Caerin 1 Antimicrobial Peptides that Inhibit HIV and Neisseria May Spare Protective Lactobacilli
Abstract
1. Introduction
2. Results
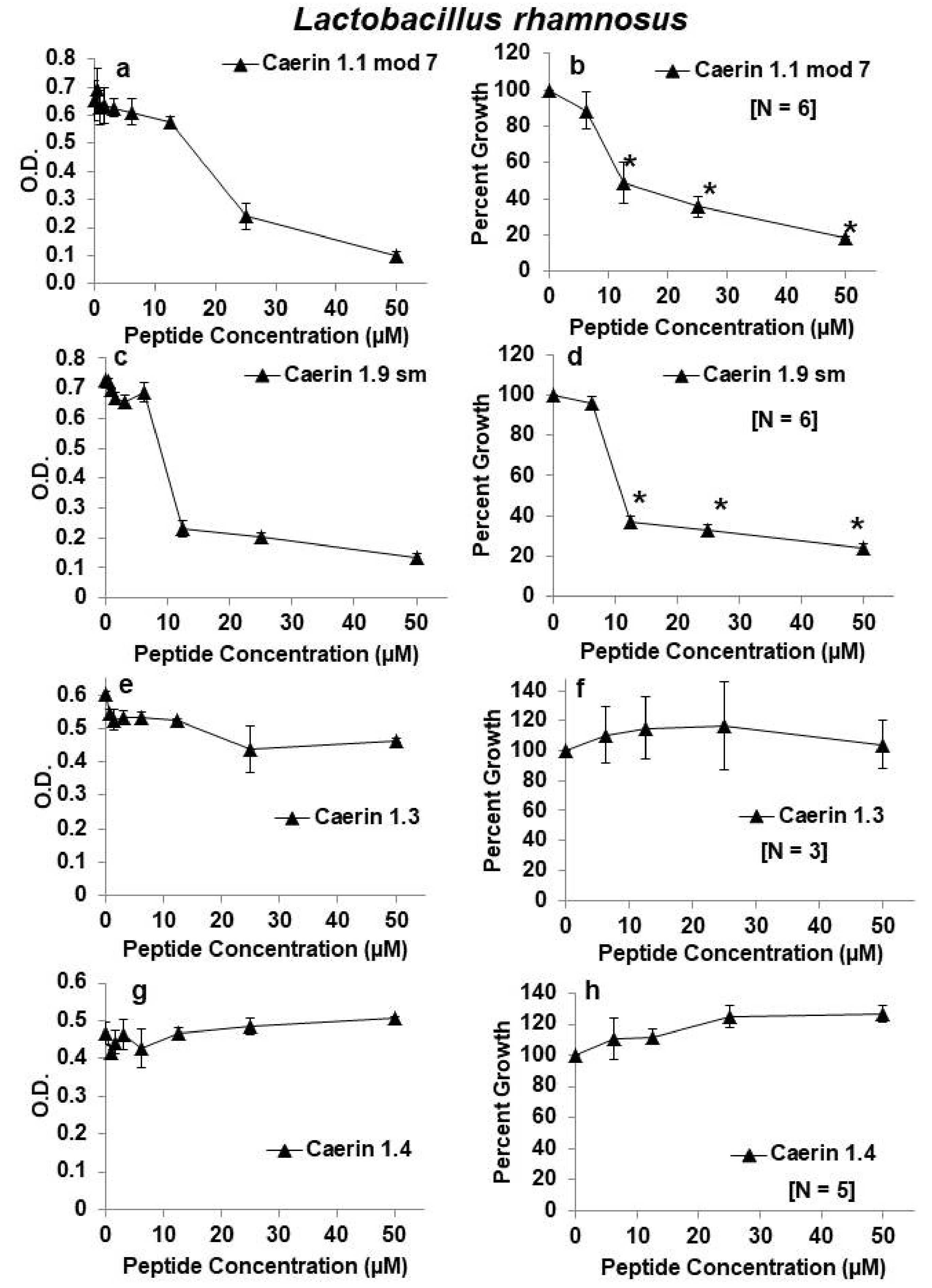
2.1. Effects of Caerin 1 Peptides on Growth of Lactobacillus rhamnosus
2.2. Effects of Caerin 1 Peptides on Growth of Lactobacillus crispatus
2.3. Comparison of the Effects of Caerin 1 Peptides on HIV Infection and Growth of Lactobacilli
2.4. Effects of Caerin 1 Peptides on Growth of Neisseria lactamica
3. Discussion
4. Materials and Methods
4.1. Peptides
4.2. Bacteria and Bacterial Growth Assays
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Available online: www.who.int (accessed on 6 July 2020).
