Combination Therapy Using Low-Concentration Oxacillin with Palmitic Acid and Span85 to Control Clinical Methicillin-Resistant Staphylococcus aureus
Abstract
1. Introduction
2. Results and Discussion
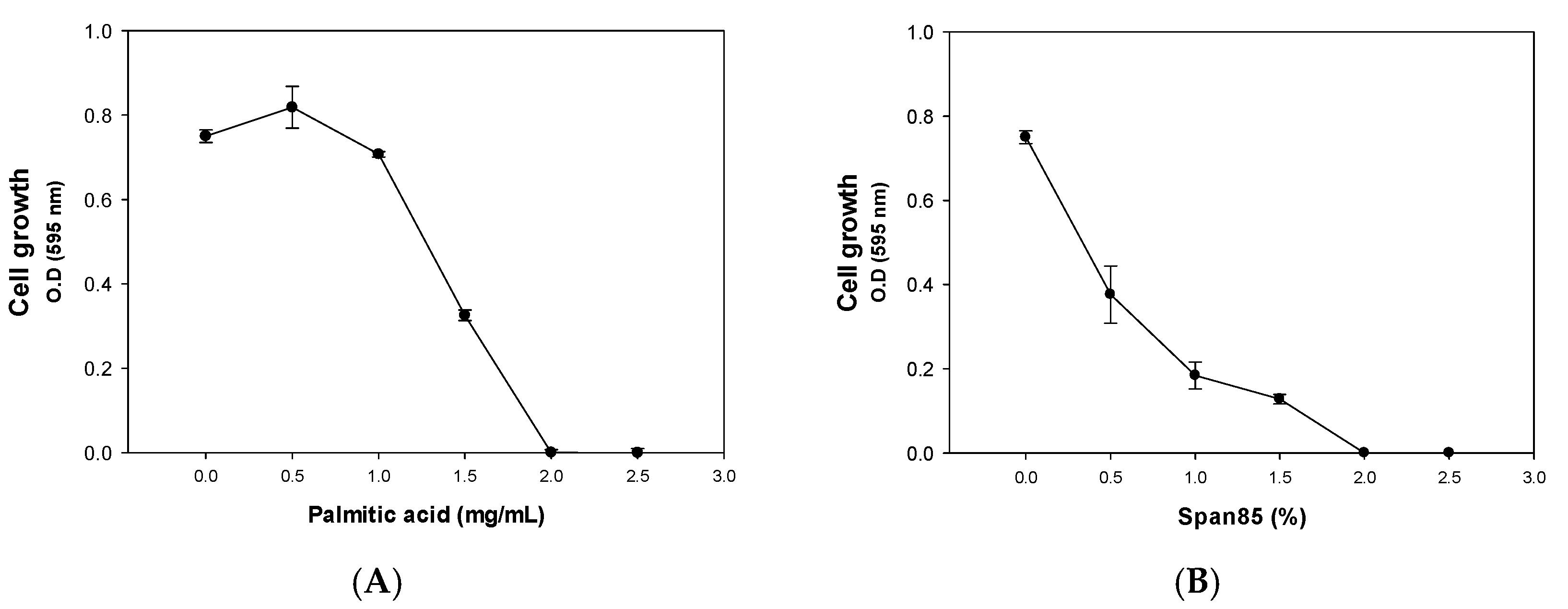
2.1. Inhibitory Effect of Palmitic Acid and Span85
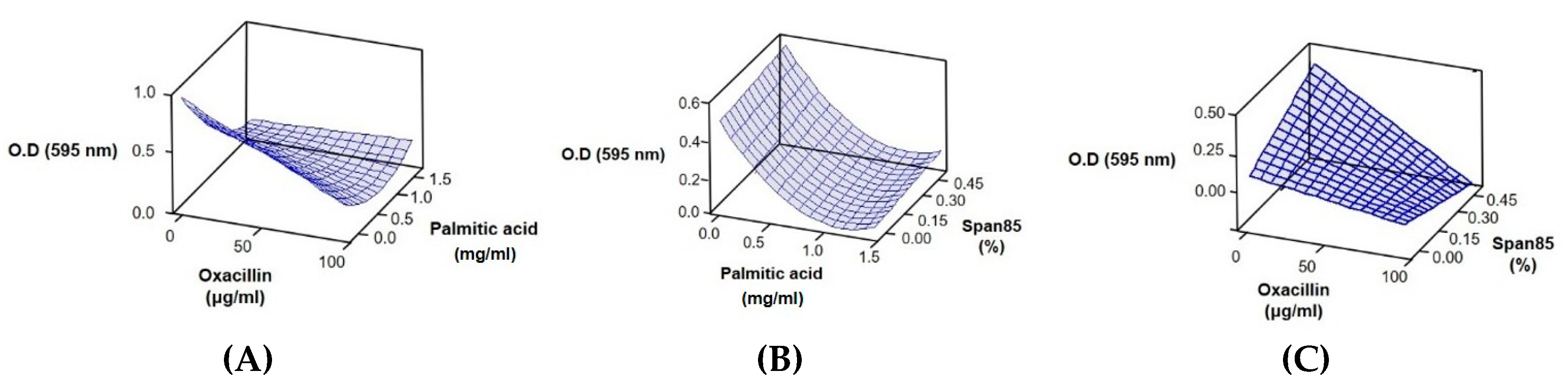
2.2. Response Surface Methodology Analysis to Study the Effect of the Interaction of Different Antibacterial Agents
0.36603X22 + 0.00565111X1X2 − 0.00884X1X3
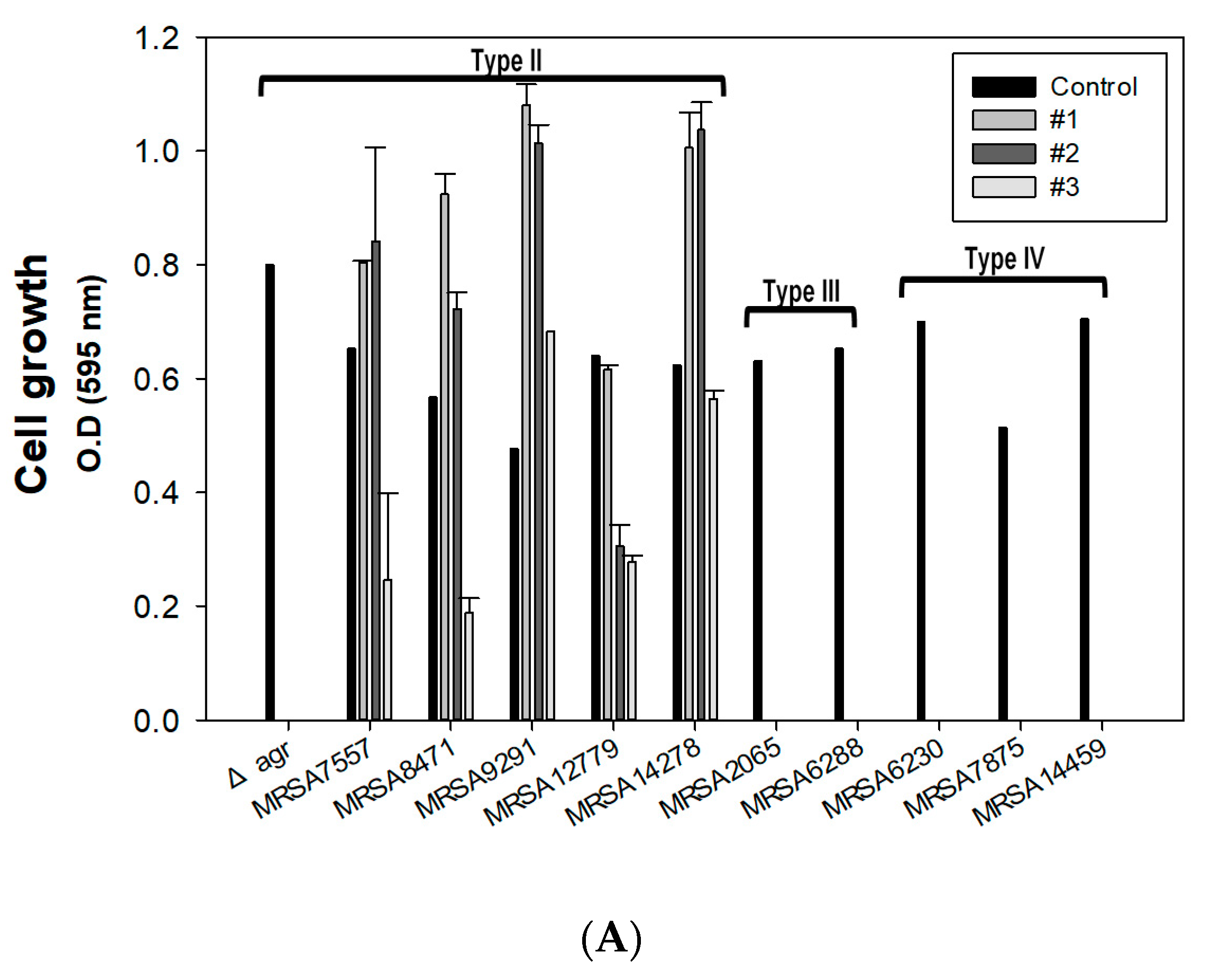
2.3. Effect of Combined Therapy on the Δagr Strain and Clinically Isolated Strains
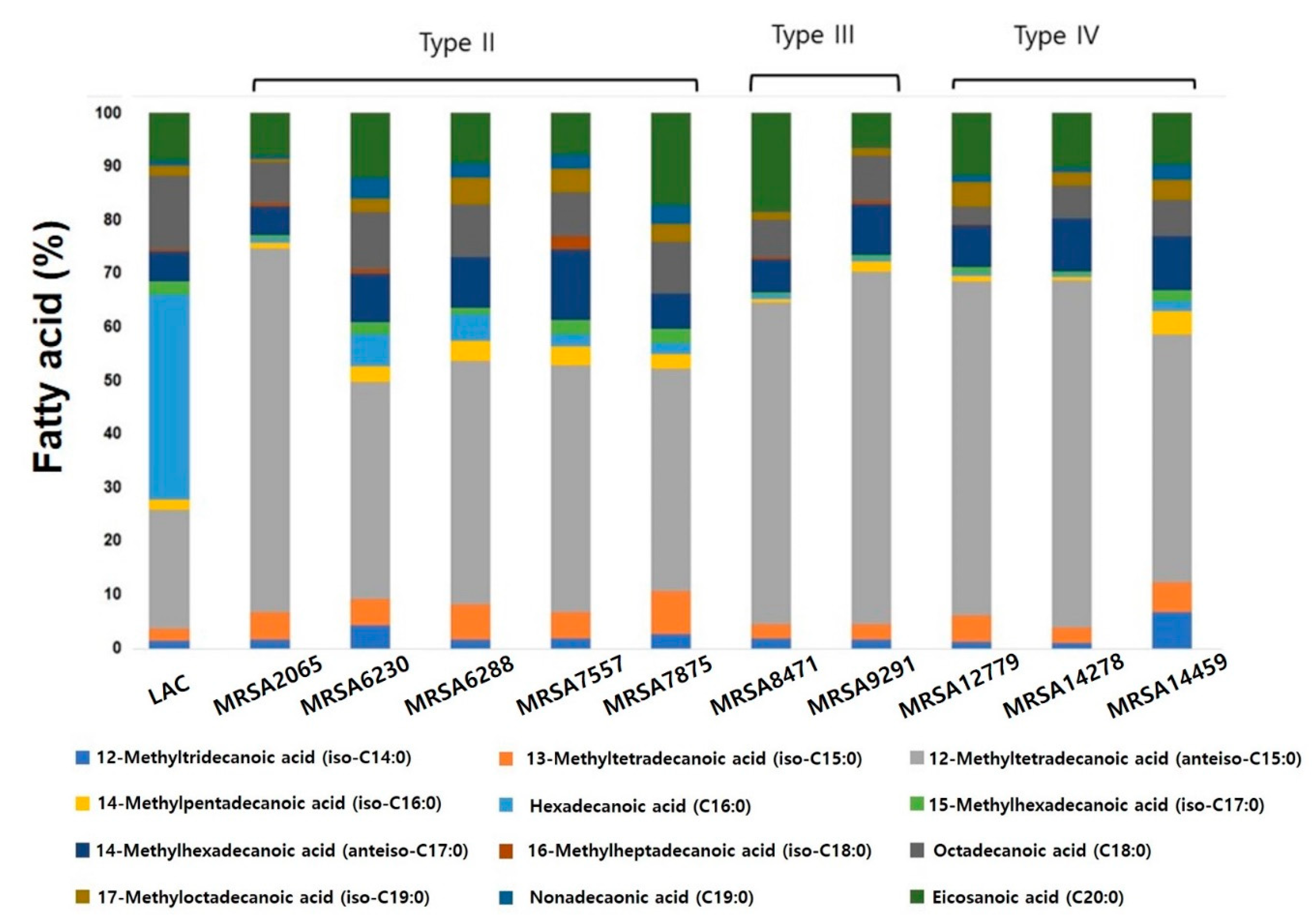
2.4. Characterization of Clinically Isolated strains with Phospholipid Fatty Acid (PLFA) Analysis
3. Materials and Methods
3.1. Bacterial Strains, Media, and Culture Conditions
3.2. Antibacterial Agents
3.3. Analysis of Cell Growth and Biofilm Formation
3.4. Response Surface Methodology Analysis
3.5. PLFA Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Davies, J.; Davies, D. Origins and evolution of antibiotic resistance. Microbiol. Mol. Biol. Rev. 2010, 74, 417–433. [Google Scholar] [CrossRef] [PubMed]
- Chambers, H.F.; Deleo, F.R. Waves of resistance: Staphylococcus aureus in the antibiotic era. Nat. Rev. Microbiol. 2009, 7, 629–641. [Google Scholar] [CrossRef] [PubMed]
- Grundstad, M.L.; Parlet, C.P.; Kwiecinski, J.M.; Kavanaugh, J.S.; Crosby, H.A.; Cho, Y.S.; Heilmann, K.; Diekema, D.J.; Horswill, A.R. Quorum Sensing, Virulence, and Antibiotic Resistance of USA100 Methicillin-Resistant Staphylococcus aureus Isolates. mSphere 2019, 4, e00553-19. [Google Scholar] [CrossRef] [PubMed]
- Salam, A.M.; Quave, C.L. Targeting Virulence in Staphylococcus aureus by Chemical Inhibition of the Accessory Gene Regulator System In Vivo. mSphere 2018, 3, e00500-17. [Google Scholar] [CrossRef] [PubMed]
