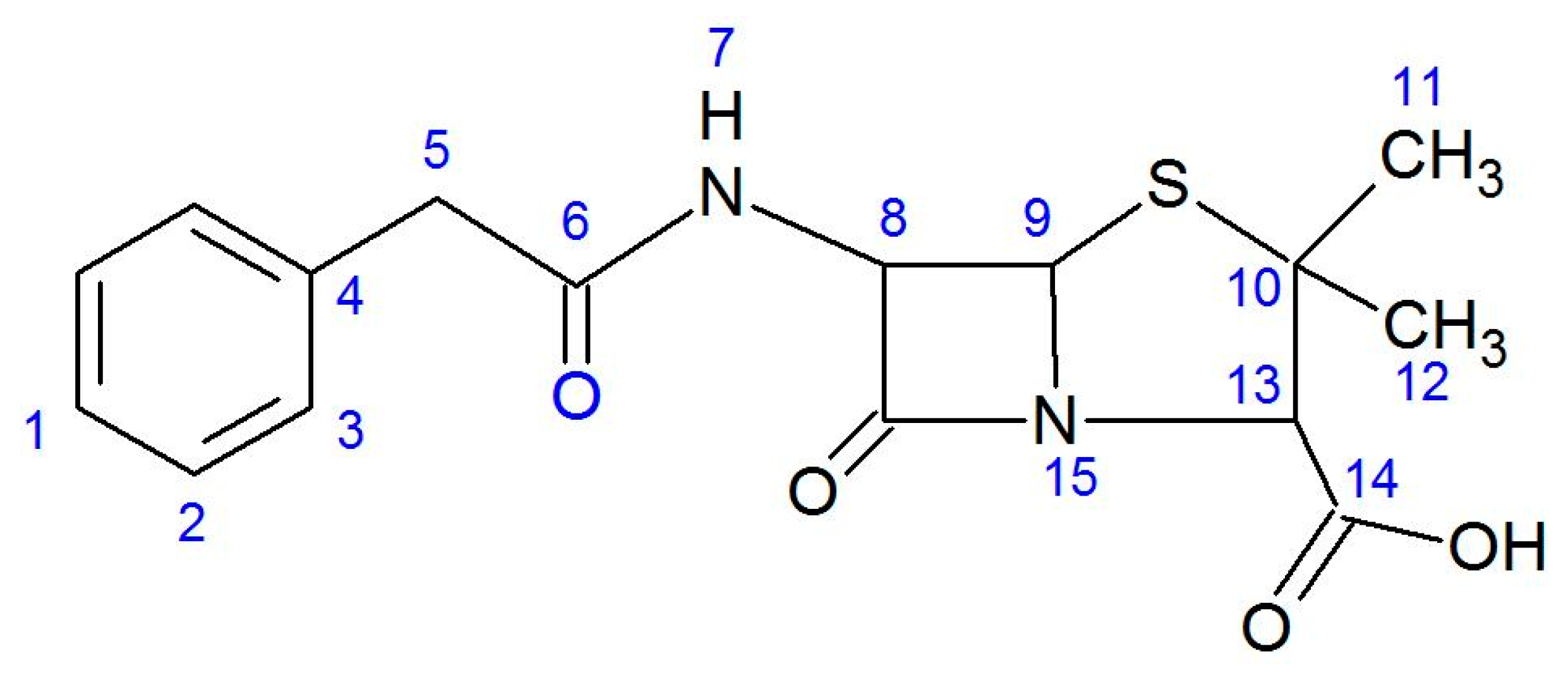
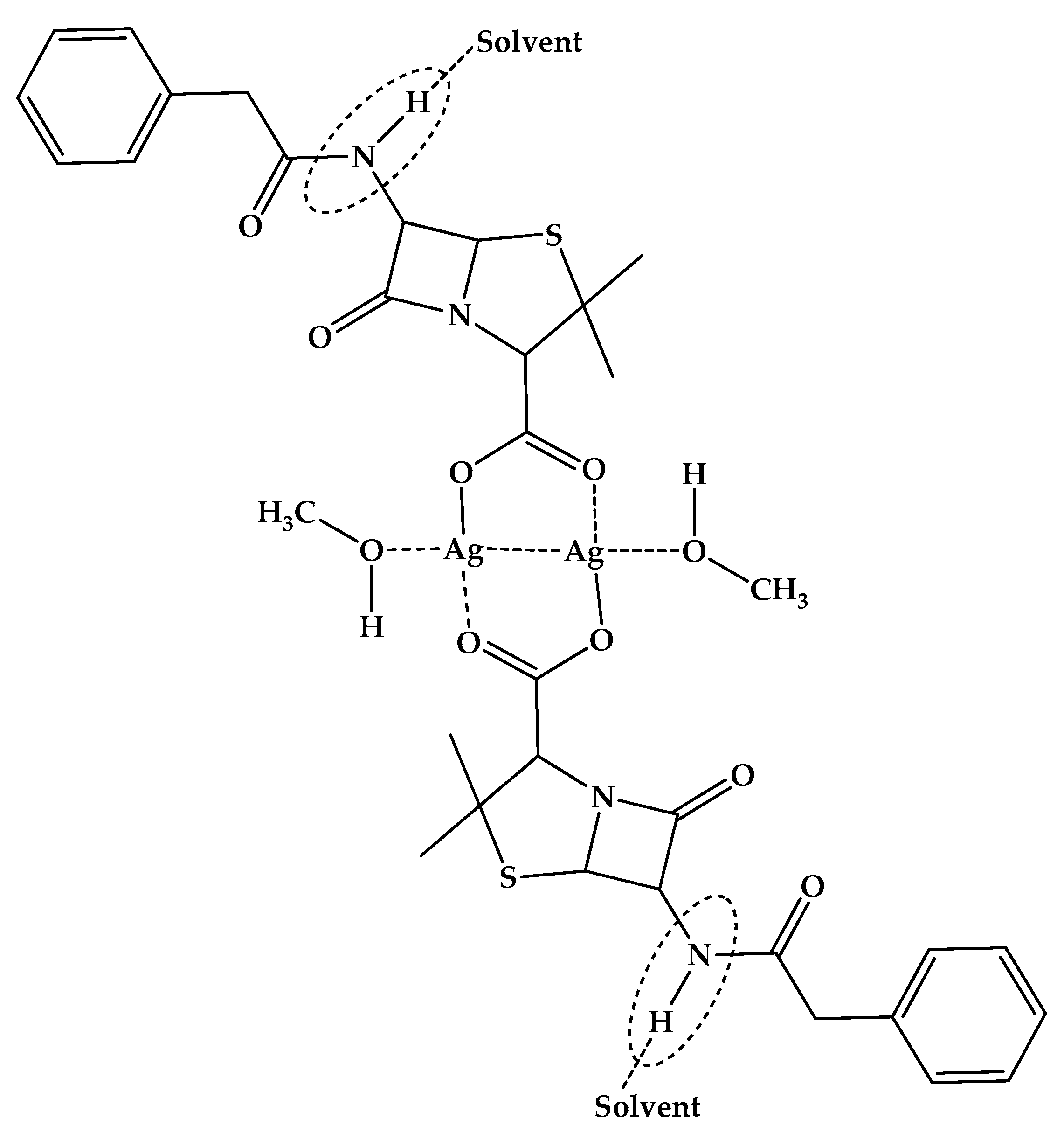
2.2. Characterization of PenAg in Solid State Studies
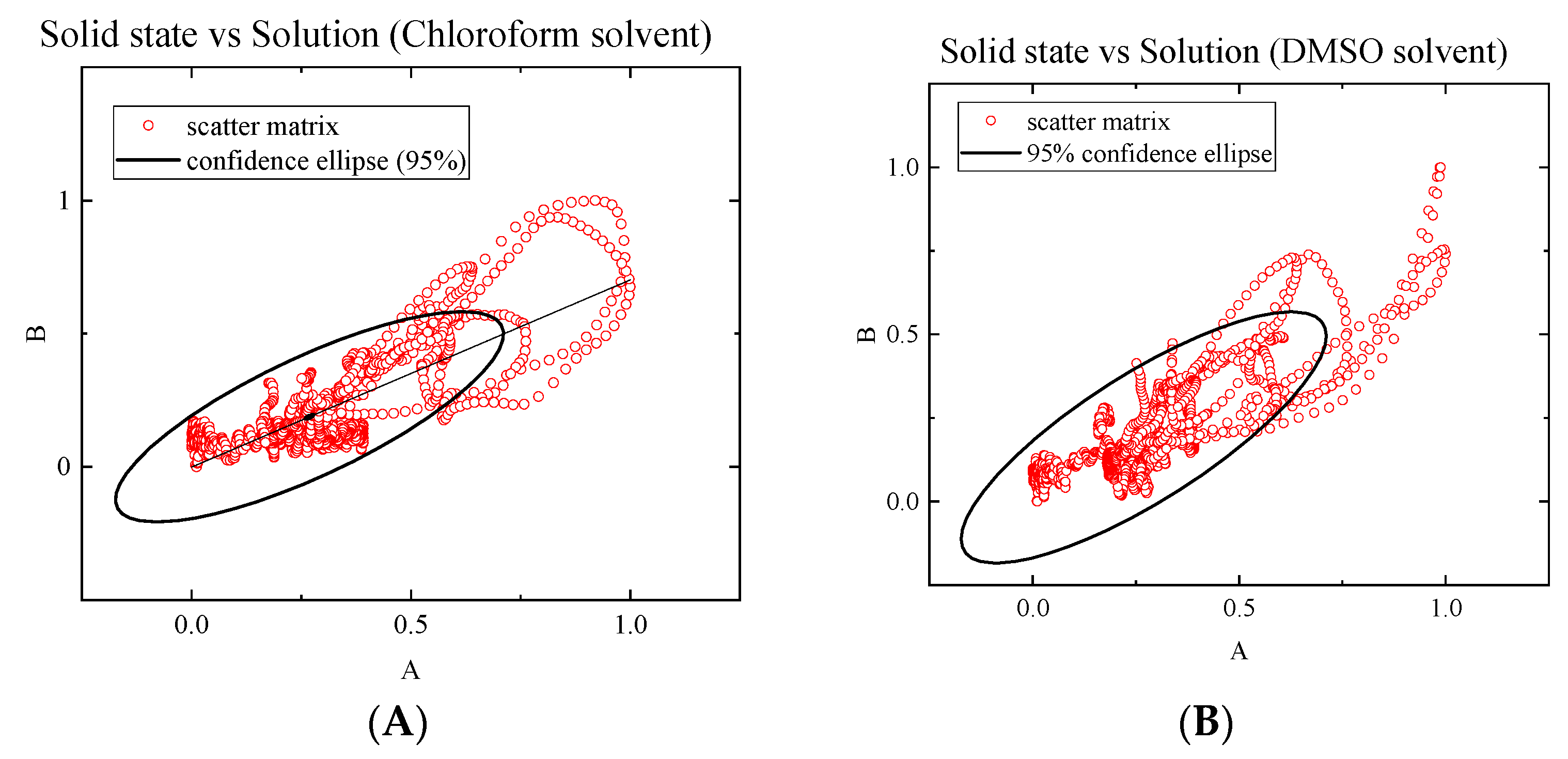
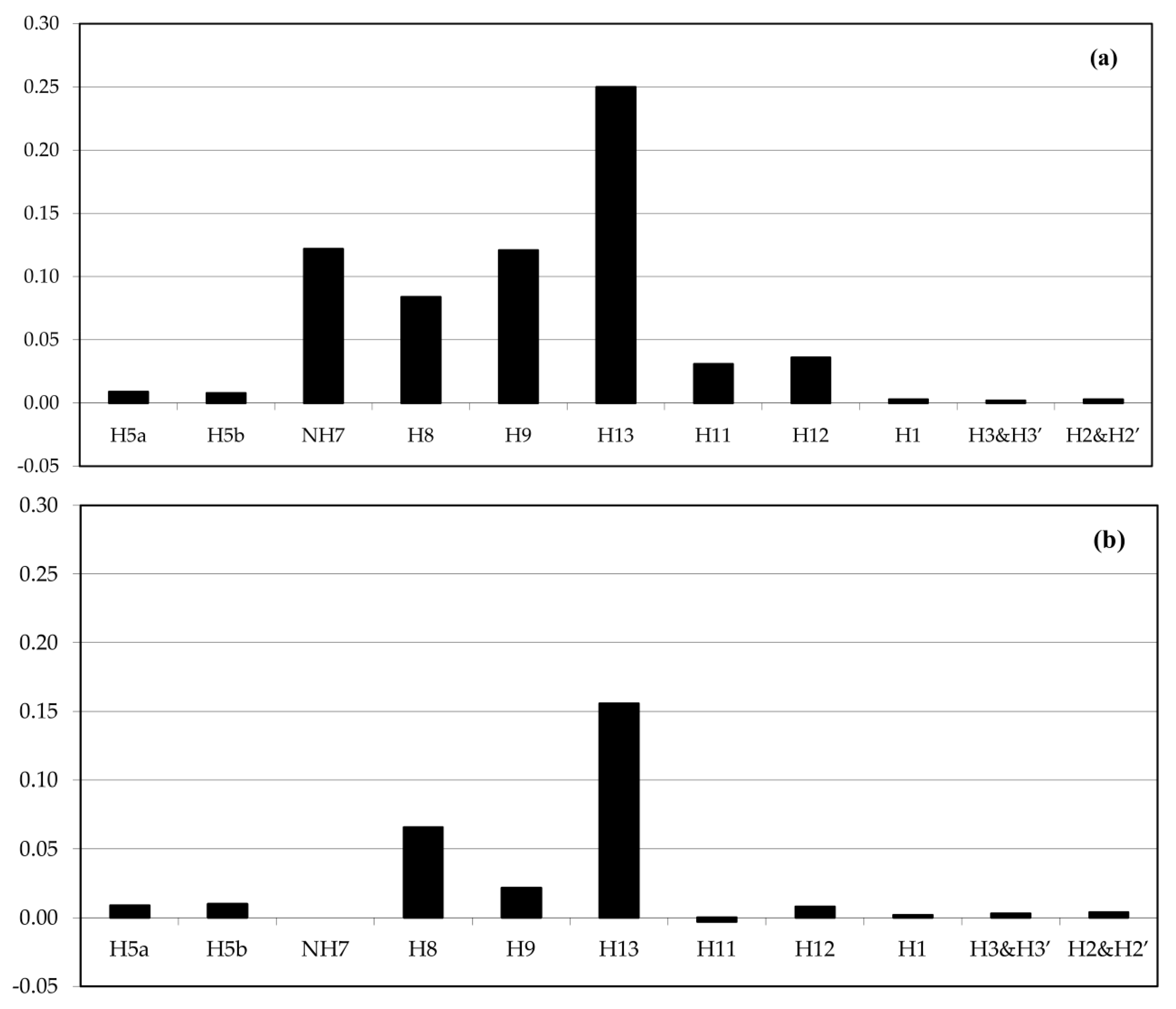
2.3. Solution Studies
2.4. Antibacterial Studies
2.5. In Vivo Toxicity Evaluation, by Brine Shrimp Artemia Salina
3. Conclusions
4. Materials and Methods
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Vardanyan, R.S.; Hruby, V.J. Synthesis of Essential Drugs; Elsevier: Amsterdam, The Netherlands, 2006. [Google Scholar]
- Morán-Díaz, J.R.; Jiménez-Vázquez, H.A.; Gómez-Pliego, R.; Arellano-Mendoza, M.G.; Quintana-Zavala, D.; Guevara-Salazar, J.A. Correlation study of antibacterial activity and spectrum of Penicillins through a structure-activity relationship analysis. Med. Chem. Res. 2019, 28, 1529–1546. [Google Scholar] [CrossRef]
- Munita, J.M.; Arias, C.A. Mechanisms of Antibiotic Resistance. Microbiol. Spectr. 2016, 4. [Google Scholar] [CrossRef]
- Randall, C.P.; Oyama, L.B.; Bostock, J.M.; Chopra, I.; O’Neill, A.J. The silver cation (Ag+): Antistaphylococcal activity, mode of action and resistance studies. J. Antimicrob. Chemother. 2013, 68, 131–138. [Google Scholar] [CrossRef]
- Muller, M. Bacterial Silver Resistance Gained by Cooperative Interspecies Redox Behavior. Antimicrob. Agents Chemother. 2018, 62, e00672-18. [Google Scholar] [CrossRef] [PubMed]
- Sainis, I.; Banti, C.N.; Owczarzak, A.M.; Kyros, L.; Kourkoumelis, N.; Kubicki, M.; Hadjikakou, S.K. New antibacterial, non-genotoxic materials, derived from the functionalization of the anti-thyroid drug methimazole with silver ions. J. Inorg. Biochem. 2016, 160, 114–124. [Google Scholar] [CrossRef] [PubMed]
