Opportunist Coinfections by Nontuberculous Mycobacteria and Fungi in Immunocompromised Patients
Abstract
:1. Introduction
2. Opportunistic Infections and Coinfection
3. The Aetiological Agents: NTM and Fungi
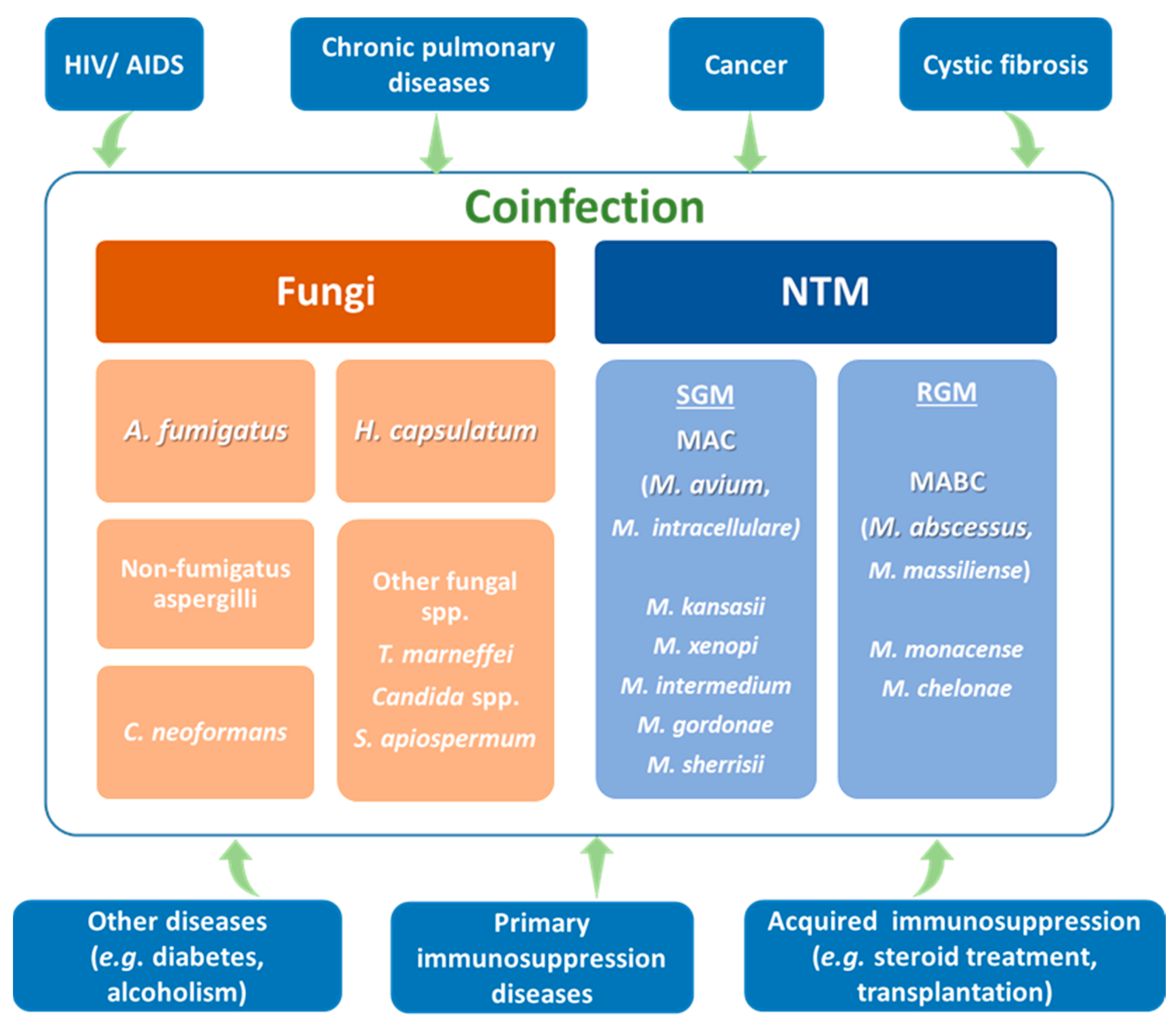
4. The Human Host: Predisposing Conditions for NTM, Fungal Infections and Coinfections
5. Coinfection by NTM and Fungi
6. Treatment: Major Challenges and Future Perspectives
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- WHO. The Top 10 Causes of Death. Available online: https://www.who.int/news-room/fact-sheets/detail/the-top-10-causes-of-death (accessed on 24 September 2020).
- Daniel-Wayman, S.; Ricotta, E.; Prevots, D.R.; Adjemian, J. Epidemiology of Nontuberculous Mycobacteriosis. Semin. Respir. Crit. Care Med. 2018, 39, 325–335. [Google Scholar] [CrossRef] [PubMed]
- Van Der Werf, M.J.; Kodmon, C.; Katalinić-Janković, V.; Kummik, T.; Soini, H.; Richter, E.; Papaventsis, D.; Tortoli, E.; Perrin, M.; Van Soolingen, D.; et al. Inventory study of non-tuberculous mycobacteria in the European Union. BMC Infect. Dis. 2014, 14, 62. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aksamit, T.R.; Philley, J.V.; Griffith, D.E. Nontuberculous mycobacterial (NTM) lung disease: The top ten essentials. Respir. Med. 2014, 108, 417–425. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Griffith, D.E.; Aksamit, T.; Brown-Elliott, B.A.; Catanzaro, A.; Daley, C.; Gordin, F.; Holland, S.M.; Horsburgh, R.; Huitt, G.; Iademarco, M.F.; et al. An Official ATS/IDSA Statement: Diagnosis, Treatment, and Prevention of Nontuberculous Mycobacterial Diseases. Am. J. Respir. Crit. Care Med. 2007, 175, 367–416. [Google Scholar] [CrossRef]
- ESCMID: ESGMYC. Available online: https://www.escmid.org/research_projects/study_groups/study_groups_g_n/mycobacterial_infection/ (accessed on 25 September 2020).