- Bard, J.D.; Hindler, J.A.; Gold, H.S.; Limbago, B. Rationale for Eliminating Staphylococcus Breakpoints for beta-Lactam Agents Other Than Penicillin, Oxacillin or Cefoxitin, and Ceftaroline. Clin. Infect. Dis. 2014, 58, 1287–1296. [Google Scholar] [CrossRef]
- Saber, H.; Jasni, A.S.; Jamaluddin, T.; Ibrahim, R. A Review of Staphylococcal Cassette Chromosome mec (SCCmec) Types in Coagulase-Negative Staphylococci (CoNS) Species. Malays. J. Med. Sci. 2017, 24, 7–18. [Google Scholar] [CrossRef]
- Gonzales, P.R.; Pesesky, M.W.; Bouley, R.; Ballard, A.; Biddy, B.A.; Suckow, M.A.; Wolter, W.R.; Schroeder, V.A.; Burnham, C.-A.D.; Mobashery, S.; et al. Synergistic, collaterally sensitive β-lactam combinations suppress resistance in MRSA. Nat. Chem. Biol. 2015, 11, 855–861. [Google Scholar] [CrossRef]
- Tyers, M.; Wright, G.D. Drug combinations: A strategy to extend the life of antibiotics in the 21st century. Nat. Rev. Microbiol. 2019, 17, 141–155. [Google Scholar] [CrossRef]
- Song, H.-S.; Choi, T.-R.; Han, Y.-H.; Park, Y.-L.; Park, J.Y.; Yang, S.-Y.; Bhatia, S.K.; Gurav, R.; Kim, Y.-G.; Kim, J.-S.; et al. Increased resistance of a methicillin-resistant Staphylococcus aureus Δagr mutant with modified control in fatty acid metabolism. AMB Express 2020, 10, 64. [Google Scholar] [CrossRef]
- Ng’uni, T.; Mothlalamme, T.; Daniels, R.; Klaasen, J.; Fielding, B. Additive antibacterial activity of naringenin and antibiotic combinations against multidrug resistant Staphylococcus aureus. Afr. J. Microbiol. Res. 2015, 9, 1513–1518. [Google Scholar] [CrossRef][Green Version]
- Desbois, A.P.; Lawlor, K.C. Antibacterial activity of long-chain polyunsaturated fatty acids against Propionibacterium acnes and Staphylococcus aureus. Mar. Drugs 2013, 11, 4544–4557. [Google Scholar] [CrossRef] [PubMed]