- Papadimitriou, A.; Ketikidis, I.; Stathopoulou, M.K.; Banti, C.N.; Papachristodoulou, C.; Zoumpoulakis, L.; Agathopoulos, S.; Vagenas, G.V.; Hadjikakou, S.K. Innovative material containing the natural product curcumin, with enhanced antimicrobial properties for active packaging. Mater. Sci. Eng. C 2018, 84, 118–122. [Google Scholar] [CrossRef] [PubMed]
- Milionis, I.; Banti, C.N.; Sainis, I.; Raptopoulou, C.P.; Psycharis, V.; Kourkoumelis, N.; Hadjikakou, S.K. Silver ciprofloxacin (CIPAG): A successful combination of antibiotics in inorganic-organic hybrid for the development of novel formulations based on chemically modified commercially available antibiotics. J. Biol. Inorg. Chem. 2018, 23, 705–723. [Google Scholar] [CrossRef] [PubMed]
- Stathopoulou, M.-E.K.; Banti, C.N.; Kourkoumelis, N.; Hatzidimitriou, A.G.; Kalampounias, A.G.; Hadjikakou, S.K. Silver complex of salicylic acid and its hydrogel-cream in wound healing chemotherapy. J. Inorg. Biochem. 2018, 181, 41–45. [Google Scholar] [CrossRef] [PubMed]
- Chrysouli, M.P.; Banti, C.N.; Milionis, Ι.; Koumasi, D.; Raptopoulou, C.P.; Psycharis, V.; Sainis, I.; Hadjikakou, S.K. A water-soluble silver (I) formulation as an effective disinfectant of contact lenses cases. Mater. Sci. Eng. C 2018, 93, 902–910. [Google Scholar] [CrossRef]
- Banti, C.N.; Poyraz, M.; Sainis, I.; Sari, M.; Rossos, G.; Kourkoumelis, N.; Hadjikakou, S.K. The periodic table of urea derivative: Small molecules of zinc (II) and nickel (II) of diverse antimicrobial and antiproliferative applications. Mol. Divers. 2019. [Google Scholar] [CrossRef]