- Gona, P.N.; Gona, C.M.; Ballout, S.; Rao, S.R.; Kimokoti, R.; Mapoma, C.C.; Mokdad, A.H. Burden and changes in HIV/AIDS morbidity and mortality in Southern Africa Development Community Countries, 1990–2017. BMC Public Health 2020, 20, 867. [Google Scholar] [CrossRef] [PubMed]
- Abdool Karim, Q.; Abdool Karim, S.S.; Frohlich, J.A.; Grobler, A.C.; Baxter, C.; Mansoor, L.E.; Kharsany, A.B.M.; Sibeko, S.; Mlisana, K.P.; Omar, Z.; et al. Effectiveness and safety of tenofovir gel, an antiretroviral microbicide, for the prevention of HIV infection in women. Science 2010, 329, 1168–1174. [Google Scholar] [CrossRef] [PubMed]
- Baeten, J.M.; Palanee-Phillips, T.; Brown, E.R.; Schwartz, K.; Soto-Torres, L.E.; Govender, V.; Jeenarain, N.; Gaffoor, A.; Martinson, F.; Makanani, B.; et al. Use of a vaginal ring containing dapivirine for HIV-1 prevention in women. N. Engl. J. Med. 2016, 37, 2121–2132. [Google Scholar] [CrossRef] [PubMed]
- Nel, A.; van Niekerk, N.; Kapiga, S.; Bekker, L.G.; Gama, C.; Gill, K.; Kamali, A.; Kotze, P.; Louw, C.; Mabude, Z.; et al. Safety and efficacy of a dapivirine vaginal ring for HIV prevention in women. N. Engl. J. Med. 2016, 375, 2133–2143. [Google Scholar] [CrossRef]
- Cohen, M.S.; Council, O.D.; Chen, J.S. Sexually transmitted infections and HIV in the era of antiretroviral treatment and prevention: The biologic basis for epidemiologic synergy. J. Int. AIDS Soc. 2019, 22, e25355. [Google Scholar] [CrossRef]
- McKinnon, L.R.; Liebenberg, L.J.; Yende-Zuma, N.; Archary, S.; Ngcapu, S.; Sivro, A.; Nagelkerke, N.; Lerma, G.G.; Kashuba, A.D.; Masson, L.; et al. Genital inflammation undermines the effectiveness of tenofovir gel in preventing HIV acquisition in women. Nat. Med. 2018, 24, 491–496. [Google Scholar] [CrossRef]
- Martin, H.L., Jr.; Richardson, B.A.; Nyange, P.M.; Lavreys, L.; Hillier, S.L.; Chohan, B.; Mandaliya, K.; Ndinya-Achola, J.L.; Bwayo, J.; Kreiss, J. Vaginal lactobacilli, microbial flora, and risk of human immunodeficiency virus type 1 and sexually transmitted disease acquisition. J. Infect. Dis. 1999, 180, 1863–1868. [Google Scholar] [CrossRef]
- McClelland, R.S.; Lingappa, J.R.; Srinivasan, S.; Kinuthia, J.; John-Steward, G.S.; Jaoko, W.; Richardson, G.A.; Yuhas, K.; Fiedler, T.L.; Mandaliya, K.N.; et al. Key vaginal bacteria associated with increased risk of HIV acquisition in African women: A nested case-control study. Lancet Infect Dis. 2018, 18, 554–564. [Google Scholar] [CrossRef]
- VanCompernolle, S.E.; Taylor, R.J.; Oswald-Richter, K.; Jiang, J.; Youree, B.E.; Bowie, J.H.; Tyler, M.J.; Conlon, J.M.; Wade, D.; KewalRamani, V.N.; et al. Amphibian antimicrobial skin peptides potently inhibit HIV infection and transfer of virus from dendritic cells to T cells. J. Virol. 2005, 79, 11598–11606. [Google Scholar] [CrossRef]
- VanCompernolle, S.; Smith, P.B.; Bowie, J.H.; Tyler, M.J.; Unutmaz, D.; Rollins-Smith, L.A. Inhibition of HIV infection by caerin 1 antimicrobial peptides. Peptides 2015, 71, 296–303. [Google Scholar] [CrossRef]
- Dizzell, A.; Nazli, A.; Reid, G.; Kaushic, C. Protective effect of probiotic bacteria and estrogen in preventing HIV-1-mediated impairment of epithelial barrier integrity in female genital tract. Cells 2019, 8, 1120. [Google Scholar] [CrossRef] [PubMed]