- Alcaraz, L.E.; Blanco, S.E.; Puig, O.N.; Tomas, F.; Ferretti, F.H. Antibacterial activity of flavonoids against methicillin-resistant Staphylococcus aureus strains. J. Theor. Biol. 2000, 205, 231–240. [Google Scholar] [CrossRef] [PubMed]
- Al-Doori, Z.; Morrison, D.; Edwards, G.; Gemmell, C. Susceptibility of MRSA to triclosan. J. Antimicrob. Chemother. 2003, 51, 185–186. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, M.T.; Hanzelmann, D.; Hartner, T.; Peschel, A.; Gotz, F. Skin-Specific Unsaturated Fatty Acids Boost the Staphylococcus aureus Innate Immune Response. Infect. Immun 2016, 84, 205–215. [Google Scholar] [CrossRef] [PubMed]
- Yoon, B.K.; Jackman, J.A.; Valle-Gonzalez, E.R.; Cho, N.J. Antibacterial Free Fatty Acids and Monoglycerides: Biological Activities, Experimental Testing, and Therapeutic Applications. Int. J. Mol. Sci 2018, 19, 1114. [Google Scholar] [CrossRef] [PubMed]
- Carta, G.; Murru, E.; Banni, S.; Manca, C. Palmitic Acid: Physiological Role, Metabolism and Nutritional Implications. Front. Physiol. 2017, 8, 902. [Google Scholar] [CrossRef]
- Ueda, Y.; Mashima, K.; Miyazaki, M.; Hara, S.; Takata, T.; Kamimura, H.; Takagi, S.; Jimi, S. Inhibitory effects of polysorbate 80 on MRSA biofilm formed on different substrates including dermal tissue. Sci. Rep. 2019, 9, 3128. [Google Scholar] [CrossRef]
- Walton, J.T.; Hill, D.J.; Protheroe, R.G.; Nevill, A.; Gibson, H. Investigation into the effect of detergents on disinfectant susceptibility of attached Escherichia coli and Listeria monocytogenes. J. Appl. Microbiol. 2008, 105, 309–315. [Google Scholar] [CrossRef]