- Karetsi, V.A.; Banti, C.N.; Kourkoumelis, N.; Papachristodoulou, C.; Stalikas, C.D.; Raptopoulou, C.P.; Psycharis, V.; Zoumpoulakis, P.; Mavromoustakos, T.; Sainis, I.; et al. An Efficient Disinfectant, Composite Material {SLS@ [Zn3 (CitH)2]} as Ingredient for Development of Sterilized and Non Infectious Contact Lens. Antibiotics 2019, 8, 213. [Google Scholar] [CrossRef]
- Renfrew, A.K. Transition metal complexes with bioactive ligands: Mechanisms for selective ligand release and applications for drug delivery. Metallomics 2014, 6, 1324. [Google Scholar] [CrossRef]
- Ma, D.-L.; Wu, C.; Cheng, S.-S.; Lee, F.-W.; Han, Q.-B.; Leung, C.-H. Development of Natural Product-Conjugated Metal Complexes as Cancer Therapies. Int. J. Mol. Sci. 2019, 20, 341. [Google Scholar] [CrossRef]
- Anacona, J.R.; Figueroa, E.M. Synthesis and Characterization of Metal Complexes with Penicillin. J. Coord. Chem. 1999, 48, 181–189. [Google Scholar] [CrossRef]
- Anacona, J.R. Synthesis and Antibacterial Activity of Some Metal Complexes of Beta-Lactamic Antibiotics. J. Coord. Chem. 2001, 54, 355–365. [Google Scholar] [CrossRef]
- Abdellah, M.A.; Hadjikakou, S.K.; Hadjiliadis, N.; Kubicki, M.; Bakas, T.; Kourkoumelis, N.; Simos, Y.V.; Karkabounas, S.; Barsan, M.; Butler, I.S. Synthesis, characterization and biological studies of organotin(IV) derivatives with o- or p-hydroxy-benzoic acids. Bioinorg. Chem. Appl. 2009, 2009, 12. [Google Scholar] [CrossRef] [PubMed]
- Noda, I.; Osaki, Y. Two-Dimensional Correlation Spectroscopy–Applications in Vibrational and Optical Spectroscopy; John Wiley & Sons Ltd.: West Sussex, UK, 2004. [Google Scholar]