- Gosmann, C.; Anahtar, M.N.; Handley, S.A.; Farcasanu, M.; Abu-Ali, G.; Bowman, B.A.; Padavattan, N.; Desai, C.; Droit, L.; Moodley, A.; et al. Lactobacillus-deficient cervicovaginal bacterial communities are associated with increased HIV acquisition in young South African women. Immunity 2017, 46, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Steinborner, S.T.; Currie, G.J.; Bowie, J.H.; Wallace, J.C.; Tyler, M.J. New antibiotic caerin 1 peptides from the skin secretion of the Australian tree frog Litoria chloris. Comparison of the activities of the caerin 1 peptides from the genus Litoria. J. Peptide Res. 1998, 51, 121–126. [Google Scholar]
- Pukala, T.L.; Bertozzi, T.; Donnellan, S.C.; Bowie, J.H.; Surinya-Johnson, K.H.; Liu, Y.; Jackway, R.J.; Doyle, J.R.; Llewellyn, L.E.; Tyler, M.J. Host-defence peptide profiles of the skin secretions of interspecific hybrid tree frogs and their parents, female Litoria splendida and male Litoria caerulea. FEBS J. 2006, 273, 3511–3519. [Google Scholar] [CrossRef]
- Wong, H.; Bowie, J.H.; Carver, J.A. The solution structure and activity of caerin 1.1, an antimicrobial peptide from the Australian green tree frog, Litoria splendida. Eur. J. Biochem. 1997, 247, 545–557. [Google Scholar] [CrossRef]
- Pukala, T.L.; Brinkworth, C.S.; Carver, J.A.; Bowie, J.H. Investigating the importance of the flexible hinge in caerin 1.1: Solution structures and activity of two synthetically modified caerin peptides. Biochemistry 2004, 43, 937–944. [Google Scholar] [CrossRef]
- Wang, T.; Andreazza, H.J.; Pukala, T.L.; Sherman, P.J.; Calabrese, A.N.; Bowie, J.H. Histidine-containing host-defence skin peptides of anurans bind Cu2+. An electrospray ionisation mass spectrometry and computational modelling study. Rapid Commun. Mass Spectrom. 2011, 25, 1209–1221. [Google Scholar] [CrossRef]
- Snyder, L.A.; Saunders, N.J. The majority of genes in the pathogenic Neisseria species are present in the non-pathogenic Neisseria lactamica, including those designated as ‘virulence genes’. BMC Genom. 2006, 7, 128. [Google Scholar] [CrossRef]
- Dover, S.E.; Aroutcheva, A.A.; Faro, S.; Chikindas, M.L. Natural antimicrobials and their role in vaginal health: A short review. Int. J. Probiotics Prebiotics 2008, 3, 219–230. [Google Scholar]
- Ravel, J.; Gajer, P.; Abdo, Z.; Schneider, G.M.; Koenig, S.S.K.; McCulle, S.L.; Karlebach, S.; Gorle, R.; Russell, J.; Tacket, C.O.; et al. Vaginal microbiome of reproductive-age women. Proc. Natl. Acad. Sci. USA 2011, 108, 4680–4687. [Google Scholar] [CrossRef]
- Oakley, B.B.; Fiedler, T.L.; Marrazzo, J.M.; Fredricks, D.N. Diversity of human vaginal bacterial communities and associations with clinically defined bacterial vaginosis. Appl. Environ. Microbiol. 2008, 74, 4898–4909. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, S.; Liu, C.; Mitchell, C.M.; Fiedler, T.L.; Thomas, K.K.; Agnew, K.J.; Marrazzo, J.M.; Fredricks, D.N. Temporal variability of human vaginal bacteria and relationship with bacterial vaginosis. PLoS ONE 2010, 5, e10197. [Google Scholar] [CrossRef] [PubMed]
- McKinnon, L.R.; Achilles, S.L.; Bradshaw, C.S.; Burgener, A.; Crucitti, T.; Fredricks, D.N.; Jaspan, H.B.; Kaul, R.; Kaushic, C.; Klatt, N.; et al. The evolving facets of bacterial vaginosis: Implications for HIV transmission. AIDS Res. Hum. Retrov. 2019, 35, 219–228. [Google Scholar] [CrossRef] [PubMed]
- Tahara, T.; Kanatani, K. Isolation and partial characterization of crispacin A, a cell-associated bacteriocin produced by Lacto bacillus crispatus JCM 2009. FEMS Microbiol. Lett. 1997, 147, 287–290. [Google Scholar] [CrossRef][Green Version]