- Bunjes, H.; Koch, M.H.; Westesen, K. Influence of emulsifiers on the crystallization of solid lipid nanoparticles. J. Pharm. Sci. 2003, 92, 1509–1520. [Google Scholar] [CrossRef]
- Rudkin, J.K.; Edwards, A.M.; Bowden, M.G.; Brown, E.L.; Pozzi, C.; Waters, E.M.; Chan, W.C.; Williams, P.; O’Gara, J.P.; Massey, R.C. Methicillin resistance reduces the virulence of healthcare-associated methicillin-resistant Staphylococcus aureus by interfering with the agr quorum sensing system. J. Infect. Dis. 2012, 205, 798–806. [Google Scholar] [CrossRef]
- Jeon, J.M.; Rajesh, T.; Song, E.; Lee, H.W.; Yang, Y.H. Media optimization of Corynebacterium glutamicum for succinate production under oxygen-deprived condition. J. Microbiol. Biotechnol. 2013, 23, 211–217. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, S.K.; Lee, B.R.; Sathiyanarayanan, G.; Song, H.S.; Kim, J.; Jeon, J.M.; Kim, J.H.; Park, S.H.; Yu, J.H.; Park, K.; et al. Medium engineering for enhanced production of undecylprodigiosin antibiotic in Streptomyces coelicolor using oil palm biomass hydrolysate as a carbon source. Bioresour. Technol. 2016, 217, 141–149. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, S.K.; Gurav, R.; Choi, T.-R.; Jung, H.-R.; Yang, S.-Y.; Moon, Y.-M.; Song, H.-S.; Jeon, J.-M.; Choi, K.-Y.; Yang, Y.-H. Bioconversion of plant biomass hydrolysate into bioplastic (polyhydroxyalkanoates) using Ralstonia eutropha 5119. Bioresour. Technol. 2019, 271, 306–315. [Google Scholar] [CrossRef] [PubMed]
- Cokol, M.; Kuru, N.; Bicak, E.; Larkins-Ford, J.; Aldridge, B.B. Efficient measurement and factorization of high-order drug interactions in Mycobacterium tuberculosis. Sci. Adv. 2017, 3, e1701881. [Google Scholar] [CrossRef]
- Cokol-Cakmak, M.; Bakan, F.; Cetiner, S.; Cokol, M. Diagonal Method to Measure Synergy Among Any Number of Drugs. J. Vis. Exp. 2018, 57713. [Google Scholar] [CrossRef]