- Kalampounias, A.G. Correlating changes in structure and dynamical properties in LnX3 (Ln = Y, Ho, Dy, Gd, Nd, La and X = Cl, Br) ionic melts. Chem. Pap. 2017, 71, 1529–1539. [Google Scholar] [CrossRef]
- Imafuku, Y.; Takeuchi, Y. Synthesis and characterization of some penicillins modified with germanium-containing moieties. Appl. Organometal. Chem. 2004, 18, 384–393. [Google Scholar] [CrossRef]
- Branch, S.K.; Casy, A.F.; Ominde, E.M.A. Application of 1H nuclear magnetic resonance spectroscopy to the analysis of 13-lactam antibiotics and their common degradation Products. J. Pharm. Biomed. Anal. 1987, 5, 73–103. [Google Scholar] [CrossRef]
- Möhler, J.S.; Kolmar, T.; Synnatschke, K.; Hergert, M.; Wilson, L.A.; Ramu, S.; Elliott, A.G.; Blaskovich, M.A.T.; Sidjabat, H.E.; Paterson, D.L.; et al. Enhancement of antibiotic-activity through complexation with metal ions-Combined ITC, NMR, enzymatic and biological studies. J. Inorg. Biochem. 2017, 167, 134–141. [Google Scholar] [CrossRef]
- Kupka, T.; Dziegielewski, J.O.; Pasterna, G. NMR studies on penicillins: Hydro-gen bonding, self-association and micellar solutions of cloxacillin Na-salt in D2O. J. Pharm. Biomed. Anal. 1993, 11, 103–116. [Google Scholar] [CrossRef]
- Baxter, N.J.; Williamson, M.P. Temperature dependence of 1H chemical shifts in proteins. J. Biomol. NMR 1997, 9, 359–369. [Google Scholar] [CrossRef] [PubMed]
- Petrova, M.; Muhamadejev, R.; Vigante, B.; Duburs, G.; Liepinsh, E. Intramolecular hydrogen bonds in 1,4-dihydropyridine derivatives. R. Soc. Open Sci. 2018, 5, 180088. [Google Scholar] [CrossRef] [PubMed]