- NTM-NET. Available online: http://www.ntm-net.org/ (accessed on 25 September 2020).
- Richardson, M. Opportunistic and pathogenic fungi. J. Antimicrob. Chemother. 1991, 28, 1–11. [Google Scholar] [CrossRef]
- Working Groups: ISHAM. Available online: https://www.isham.org/working-groups (accessed on 25 September 2020).
- ESCMID: Mission & Objectives. Available online: https://www.escmid.org/research_projects/study_groups/study_groups_a_f/fungal_infection/mission_objectives/ (accessed on 25 September 2020).
- Reedy, J.L.; Bastidas, R.J.; Heitman, J. The Virulence of Human Pathogenic Fungi: Notes from the South of France. Cell Host Microbe 2007, 2, 77–83. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muskett, H.; Shahin, J.; Eyres, G.; Harvey, S.; Rowan, K.M.; Harrison, D. Risk factors for invasive fungal disease in critically ill adult patients: A systematic review. Crit. Care 2011, 15, R287. [Google Scholar] [CrossRef] [Green Version]
- Dekhuijzen, P.R.; Batsiou, M.; Bjermer, L.; Bosnic-Anticevich, S.; Chrystyn, H.; Papi, A.; Rodríguez-Roisin, R.; Fletcher, M.; Wood, L.; Cifra, A.; et al. Incidence of oral thrush in patients with COPD prescribed inhaled corticosteroids: Effect of drug, dose, and device. Respir. Med. 2016, 120, 54–63. [Google Scholar] [CrossRef] [Green Version]
- Ferreira, G.D.S.A.; Watanabe, A.L.C.; Trevizoli, N.D.C.; Jorge, F.M.F.; De Camposa, P.B.; Couto, C.D.F.; De Lima, L.V.; Raupp, D.R.L. Colonic Infection by Histoplasma capsulatum in a Liver Transplant Patient: A Case Report. Transplant. Proc. 2020, 52, 1413–1416. [Google Scholar] [CrossRef]
- Lim, S.M.S.; Sinnollareddy, M.; Sime, F.B. Challenges in Antifungal Therapy in Diabetes Mellitus. J. Clin. Med. 2020, 9, 2878. [Google Scholar] [CrossRef]
- Mann, S.; Tobolowsky, F.; Purohit, S.; Henao-Martínez, A.; Bajrovic, V.; Ramanan, P.; Wolfel, E.; Khazanie, P.; Barron, M.; Madinger, N.; et al. Cryptococcal pericarditis in a heart transplant recipient. Transpl. Infect. Dis. 2020, e13366. [Google Scholar] [CrossRef]
- Zarei, F.; Hashemi, S.J.; Salehi, M.; Mahmoudi, S.; Zibafar, E.; Ahmadinejad, Z.; Foroushani, A.R.; Ardi, P.; Ghazvini, R.D. Molecular characterization of fungi causing colonization and infection in organ transplant recipients: A one-year prospective study. Curr. Med. Mycol. 2020, 6, 30–35. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.-L.; Hospitals, T.; Hsieh, L.-Y.; Wang, A.-H.; Lo, H.-J. Characterization of Candida Species from Different Populations in Taiwan. Mycopathologia 2011, 172, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Vainionpää, A.; Tuomi, J.; Kantola, S.; Anttonen, V. Neonatal thrush of newborns: Oral candidiasis? Clin. Exp. Dent. Res. 2019, 5, 580–582. [Google Scholar] [CrossRef] [Green Version]
- Xiao, J.; Fogarty, C.T.; Wu, T.; Alkhers, N.; Zeng, Y.; Thomas, M.; Youssef, M.; Wang, L.; Cowen, L.; Abdelsalam, H.; et al. Oral health and Candida carriage in socioeconomically disadvantaged US pregnant women. BMC Pregnancy Childbirth 2019, 19, 1–13. [Google Scholar] [CrossRef]
- Waikhom, S.D.; Afeke, I.; Kwawu, G.S.; Mbroh, H.K.; Osei, G.Y.; Louis, B.; Deku, J.G.; Kasu, E.S.; Mensah, P.; Agede, C.Y.; et al. Prevalence of vulvovaginal candidiasis among pregnant women in the Ho municipality, Ghana: Species identification and antifungal susceptibility of Candida isolates. BMC Pregnancy Childbirth 2020, 20, 1–14. [Google Scholar] [CrossRef]
- Pang, W.; Shang, P.; Li, Q.; Xu, J.; Bi, L.; Zhong, J.; Pei, X. Prevalence of Opportunistic Infections and Causes of Death among Hospitalized HIV-Infected Patients in Sichuan, China. Tohoku J. Exp. Med. 2018, 244, 231–242. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fauchier, T.; Hasseine, L.; Gari-Toussaint, M.; Casanova, V.; Marty, P.M.; Pomares, C. Detection of Pneumocystis jirovecii by Quantitative PCR To Differentiate Colonization and Pneumonia in Immunocompromised HIV-Positive and HIV-Negative Patients. J. Clin. Microbiol. 2016, 54, 1487–1495. [Google Scholar] [CrossRef] [Green Version]
- José, R.J.; Brown, J.S. Opportunistic bacterial, viral and fungal infections of the lung. Medicine 2016, 44, 378–383. [Google Scholar] [CrossRef]
- Sobel, J. Vaginal Infections in Adult Women. Med. Clin. N. Am. 1990, 74, 1573–1602. [Google Scholar] [CrossRef]