- Enright, M.C.; Day, N.P.; Davies, C.E.; Peacock, S.J.; Spratt, B.G. Multilocus sequence typing for characterization of methicillin-resistant and methicillin-susceptible clones of Staphylococcus aureus. J. Clin. Microbiol. 2000, 38, 1008–1015. [Google Scholar] [CrossRef]
- Harmsen, D.; Claus, H.; Witte, W.; Rothganger, J.; Claus, H.; Turnwald, D.; Vogel, U. Typing of methicillin-resistant Staphylococcus aureus in a university hospital setting by using novel software for spa repeat determination and database management. J. Clin. Microbiol. 2003, 41, 5442–5448. [Google Scholar] [CrossRef]
- Sanchez, C.J., Jr.; Mende, K.; Beckius, M.L.; Akers, K.S.; Romano, D.R.; Wenke, J.C.; Murray, C.K. Biofilm formation by clinical isolates and the implications in chronic infections. BMC Infect. Dis. 2013, 13, 47. [Google Scholar] [CrossRef]
- Garcia-Fernandez, E.; Koch, G.; Wagner, R.M.; Fekete, A.; Stengel, S.T.; Schneider, J.; Mielich-Suss, B.; Geibel, S.; Markert, S.M.; Stigloher, C.; et al. Membrane Microdomain Disassembly Inhibits MRSA Antibiotic Resistance. Cell 2017, 171, 1354–1367.e20. [Google Scholar] [CrossRef]
- Keren, I.; Kaldalu, N.; Spoering, A.; Wang, Y.; Lewis, K. Persister cells and tolerance to antimicrobials. Fems. Microbiol. Lett. 2004, 230, 13–18. [Google Scholar] [CrossRef]
- Bisignano, C.; Ginestra, G.; Smeriglio, A.; La Camera, E.; Crisafi, G.; Franchina, F.A.; Tranchida, P.Q.; Alibrandi, A.; Trombetta, D.; Mondello, L.; et al. Study of the Lipid Profile of ATCC and Clinical Strains of Staphylococcus aureus in Relation to Their Antibiotic Resistance. Molecules 2019, 24, 1276. [Google Scholar] [CrossRef] [PubMed]