- Sośnicki, J.G.; Hansen, P.E. Temperature coefficient of NH chemical shifts of thioamides and amides in relation to structure. J. Mol. Struct. 2004, 700, 91–103. [Google Scholar] [CrossRef]
- Newberry, R.W.; Raines, R.T. A prevalent intraresidue hydrogen bond stabilizes proteins. Nat. Chem. Biol. 2006, 12, 1084–1088. [Google Scholar] [CrossRef]
- Mitra, A.; Seaton, P.J.; Assarpour, R.A.; Williamson, T. Unprecedented concen-tration dependent chemical shift variation in 1H-NMR studies: A caveat in the investigations of molecular recognition and structure elucidation. Tetrahedron 1998, 54, 15489–15498. [Google Scholar] [CrossRef]
- Tagawa, A.; Shikata, T. Anti-parallel dimer and tetramer formation of cyclic and open structure tertiary amides, N-methyl-2-pyrrolidone and N, N-dimethylacetamide, in so-lution of a non-polar solvent, benzene. Phys. Chem. Chem. Phys. 2019, 21, 22081–22091. [Google Scholar] [CrossRef]
- Wiegand, I.; Hilpert, K.; Hancock, R.E.W. Agar and broth dilution methods to determine the minimal inhibitory concentration (MIC) of antimicrobial substances. Nat. Protoc. 2008, 3, 163–175. [Google Scholar] [CrossRef]
- Kostenko, V.; Ceri, H.; Martinuzzi, R.J. Increased tolerance of Staphylococcus aureus to vancomycin in Viscous media. FEMS Immunol. Med. Microbiol. 2007, 51, 277–288. [Google Scholar]
- Matuschek, E.; Brown, D.F.J.; Kahlmeter, G. Development of the EUCAST disk di_usion antimicrobial susceptibility testing method and its implementation in routine microbiology laboratories. Clin. Microbiol. Infect. 2014, 20, o255–o266. [Google Scholar] [CrossRef]
- Lee, S.M.; Min, B.S.; Kho, Y.H. Brine Shrimp Lethality of the Compounds from Phryma leptostachya L. Arch. Pharm. Res. 2002, 25, 652. [Google Scholar] [CrossRef] [PubMed]