- Meersseman, W.; Lagrou, K.; Spriet, I.; Maertens, J.; Verbeken, E.; Peetermans, W.E.; Van Wijngaerden, E. Significance of the isolation of Candida species from airway samples in critically ill patients: A prospective, autopsy study. Intensiv. Care Med. 2009, 35, 1526–1531. [Google Scholar] [CrossRef]
- Drell, T.; Lillsaar, T.; Tummeleht, L.; Simm, J.; Aaspõllu, A.; Väin, E.; Saarma, I.; Salumets, A.; Donders, G.G.G.; Metsis, M. Characterization of the Vaginal Micro- and Mycobiome in Asymptomatic Reproductive-Age Estonian Women. PLoS ONE 2013, 8, e54379. [Google Scholar] [CrossRef]
- Garnacho-Montero, J.; Olaechea, P.; Alvarez-Lerma, F.; Alvarez-Rocha, L.; Blanquer, J.; Galván, B.; Rodriguez, A.; Zaragoza, R.; Aguado, J.-M.; Mensa, J.; et al. Epidemiology, diagnosis and treatment of fungal respiratory infections in the critically ill patient. Rev. Esp. Quimioter. 2013, 26, 173–188. [Google Scholar]
- Neville, B.A.; D’Enfert, C.; Bougnoux, M.-E. Candida albicanscommensalism in the gastrointestinal tract. FEMS Yeast Res. 2015, 15, 15. [Google Scholar] [CrossRef] [Green Version]
- Bertolini, M.; Dongari-Bagtzoglou, A. The Relationship of Candida albicans with the Oral Bacterial Microbiome in Health and Disease. Adv. Exp. Med. Biol. 2019, 69–78. [Google Scholar] [CrossRef]
- Gow, N.A.R.; Hube, B. Importance of the Candida albicans cell wall during commensalism and infection. Curr. Opin. Microbiol. 2012, 15, 406–412. [Google Scholar] [CrossRef] [Green Version]
- Griffiths, E.C.; Pedersen, A.B.; Fenton, A.; Petchey, O.L. The nature and consequences of coinfection in humans. J. Infect. 2011, 63, 200–206. [Google Scholar] [CrossRef] [Green Version]
- Gorsich, E.; Etienne, R.S.; Medlock, J.; Beechler, B.R.; Spaan, J.M.; Spaan, R.S.; Ezenwa, V.O.; Jolles, A.E. Opposite outcomes of coinfection at individual and population scales. Proc. Natl. Acad. Sci. USA 2018, 115, 7545–7550. [Google Scholar] [CrossRef] [Green Version]
- Petney, T.N.; Andrews, R.H. Multiparasite communities in animals and humans: Frequency, structure and pathogenic significance. Int. J. Parasitol. 1998, 28, 377–393. [Google Scholar] [CrossRef]
- Canetti, D.; Riccardi, N.; Martini, M.; Villa, S.; Di Biagio, A.; Codecasa, L.; Castagna, A.; Barberis, I.; Gazzaniga, V.; Besozzi, G. HIV and Tuberculosis: The Paradox of Dual Illnesses and the Challenges of Their Fighting in the History; Churchill Livingstone: London, UK, 2020; Volume 122. [Google Scholar]
- Sharan, R.; Bucşan, A.N.; Ganatra, S.; Paiardini, M.; Mohan, M.; Mehra, S.; Khader, S.A.; Kaushal, D. Chronic Immune Activation in TB/HIV Co-Infection; Elsevier Ltd.: Amsterdam, The Netherlands, 2020. [Google Scholar]
- Birger, R.; Kouyos, R.D.; Cohen, T.; Griffiths, E.C.; Huijben, S.; Mina, M.J.; Volkova, V.; Grenfell, B.; Metcalf, C.J.E. The potential impact of coinfection on antimicrobial chemotherapy and drug resistance. Trends Microbiol. 2015, 23, 537–544. [Google Scholar] [CrossRef] [Green Version]
- McArdle, A.J.; Turkova, A.; Cunnington, A.J. When do co-infections matter? Curr. Opin. Infect. Dis. 2018, 31, 209–215. [Google Scholar] [CrossRef]
- Zhang, X.-S.; Cao, K.-F. The Impact of Coinfections and Their Simultaneous Transmission on Antigenic Diversity and Epidemic Cycling of Infectious Diseases. BioMed Res. Int. 2014, 2014, 1–23. [Google Scholar] [CrossRef]
- Dietrich, A.; Hermans, C.; Heppt, M.V.; Ruzicka, T.; Schauber, J.; Reinholz, M. Human papillomavirus status, anal cytology and histopathological outcome in HIV-positive patients. J. Eur. Acad. Dermatol. Venereol. 2015, 29, 2011–2018. [Google Scholar] [CrossRef]
- Henn, G.A.D.L.; Júnior, A.N.R.; Colares, J.K.B.; Mendes, L.P.; Silveira, J.G.C.; Lima, A.A.F.; Aires, B.P.; Facanha, M.C. Is Visceral Leishmaniasis the same in HIV-coinfected adults? Braz. J. Infect. Dis. 2018, 22, 92–98. [Google Scholar] [CrossRef]
- Nunes, J.D.O.; Bizerra, P.L.; Paniago, A.M.M.; Mendes, R.P.; Chang, M.R.; Pillon, K.R.A.P. The Simultaneous Occurrence of Histoplasmosis and Cryptococcal Fungemia: A Case Report and Review of the Literature. Mycopathologia 2016, 181, 891–897. [Google Scholar] [CrossRef]
- Ioannou, P.; Papakitsou, I.; Kofteridis, D.P. Fungal endocarditis in transplant recipients: A systematic review. Mycoses 2020, 63, 952–963. [Google Scholar] [CrossRef]