- Poger, D.; Caron, B.; Mark, A.E. Effect of methyl-branched fatty acids on the structure of lipid bilayers. J. Phys. Chem. B 2014, 118, 13838–13848. [Google Scholar] [CrossRef] [PubMed]
- Cheung, G.Y.; Wang, R.; Khan, B.A.; Sturdevant, D.E.; Otto, M. Role of the accessory gene regulator agr in community-associated methicillin-resistant Staphylococcus aureus pathogenesis. Infect. Immun. 2011, 79, 1927–1935. [Google Scholar] [CrossRef] [PubMed]


| Factor | Level of Factor | ||
|---|---|---|---|
| −1 | 0 | 1 | |
| Oxacillin (X1, μg/mL) | 0 | 50 | 100 |
| Palmitic acid (X2, mg/mL) | 0 | 0.75 | 1.5 |
| Span85 (X3, %) | 0 | 0.25 | 0.5 |
| Runs | Oxacillin (μg/mL) | Palmitic Acid (mg/mL) | Span85 (% v/v) | OD (595 nm) |
|---|---|---|---|---|
| 1 | 0 | 0 | 0.25 | 1.07 ± 0.03 |
| 2 | 100 | 0 | 0.25 | 0.12 ± 0.03 |
| 3 | 0 | 1.5 | 0.25 | 0.10 ± 0.03 |
| 4 | 100 | 1.5 | 0.25 | 0.00 ± 0.00 |
| 5 | 0 | 0.75 | 0 | 0.02 ± 0.02 |
| 6 | 100 | 0.75 | 0 | 0.02 ± 0.00 |
| 7 | 0 | 0.75 | 0.5 | 0.44 ± 0.00 |
| 8 | 100 | 0.75 | 0.5 | 0.00 ± 0.00 |
| 9 | 50 | 0 | 0 | 0.51 ± 0.07 |
| 10 | 50 | 1.5 | 0 | 0.10 ± 0.00 |
| 11 | 50 | 0 | 0.5 | 0.43 ± 0.02 |
| 12 | 50 | 1.5 | 0.5 | 0.05 ± 0.03 |
| 13 | 50 | 0.75 | 0.25 | 0.08 ± 0.02 |
| 14 | 50 | 0.75 | 0.25 | 0.01 ± 0.00 |
| 15 | 50 | 0.75 | 0.25 | 0.07 ± 0.00 |
| Source | DF | SS | MS | F-Value | Prob > F |
|---|---|---|---|---|---|
| Model | 6 | 3.36120 | 0.56020 | 55.67 | 0.000 |
| Error | 38 | 0.38242 | 0.01006 | ||
| Total | 44 | 3.74362 |
| Entry | Oxacillin (μg/mL) | Palmitic Acid (mg/mL) | Span85 (%) |
|---|---|---|---|
| #1 | 15 | 1.3 | 0.1 |
| #2 | 50 | 1 | 0.08 |
| #3 | 100 | 0.3 | 0.4 |
| Name | Type | SCCmec Type | Oxacillin MIC (μg/mL) | Spa Type | MLST (ST) |
|---|---|---|---|---|---|
| LAC | MRSA | IV | 20 | t008 | 8 |
| 2065 | MRSA | III | 1024 | t037 | 239 |
| 6230 | MRSA | IV | 128 | t324 | 72 |