- Gajera, S.B.; Mehta, J.V.; Patel, M.N. Design of Multifunctional IridiumIII Compounds as a Potential Therapeutic Agents from Basic Molecular Scaffolds. ChemistrySelect 2016, 1, 3966. [Google Scholar] [CrossRef]
- Neves, R.A.F.; Fernandes, T.; dos Santos, L.N.; Nascimento, S.M. Toxicity of benthic dinoflagellates on grazing, behavior and survival of the brine shrimp Artemia salina. PLoS ONE 2017, 12, e0175168. [Google Scholar] [CrossRef] [PubMed]
- Faimali, M.; Giussani, V.; Piazza, V.; Garaventa, F.; Corrà, C.; Asnaghi, V.; Privitera, D.; Gallus, L.; Cattaneo-Vietti, R.; Mangialajo, L.; et al. Toxic effects of harmful benthic dinoflagellate Ostreopsis ovata on invertebrate and vertebrate marine organisms. Mar. Environ. Res. 2012, 76, 97. [Google Scholar] [CrossRef] [PubMed]
- Morones-Ramirez, J.R.; Winkler, J.A.; Spina, C.S.; Collins, J.J. Silver Enhances Antibiotic Activity Against Gram-negative Bacteria. Sci. Transl. Med. 2013, 5, 190ra81. [Google Scholar] [CrossRef] [PubMed]
- Rahman, K.; Ullah Khan, S.; Fahad, S.; Khan Shinwari, Z.; Khan, D.; Kamal, S.; Ullah, I.; Ishtiaq Anjum, S.; Man, S.; Jamil Khan, A.; et al. In vitro biological screening of a critically endangered medicinal plant, Atropa acuminata Royle Ex Lindl of north western Himalaya. Sci. Rep. 2018, 8, 11028. [Google Scholar] [CrossRef]
- Arun, V.V.; Saharan, N.; Ramasubramanian, V.; Babitha Rani, A.M.; Salin, K.R.; Sontakke, R.; Haridas, H.; George Pazhayamadom, D. Multi-response optimization of Artemia hatching process using split-split-plot design based response surface methodology. Sci. Rep. 2017, 7, 40394. [Google Scholar] [CrossRef]