- Shah, M.M.; Hsiao, E.I.; Kirsch, C.M.; Gohil, A.; Narasimhan, S.; Stevens, D.A. Invasive pulmonary aspergillosis and influenza co-infection in immunocompetent hosts: Case reports and review of the literature. Diagn. Microbiol. Infect. Dis. 2018, 91, 147–152. [Google Scholar] [CrossRef]
- Reece, E.; Segurado, R.; Jackson, A.; McClean, S.; Renwick, J.; Greally, P. Co-colonisation with Aspergillus fumigatus and Pseudomonas aeruginosa is associated with poorer health in cystic fibrosis patients: An Irish registry analysis. BMC Pulm. Med. 2017, 17, 70. [Google Scholar] [CrossRef] [Green Version]
- Chermetz, M.; Gobbo, M.; Rupel, K.; Ottaviani, G.; Tirelli, G.; Bussani, R.; Luzzati, R.; Di Lenarda, R.; Biasotto, M. Combined Orofacial Aspergillosis and Mucormycosis: Fatal Complication of a Recurrent Paediatric Glioma—Case Report and Review of Literature. Mycopathologia 2016, 181, 723–733. [Google Scholar] [CrossRef]
- Maalouly, C.; Devresse, A.; Martin, A.; Rodriguez-Villalobos, H.; Kanaan, N.; Belkhir, L. Coinfection of Mycobacterium malmoense and Mycobacterium chimaera in a kidney transplant recipient: A case report and review of the literature. Transpl. Infect. Dis. 2020, e13241. [Google Scholar] [CrossRef]
- Hirabayashi, R.; Nakagawa, A.; Takegawa, H.; Tomii, K. A case of pleural effusion caused by Mycobacterium fortuitum and Mycobacterium mageritense coinfection. BMC Infect. Dis. 2019, 19, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.H.; Jhun, B.W.; Kim, S.-Y.; Choe, J.; Jeon, K.; Huh, H.J.; Ki, C.-S.; Lee, N.Y.; Shin, S.J.; Daley, C.L.; et al. Nontuberculous Mycobacterial Lung Diseases Caused by Mixed Infection with Mycobacterium avium Complex and Mycobacterium abscessus Complex. Antimicrob. Agents Chemother. 2018, 62. [Google Scholar] [CrossRef] [Green Version]
- Furuta, K.; Ito, A.; Ishida, T.; Ito, Y.; Sone, N.; Takaiwa, T.; Yokoyama, T.; Tachibana, H.; Arita, M.; Hashimoto, T. 18 Cases of pulmonary Mycobacterium abscessus: Clinical difference depending on the presence or absence of Mycobacterium avium complex. J. Infect. Chemother. 2016, 22, 622–628. [Google Scholar] [CrossRef]
- Naito, K.; Noguchi, S.; Yatera, K.; Kawanami, T.; Yamasaki, K.; Fukuda, K.; Ikegami, H.; Akata, K.; Kido, T.; Sakamoto, N.; et al. Coinfection With Multiple Nontuberculous Mycobacteria as a Possible Exacerbating Factor in Pulmonary Nontuberculous Mycobacteriosis. Chest 2020. [Google Scholar] [CrossRef]
- Lai, C.-C.; Wang, C.-Y.; Hsueh, P.-R. Co-infections among patients with COVID-19: The need for combination therapy with non-anti-SARS-CoV-2 agents? J. Microbiol. Immunol. Infect. 2020, 53, 505–512. [Google Scholar] [CrossRef]
- Genus: Mycobacterium. Available online: https://www.bacterio.net/genus/mycobacterium (accessed on 26 September 2020).
- Swift, S.; Cohen, H. Granulomas of the Skin Due toMycobacterium balneiafter Abrasions from a Fish Tank. N. Engl. J. Med. 1962, 267, 1244–1246. [Google Scholar] [CrossRef]
- Walsh, U.S.; Meyers, W.M.; Abalos, R.M.; Portaels, F.; Cruz, E.C.D.; Walsh, G.P.; Tan, E.V. CLINICAL AND HISTOLOGIC FEATURES OF SKIN LESIONS IN A CYNOMOLGUS MONKEY EXPERIMENTALLY INFECTED WITH MYCOBACTERIUM ULCERANS (BURULI ULCER) BY INTRADERMAL INOCULATION. Am. J. Trop. Med. Hyg. 2007, 76, 132–134. [Google Scholar] [CrossRef]
- Walsh, D.S.; Portaels, F.; Meyers, W.M. Buruli ulcer (Mycobacterium ulcerans infection). Trans. R. Soc. Trop. Med. Hyg. 2008, 102, 969–978. [Google Scholar] [CrossRef]
- Swenson, C.; Zerbe, C.S.; Fennelly, K. Host Variability in NTM Disease: Implications for Research Needs. Front. Microbiol. 2018, 9, 2901. [Google Scholar] [CrossRef]
- Falkinham, J.O. Environmental Sources of Nontuberculous Mycobacteria. Clin. Chest Med. 2015, 36, 35–41. [Google Scholar] [CrossRef]
- Edwards, A.J.; Chen, C.; Kemski, M.M.; Hu, J.; Mitchell, T.; Rappleye, C.A. Histoplasma yeast and mycelial transcriptomes reveal pathogenic-phase and lineage-specific gene expression profiles. BMC Genom. 2013, 14, 695. [Google Scholar] [CrossRef] [Green Version]
- Cauchie, M.; Desmet, S.; Lagrou, K. Candida and its dual lifestyle as a commensal and a pathogen. Res. Microbiol. 2017, 168, 802–810. [Google Scholar] [CrossRef]
- Kullberg, B.J.; Arendrup, M.C. Invasive Candidiasis. N. Engl. J. Med. 2015, 373, 1445–1456. [Google Scholar] [CrossRef] [Green Version]