| 6288 | MRSA | III | 1024 | t037 | 239 |
| 7557 | MRSA | II | 1024 | t9353 | 5 |
| 7875 | MRSA | IV | 128 | t664 | 72 |
| 8471 | MRSA | II | 1024 | t9353 | 5 |
| 9291 | MRSA | II | 1024 | t601 | 5 |
| 12779 | MRSA | II | 1024 | t2460 | 5 |
| 14278 | MRSA | II | 1024 | t9353 | 5 |
| 14459 | MRSA | IV | 1024 | t324 | 72 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, H.-S.; Choi, T.-R.; Bhatia, S.K.; Lee, S.M.; Park, S.L.; Lee, H.S.; Kim, Y.-G.; Kim, J.-S.; Kim, W.; Yang, Y.-H. Combination Therapy Using Low-Concentration Oxacillin with Palmitic Acid and Span85 to Control Clinical Methicillin-Resistant Staphylococcus aureus. Antibiotics 2020, 9, 682. https://doi.org/10.3390/antibiotics9100682
Song H-S, Choi T-R, Bhatia SK, Lee SM, Park SL, Lee HS, Kim Y-G, Kim J-S, Kim W, Yang Y-H. Combination Therapy Using Low-Concentration Oxacillin with Palmitic Acid and Span85 to Control Clinical Methicillin-Resistant Staphylococcus aureus. Antibiotics. 2020; 9(10):682. https://doi.org/10.3390/antibiotics9100682
Chicago/Turabian StyleSong, Hun-Suk, Tae-Rim Choi, Shashi Kant Bhatia, Sun Mi Lee, Sol Lee Park, Hye Soo Lee, Yun-Gon Kim, Jae-Seok Kim, Wooseong Kim, and Yung-Hun Yang. 2020. "Combination Therapy Using Low-Concentration Oxacillin with Palmitic Acid and Span85 to Control Clinical Methicillin-Resistant Staphylococcus aureus" Antibiotics 9, no. 10: 682. https://doi.org/10.3390/antibiotics9100682
APA StyleSong, H.-S., Choi, T.-R., Bhatia, S. K., Lee, S. M., Park, S. L., Lee, H. S., Kim, Y.-G., Kim, J.-S., Kim, W., & Yang, Y.-H. (2020). Combination Therapy Using Low-Concentration Oxacillin with Palmitic Acid and Span85 to Control Clinical Methicillin-Resistant Staphylococcus aureus. Antibiotics, 9(10), 682. https://doi.org/10.3390/antibiotics9100682