- Rani Muhamad Syahmi, A.; Vijayaratna, S.; Sasidharan, S.; Yoga Latha, L.; Ping Kwan, Y.; Ling Lau, Y.; Ngit Shin, L.; Chen, Y. Acute Oral Toxicity and Brine Shrimp Lethality of Elaeis guineensis Jacq., (Oil Palm Leaf) Methanol Extract. Molecules 2010, 15, 8111. [Google Scholar] [CrossRef]


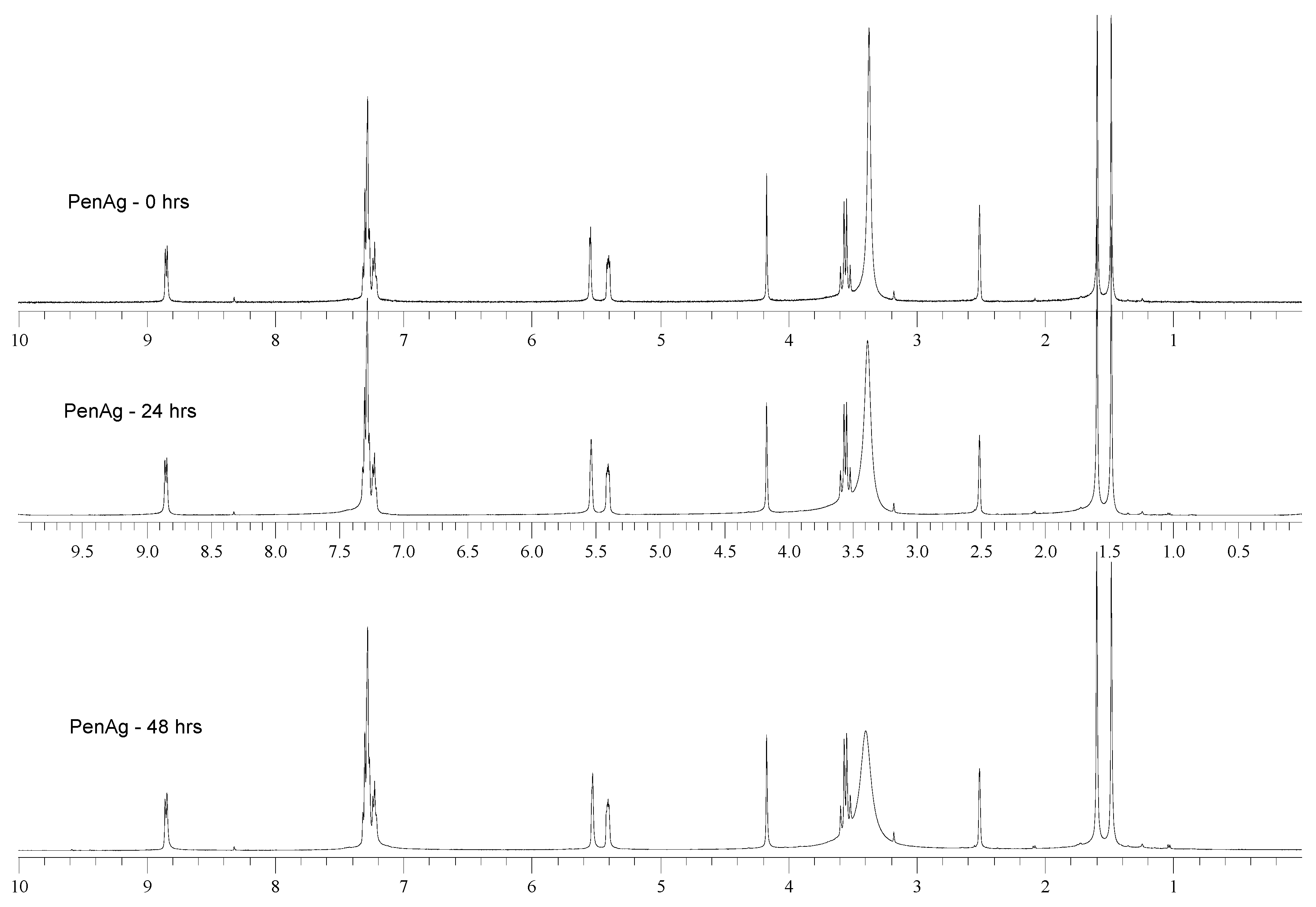



| Solid/KBr | CHCl3 | Solid/KBr | DMSO | |||
|---|---|---|---|---|---|---|
| Solid/KBr | 1 | 0.78715 | Solid/KBr | 1 | 0.80955 | |
| CH3Cl | 0.78715 | 1 | DMSO | 0.80955 | 1 |
| Compound | MIC values (μΜ) | Ref. | ||
|---|---|---|---|---|
| P. aeruginosa | S. epidermidis | S. aureus | ||
| PenAg | 23.00 ± 2.29 | 2.41 ± 0.88 | 0.08 ± 0.02 | [*] |
| PenNa | >2000 | 3.76 ± 1.14 | 0.14 ± 0.02 | [*] |
| CIPAG | 0.61 ± 0.14 | 0.46 ± 0.08 | 0.54 ± 0.07 | [8] |
| ([CIPH2+]·Cl−) | 1.17 ± 0.22 | 1.08 ± 0.12 | 1.45 ± 0.12 | [8] |
| AGSAL-1 | 28 | 50 | 42 | [9] |
| salH2 | >1250 | >1250 | >1250 | [9] |
| AGMNA | 25.7 ± 2.4 | - | 42.0 ± 0.3 | [10] |
| MNAH2 | >300 | - | >300 | [10] |
| [Zn3(CitH)2] | >250 | 119.2 ± 18.1 | 183.3 ± 31.4 | [12] |
| CitH4 | >250 | >250 | >250 | [12] |
| MBC values (μΜ) | ||||
| PenAg | 46.6 | - | - | [*] |
| PenNa | 2000 | - | - | [*] |
| CIPAG | 0.7 | 0.8 | 1.0 | [8] |
| ([CIPH2+]·Cl−) | 1.60 | 1.60 | 2.00 | [8] |
| AGSAL-1 | 33.0 | 90.0 | 50.0 | [9] |
| salH2 | ND | ND | ND | [9] |
| AGMNA | 61.1 | - | 85.7 | [10] |
| MNAH2 | 153.3 | - | 135.0 | [10] |
| [Zn3(CitH)2] | >250 | 201.7 | >250 | [12] |
| CitH4 | >250 | >250 | >250 | [12] |
| Inhibition zone (mm) | ||||
| Control/DMSO | 10 | 10 | 10 | |
| PenAg | 17 | 34 | 57 | [*] |
| PenNa | 10 | 34 | 60 | [*] |