- De Medeiros, M.A.P.; De Melo, A.P.V.; Bento, A.D.O.; De Souza, L.B.F.C.; Neto, F.D.A.B.; Garcia, J.B.-L.; Zuza-Alves, D.L.; Francisco, E.C.; Melo, A.S.D.A.; Chaves, G.M. Epidemiology and prognostic factors of nosocomial candidemia in Northeast Brazil: A six-year retrospective study. PLoS ONE 2019, 14, e0221033. [Google Scholar] [CrossRef]
- Koehler, P.; Stecher, M.; Cornely, O.; Vehreschild, M.; Bohlius, J.; Wisplinghoff, H.; Vehreschild, J. Morbidity and mortality of candidaemia in Europe: An epidemiologic meta-analysis. Clin. Microbiol. Infect. 2019, 25, 1200–1212. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, D.; Xue, X.; Yang, L.; Chen, L.; Pan, L. Clinical analysis of 16 cases of pulmonary cryptococcosis in patients with normal immune function. Ann. Palliat. Med. 2020, 9, 1117–1124. [Google Scholar] [CrossRef]
- Moretti, M.L.; Busso-Lopes, A.F.; Tararam, C.A.; Moraes, R.; Muraosa, Y.; Mikami, Y.; Gonoi, T.; Taguchi, H.; Lyra, L.; Reichert-Lima, F.; et al. Airborne transmission of invasive fusariosis in patients with hematologic malignancies. PLoS ONE 2018, 13, e0196426. [Google Scholar] [CrossRef] [Green Version]
- Prigitano, A.; Cavanna, C.; Passera, M.; Gelmi, M.; Sala, E.; Ossi, C.; Grancini, A.; Calabrò, M.; Bramati, S.; Tejada, M.; et al. Evolution of fungemia in an Italian region. J. Mycol. Médicale 2020, 30, 100906. [Google Scholar] [CrossRef]
- Faria, S.; Joao, I.; Jordao, L. General Overview on Nontuberculous Mycobacteria, Biofilms, and Human Infection. J. Pathog. 2015, 2015, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Ratnatunga, C.N.; Lutzky, V.P.; Kupz, A.; Doolan, D.L.; Reid, D.W.; Field, M.; Bell, S.C.; Thomson, R.M.; Miles, J.J. The Rise of Non-Tuberculosis Mycobacterial Lung Disease. Front. Immunol. 2020, 11, 303. [Google Scholar] [CrossRef] [Green Version]
- Bryant, J.M.; Grogono, D.M.; Greaves, D.; Foweraker, J.; Roddick, I.; Inns, T.; Reacher, M.; Haworth, C.S.; Curran, M.D.; Harris, S.R.; et al. Whole-genome sequencing to identify transmission of Mycobacterium abscessus between patients with cystic fibrosis: A retrospective cohort study. Lancet 2013, 381, 1551–1560. [Google Scholar] [CrossRef] [Green Version]
- Lutzky, V.P.; Ratnatunga, C.N.; Smith, D.J.; Kupz, A.; Rn, D.M.D.; Reid, D.W.; Thomson, R.M.; Bell, S.C.; Miles, J.J. Anomalies in T Cell Function Are Associated With Individuals at Risk of Mycobacterium abscessus Complex Infection. Front. Immunol. 2018, 9, 9. [Google Scholar] [CrossRef] [Green Version]
- Dellière, S.; Angebault, C.; Fihman, V.; Foulet, F.; Lepeule, R.; Maitre, B.; Schlemmer, F.; Botterel, F. Concomitant Presence of Aspergillus Species and Mycobacterium Species in the Respiratory Tract of Patients: Underestimated Co-occurrence? Front. Microbiol. 2020, 10, 2980. [Google Scholar] [CrossRef]
- Totaro, M.; Costa, A.L.; Casini, B.; Profeti, S.; Gallo, A.; Frendo, L.; Porretta, A.; Valentini, P.; Privitera, G.P.; Baggiani, A. Microbiological Air Quality in Heating, Ventilation and Air Conditioning Systems of Surgical and Intensive Care Areas: The Application of a Disinfection Procedure for Dehumidification Devices. Pathogens 2019, 8, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Romo, J.A.; Kumamoto, C.A. On Commensalism of Candida. J. Fungi 2020, 6, 16. [Google Scholar] [CrossRef] [Green Version]
- Hoefsloot, W.; Van Ingen, J.; Andrejak, C.; Ängeby, K.; Bauriaud, R.; Bemer, P.; Beylis, N.; Boeree, M.J.; Cacho, J.; Chihota, V.; et al. The geographic diversity of nontuberculous mycobacteria isolated from pulmonary samples: An NTM-NET collaborative study. Eur. Respir. J. 2013, 42, 1604–1613. [Google Scholar] [CrossRef]
- Wagner, D.; Lipman, M.; Cooray, S.; Ringshausen, F.C.; Morimoto, K.; Koh, W.-J.; Thomson, R.M. Global Epidemiology of NTM Disease (Except Northern America). Ultrasound Intensive Care Unit 2018, 163–260. [Google Scholar] [CrossRef]
- Varghese, B.; Memish, Z.; Abuljadayel, N.; Al-Hakeem, R.; Alrabiah, F.; Al-Hajoj, S. Emergence of Clinically Relevant Non-Tuberculous Mycobacterial Infections in Saudi Arabia. PLoS Negl. Trop. Dis. 2013, 7, e2234. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Simons, S.O.; Van Ingen, J.; Hsueh, P.-R.; Van Hung, N.; Dekhuijzen, P.R.; Boeree, M.J.; Van Soolingen, D. Nontuberculous Mycobacteria in Respiratory Tract Infections, Eastern Asia. Emerg. Infect. Dis. 2011, 17, 343–349. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mortazavi, Z.; Bahrmand, A.; Sakhaee, F.; Doust, R.H.; Vaziri, F.; Siadat, S.D.; Fateh, A. Evaluating the clinical significance of nontuberculous mycobacteria isolated from respiratory samples in Iran: An often overlooked disease. Infect. Drug Resist. 2019, 12, 1917–1927. [Google Scholar] [CrossRef] [Green Version]