- Stoyancheva, G.; Marzotto, M.; Dellaglio, F.; Torriani, S. Bacteriocin production and gene sequencing analysis from vaginal Lactobacillus strains. Arch. Microbiol. 2014, 196, 645–653. [Google Scholar] [CrossRef]
- Mokoena, M.P. Lactic acid bacteria and their bacteriocins: Classification, biosynthesis, and applications against uropathogens: A mini-review. Molecules 2017, 22, 1255. [Google Scholar] [CrossRef]
- Pusch, O.; Kalyanaraman, R.; Tucker, L.D.; Wells, J.M.; Ramratnam, B.; Boden, D. An anti-HIV microbicide engineered in commensal bacteria: Secretion of HIV-1 fusion inhibitors by lactobacilli. AIDS 2006, 20, 1917–1922. [Google Scholar] [CrossRef]
- Lagenaur, L.A.; Sanders-Beer, B.E.; Brichacek, B.; Pal, R.; Liu, X.; Liu, Y.; Yu, R.; Venzon, D.; Lee, P.P.; Hamer, D.H. Prevention of vaginal SHIV transmission in macaques by a live recombinant Lactobacillus. Mucosal Immunol. 2011, 4, 648–657. [Google Scholar] [CrossRef]
- Zuend, C.F.; Nomellini, J.F.; Smit, J.; Horwitz, M.S. A Caulobacter crescentus microbicide protects from vaginal infection with HIV-1JR-CSF in humanized bone marrow-liver-thymus mice. J. Virol. 2019, 93, e00614-19. [Google Scholar] [CrossRef]
- Pask, J.D.; Woodhams, D.C.; Rollins-Smith, L.A. The ebb and flow of antimicrobial skin peptides defends northern leopard frogs (Rana pipiens) against chytridiomycosis. Global Change Biol. 2012, 18, 1231–1238. [Google Scholar] [CrossRef]
- De Man, J.D.; Rogosa, M.; Sharpe, M.E. A medium for the cultivation of Lactobacilli. J. Appl. Bacteriol. 1960, 23, 130–135. [Google Scholar] [CrossRef]
- Cartwright, C.P.; Stock, F.; Gill, V.J. Improved enrichment broth for cultivation of fastidious organisms. J. Clin. Microbiol. 1994, 32, 1825–1826. [Google Scholar] [CrossRef] [PubMed]






| Peptide | Species of Origin | Sequence | Mass |
|---|---|---|---|
| Caerin 1.1 | Litoria caerulea | GLLSVLGSVAKHVLPHVVPVIAEHL-NH2 | 2582 |
| Caerin 1.1 mod 7 | synthetic | GLLSVLGSVAKHVLPHVVPVIAAAL-NH2 | 2458 |
| Caerin 1.2 | L. caerulea | GLLGVLGSVAKHVLPHVVPVIAEHL-NH2 | 2552 |
| Caerin 1.3 | L. caerulea | GLLSVLGSVAQHVLPHVVPVIAEHL-NH2 | 2582 |
| Caerin 1.4 | L. caerulea, L. gilleni | GLLSSLSSVAKHVLPHVVPVIAEHL-NH2 | 2600 |
| Caerin 1.9 | L. chloris | GLFGVLGSIAKHVLPHVVPVIAEKL-NH2 | 2591 |
| Caerin 1.9 sm | synthetic | GLFGVLGSIAKHLLPHVVPVIAEKL-NH2 | 2605 |
| Caerin 1.10 | L. splendida | GLLSVLGSVAKHVLPHVVPVIAEKL-NH2 | 2573 |
| Caerin 1.20 | L. caerulea/L. splendida | GLFGILGSVAKHVLPHVIPVVAEHL-NH2 | 2600 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rollins-Smith, L.A.; Smith, P.B.; Ledeczi, A.M.; Rowe, J.M.; Reinert, L.K. Caerin 1 Antimicrobial Peptides that Inhibit HIV and Neisseria May Spare Protective Lactobacilli. Antibiotics 2020, 9, 661. https://doi.org/10.3390/antibiotics9100661
Rollins-Smith LA, Smith PB, Ledeczi AM, Rowe JM, Reinert LK. Caerin 1 Antimicrobial Peptides that Inhibit HIV and Neisseria May Spare Protective Lactobacilli. Antibiotics. 2020; 9(10):661. https://doi.org/10.3390/antibiotics9100661
Chicago/Turabian StyleRollins-Smith, Louise A., Patricia B. Smith, Anna M. Ledeczi, Julia M. Rowe, and Laura K. Reinert. 2020. "Caerin 1 Antimicrobial Peptides that Inhibit HIV and Neisseria May Spare Protective Lactobacilli" Antibiotics 9, no. 10: 661. https://doi.org/10.3390/antibiotics9100661
APA StyleRollins-Smith, L. A., Smith, P. B., Ledeczi, A. M., Rowe, J. M., & Reinert, L. K. (2020). Caerin 1 Antimicrobial Peptides that Inhibit HIV and Neisseria May Spare Protective Lactobacilli. Antibiotics, 9(10), 661. https://doi.org/10.3390/antibiotics9100661