| CIPAG | 32 | 34 | 28 | [8] |
| ([CIPH2+]·Cl−) | 30 | 36 | 24 | [8] |
| AGSAL-1 | 13 | 13 | 12 | [9] |
| salH2 | 10 | 10 | 10 | [9] |
| AGMNA | 21.8 | - | 22.1 | [10] |
| MNAH2 | 10 | 10 | 10 | [10] |
| [Zn3(CitH)2] | 10 | 10 | 10 | [12] |
| CitH4 | 10 | 10 | 10 | [12] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ketikidis, I.; Banti, C.N.; Kourkoumelis, N.; Tsiafoulis, C.G.; Papachristodoulou, C.; Kalampounias, A.G.; Hadjikakou, S.K. Conjugation of Penicillin-G with Silver(I) Ions Expands Its Antimicrobial Activity against Gram Negative Bacteria. Antibiotics 2020, 9, 25. https://doi.org/10.3390/antibiotics9010025
Ketikidis I, Banti CN, Kourkoumelis N, Tsiafoulis CG, Papachristodoulou C, Kalampounias AG, Hadjikakou SK. Conjugation of Penicillin-G with Silver(I) Ions Expands Its Antimicrobial Activity against Gram Negative Bacteria. Antibiotics. 2020; 9(1):25. https://doi.org/10.3390/antibiotics9010025
Chicago/Turabian StyleKetikidis, Ioannis, Christina N. Banti, Nikolaos Kourkoumelis, Constantinos G. Tsiafoulis, Christina Papachristodoulou, Angelos G. Kalampounias, and Sotiris K. Hadjikakou. 2020. "Conjugation of Penicillin-G with Silver(I) Ions Expands Its Antimicrobial Activity against Gram Negative Bacteria" Antibiotics 9, no. 1: 25. https://doi.org/10.3390/antibiotics9010025
APA StyleKetikidis, I., Banti, C. N., Kourkoumelis, N., Tsiafoulis, C. G., Papachristodoulou, C., Kalampounias, A. G., & Hadjikakou, S. K. (2020). Conjugation of Penicillin-G with Silver(I) Ions Expands Its Antimicrobial Activity against Gram Negative Bacteria. Antibiotics, 9(1), 25. https://doi.org/10.3390/antibiotics9010025