- Tortoli, E. Clinical manifestations of nontuberculous mycobacteria infections. Clin. Microbiol. Infect. 2009, 15, 906–910. [Google Scholar] [CrossRef] [Green Version]
- Bahr, N.C.; Antinori, S.; Wheat, L.J.; Sarosi, G.A. Histoplasmosis Infections Worldwide: Thinking Outside of the Ohio River Valley. Curr. Trop. Med. Rep. 2015, 2, 70–80. [Google Scholar] [CrossRef] [Green Version]
- Baker, J.; Setianingrum, F.; Wahyuningsih, R.; Denning, D.W. Mapping histoplasmosis in South East Asia—Implications for diagnosis in AIDS. Emerg. Microbes Infect. 2019, 8, 1139–1145. [Google Scholar] [CrossRef] [Green Version]
- Brescini, L.; Mazzanti, S.; Orsetti, E.; Morroni, G.; Masucci, A.; Pocognoli, A.; Barchiesi, F. Species distribution and antifungal susceptibilities of bloodstream Candida isolates: A nine-years single center survey. J. Chemother. 2020, 32, 244–250. [Google Scholar] [CrossRef]
- Muderris, T.; Kaya, S.; Ormen, B.; Gokmen, A.A.; Akpinar, C.V.; Gul, S.Y. Mortality and risk factor analysis for Candida blood stream infection: A three-year retrospective study. J. Mycol. Médicale 2020, 30, 101008. [Google Scholar] [CrossRef]
- Ricotta, E.E.; Lai, Y.L.; Babiker, A.; Strich, J.R.; Kadri, S.S.; Lionakis, M.S.; Prevots, D.R.; Adjemian, J. Invasive candidiasis species distribution and trends, United States, 2009–2017. J. Infect. Dis. 2020. [Google Scholar] [CrossRef]
- Pinto-Magalhães, S.; Martins, A.; Lacerda, S.; Filipe, R.; Prista-Leão, B.; Pinheiro, D.; Silva-Pinto, A.; Santos, L. Candidemia in a Portuguese tertiary care hospital: Analysis of a 2-year period. J. Mycol. Médicale 2019, 29, 320–324. [Google Scholar] [CrossRef]
- Warris, A.; Pana, Z.-D.; Oletto, A.; Lundin, R.; Castagnola, E.; Lehrnbecher, T.; Groll, A.H.; Roilides, E.; Andersen, C.T.; Arendrup, M.C.; et al. Etiology and Outcome of Candidemia in Neonates and Children in Europe. Pediatr. Infect. Dis. J. 2020, 39, 114–120. [Google Scholar] [CrossRef]
- Adenis, A.A.; Valdes, A.; Cropet, C.; McCotter, O.Z.; Derado, G.; Couppié, P.; Chiller, T.; Nacher, M. Burden of HIV-associated histoplasmosis compared with tuberculosis in Latin America: A modelling study. Lancet Infect. Dis. 2018, 18, 1150–1159. [Google Scholar] [CrossRef]
- Lahiri, S.; Manjunath, N.; Bhat, M.; Hagen, F.; Bahubali, V.H.; Palaniappan, M.; Maji, S.; Chandrashekar, N. Clinical insights and epidemiology of central nervous system infection due to Cryptococcus neoformans/gattii species complexes: A prospective study from South India. Med. Mycol. 2019, 58, 600–608. [Google Scholar] [CrossRef]
- Zhou, J.; Tan, J.; Gong, Y.; Li, N.; Luo, G. Candidemia in major burn patients and its possible risk factors: A 6-year period retrospective study at a burn ICU. Burns 2019, 45, 1164–1171. [Google Scholar] [CrossRef]
- Martini, C.; Torelli, R.; De Groot, T.; De Carolis, E.; Morandotti, G.A.; De Angelis, G.; Posteraro, B.; Meis, J.F.; Sanguinetti, M. Prevalence and Clonal Distribution of Azole-Resistant Candida parapsilosis Isolates Causing Bloodstream Infections in a Large Italian Hospital. Front. Cell. Infect. Microbiol. 2020, 10, 232. [Google Scholar] [CrossRef]
- Prevots, D.R.; Marras, T.K. Epidemiology of human pulmonary infection with nontuberculous mycobacteria a review. Clin. Chest Med. 2015, 36, 13–34. [Google Scholar] [CrossRef] [Green Version]
- Iseman, M.D.; Chan, E.D. Underlying Host Risk Factors for Nontuberculous Mycobacterial Lung Disease. Semin. Respir. Crit. Care Med. 2013, 34, 110–123. [Google Scholar] [CrossRef]
- Kousha, M.; Tadi, R.; Soubani, O.A. Pulmonary aspergillosis: A clinical review. Eur. Respir. Rev. 2011, 20, 156–174. [Google Scholar] [CrossRef] [Green Version]
- Honda, J.R.; Alper, S.; Bai, X.; Chan, E.D. Acquired and genetic host susceptibility factors and microbial pathogenic factors that predispose to nontuberculous mycobacterial infections. Curr. Opin. Immunol. 2018, 54, 66–73. [Google Scholar] [CrossRef] [PubMed]
- Marochi-Telles, J.P.; Muniz, R., Jr.; Sztajnbok, J.; Oliveira, A.C.-D. Disseminated Mycobacterium avium on HIV/AIDS: Historical and Current Literature Review. Aids Rev. 2020, 22, 9–15. [Google Scholar] [CrossRef]
- Siberry, G.K.; Abzug, M.J.; Nachman, S.; Brady, M.T.; Dominguez, K.L.; Handelsman, E.; Mofenson, L.M.; Nesheim, S.; Children, P.O.O.I.I.H.-E.A.H.-I. Guidelines for the Prevention and Treatment of Opportunistic Infections in HIV-Exposed and HIV-Infected Children. Pediatr. Infect. Dis. J. 2013, 32. [Google Scholar] [CrossRef]
- Kaplan, J.E.; Benson, C.; Holmes, K.K.; Brooks, J.T.; Pau, A.; Masur, H.; Centers for Disease Control and Prevention (CDC); National Institutes of Health; HIV Medicine Association of the Infectious Diseases Society of America. Guidelines for prevention and treatment of opportunistic infections in HIV-infected adults and adolescents: Recommendations from CDC, the National Institutes of Health, and the HIV Medicine Association of the Infectious Diseases Society of America. MMWR Recomm. Rep. Morb. Mortal. Wkly. Rep. Recomm. Rep. 2009, 58, 1–207. [Google Scholar]
- Browne, S.K.; Burbelo, P.D.; Chetchotisakd, P.; Suputtamongkol, Y.; Kiertiburanakul, S.; Shaw, P.A.; Kirk, J.L.; Jutivorakool, K.; Zaman, R.; Ding, L.; et al. Adult-Onset Immunodeficiency in Thailand and Taiwan. N. Engl. J. Med. 2012, 367, 725–734. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sampaio, E.P.; Hsu, A.P.; Pechacek, J.; Bax, H.I.; Dias, D.L.; Paulson, M.L.; Chandrasekaran, P.; Rosen, L.B.; Carvalho, D.S.; Ding, L.; et al. Signal transducer and activator of transcription 1 (STAT1) gain-of-function mutations and disseminated coccidioidomycosis and histoplasmosis. J. Allergy Clin. Immunol. 2013, 131, 1624–1634. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, J.; Kofod-Olsen, E.; Spaun, E.; Larsen, C.S.; Christiansen, M.; Mogensen, T.H. A STAT1-gain-of-function mutation causing Th17 deficiency with chronic mucocutaneous candidiasis, psoriasiform hyperkeratosis and dermatophytosis. BMJ Case Rep. 2015, 2015. [Google Scholar] [CrossRef]
- Odio, C.D.; Milligan, K.L.; E McGowan, K.; Spergel, A.K.R.; Bishop, R.J.; Boris, L.; Urban, A.; Welch, P.; Heller, T.; Kleiner, E.D.; et al. Endemic mycoses in patients with STAT3-mutated hyper-IgE (Job) syndrome. J. Allergy Clin. Immunol. 2015, 136, 1411–1413. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Q.; Boisson, B.; Béziat, V.; Puel, A.; Casanova, J.-L. Human hyper-IgE syndrome: Singular or plural? Mamm. Genome 2018, 29, 603–617. [Google Scholar] [CrossRef]
- Merkhofer, R.M.; Klein, B.S. Advances in Understanding Human Genetic Variations That Influence Innate Immunity to Fungi. Front. Cell. Infect. Microbiol. 2020, 10, 69. [Google Scholar] [CrossRef]
- Vaezi, A.; Fakhim, H.; Abtahian, Z.; Khodavaisy, S.; Geramishoar, M.; Alizadeh, A.; Meis, J.F.; Badali, H. Frequency and Geographic Distribution of CARD9 Mutations in Patients With Severe Fungal Infections. Front. Microbiol. 2018, 9, 2434. [Google Scholar] [CrossRef]
- Lionakis, M.S.; Levitz, S.M. Host Control of Fungal Infections: Lessons from Basic Studies and Human Cohorts. Annu. Rev. Immunol. 2018, 36, 157–191. [Google Scholar] [CrossRef] [PubMed]
- Smith, N.L.D.; Hankinson, J.; Simpson, A.; Denning, D.W.; Bowyer, P. Reduced expression of TLR3, TLR10 and TREM1 by human macrophages in Chronic cavitary pulmonary aspergillosis, and novel associations of VEGFA, DENND1B and PLAT. Clin. Microbiol. Infect. 2014, 20, O960–O968. [Google Scholar] [CrossRef] [Green Version]
- Smith, N.; Hankinson, J.; Simpson, A.; Bowyer, P.; Denning, D.W. A prominent role for the IL1 pathway and IL15 in susceptibility to chronic cavitary pulmonary aspergillosis. Clin. Microbiol. Infect. 2014, 20, O480–O488. [Google Scholar] [CrossRef] [Green Version]
- Henkle, E.; Winthrop, K.L. Nontuberculous mycobacteria infections in immunosuppressed hosts. Clin. Chest Med. 2015, 36, 91–99. [Google Scholar] [CrossRef] [Green Version]
- Tufariello, J.M.; Chan, J.; Flynn, J.L. Latent tuberculosis: Mechanisms of host and bacillus that contribute to persistent infection. Lancet Infect. Dis. 2003, 3, 578–590. [Google Scholar] [CrossRef]
- Abe, Y.; Fukushima, K.; Hosono, Y.; Matsumoto, Y.; Motooka, D.; Ose, N.; Nakamura, S.; Kitada, S.; Kida, H.; Kumanogoh, A. Host Immune Response and Novel Diagnostic Approach to NTM Infections. Int. J. Mol. Sci. 2020, 21. 4351. [Google Scholar] [CrossRef]
- Luckett, K.; Dummer, J.S.; Miller, G.; Hester, S.; Thomas, L.D. Histoplasmosis in Patients With Cell-Mediated Immunodeficiency: Human Immunodeficiency Virus Infection, Organ Transplantation, and Tumor Necrosis Factor-α Inhibition. Open Forum Infect. Dis. 2015, 2. [Google Scholar] [CrossRef] [Green Version]
- Herring, A.C.; Lee, J.; McDonald, R.A.; Toews, G.B.; Huffnagle, G.B. Induction of Interleukin-12 and Gamma Interferon Requires Tumor Necrosis Factor Alpha for Protective T1-Cell-Mediated Immunity to Pulmonary Cryptococcus neoformans Infection. Infect. Immun. 2002, 70, 2959–2964. [Google Scholar] [CrossRef] [Green Version]
- Chandra, J.; McCormick, T.S.; Imamura, Y.; Mukherjee, P.K.; Ghannoum, M.A. Interaction of Candida albicans with Adherent Human Peripheral Blood Mononuclear Cells Increases C. albicans Biofilm Formation and Results in Differential Expression of Pro- and Anti-Inflammatory Cytokines. Infect. Immun. 2007, 75, 2612–2620. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rosati, D.; Bruno, M.; Jaeger, M.; Kullberg, B.-J.; Van De Veerdonk, F.; Netea, M.G.; Oever, J.T. An Exaggerated Monocyte-Derived Cytokine Response to Candida Hyphae in Patients With Recurrent Vulvovaginal Candidiasis. J. Infect. Dis. 2020. [Google Scholar] [CrossRef]
- Carrion, S.D.J.; Leal, S.M.; Ghannoum, M.A.; Aimanianda, V.; Latgé, J.-P.; Pearlman, E. The RodA Hydrophobin on Aspergillus fumigatus Spores Masks Dectin-1– and Dectin-2–Dependent Responses and Enhances Fungal Survival In Vivo. J. Immunol. 2013, 191, 2581–2588. [Google Scholar] [CrossRef] [Green Version]
- Murayama, T.; Amitani, R.; Tsuyuguchi, K.; Watanabe, I.; Kimoto, T.; Suzuki, K.; Tanaka, E.; Kamei, K.; Nishimura, K. Polypoid bronchial lesions due to Scedosporium apiospermum in a patient with Mycobacterium avium complex pulmonary disease. Eur. Respir. J. 1998, 12, 745–747. [Google Scholar] [CrossRef] [Green Version]
- Gifford, A.H.; Lahey, T.; Von Reyn, C.F. Fatal hemoptysis from invasiveAspergillus nigerin a patient with cavitary lung disease andMycobacterium avium complexinfection. Med. Mycol. 2006, 44, 557–560. [Google Scholar] [CrossRef] [Green Version]
- Joury, A.; AlShehri, M.; Alhasan, M. Concurrent Persistent Cryptococcosis and Mycobacterium avium Complex Infections in a Patient With Human Immunodeficiency Virus. Ochsner J. 2019, 19, 169–173. [Google Scholar] [CrossRef]
- Kaneko, T.; Jr, D.A.M.; Marty, F.M.; Colson, Y. Triple opportunistic pulmonary cavitary disease after cord blood transplantation. Transpl. Infect. Dis. 2014, 16, 818–821. [Google Scholar] [CrossRef] [PubMed]
- Kobashi, Y.; Yoshida, K.; Miyashita, N.; Niki, Y.; Matsushima, T. Chronic Necrotizing Pulmonary Aspergillosis Complicated by a Cavitary Lesion Caused by Pulmonary Mycobacterium-avium Complex Disease. Intern. Med. 2005, 44, 246–250. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, J.; Huang, X.; Zhang, X.; Zhu, Y.; Liao, K.; Ma, J.; Wang, G.; Guo, Y.; Xie, C. Coinfection of disseminated Talaromyces marneffei and Mycobacteria kansasii in a patient with papillary thyroid cancer. Medicine 2017, 96, e9072. [Google Scholar] [CrossRef]
- Rujirachun, P.; Sangwongwanich, J.; Chayakulkeeree, M. Triple infection with Cryptococcus, varicella-zoster virus, and Mycobacterium abscessus in a patient with anti-interferon-gamma autoantibodies: A case report. BMC Infect. Dis. 2020, 20, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Pietras, T.A.; Baum, C.L.; Swick, B.L. Coexistent Kaposi sarcoma, cryptococcosis, and Mycobacterium avium intracellulare in a solitary cutaneous nodule in a patient with AIDS: Report of a case and literature review. J. Am. Acad. Dermatol. 2010, 62, 676–680. [Google Scholar] [CrossRef]
- Seok, H.; Ko, J.-H.; Shin, I.; Eun, Y.H.; Lee, S.-E.; Lee, Y.-B.; Peck, K.R. Disseminated Talaromyces marneffei and Mycobacterium intracellulare coinfection in an HIV-infected patient. Int. J. Infect. Dis. 2015, 38, 86–88. [Google Scholar] [CrossRef] [Green Version]
- Yuan, C.; Lu, H.; Yang, C.; Gao, W.; Wang, H.; Wu, G. Case report: Mycobacterium monacense isolated from the blood culture of a patient with pulmonary infection. BMC Infect. Dis. 2020, 20, 1–5. [Google Scholar] [CrossRef]
- Kim, J.S.; Choi, M.; Nam, C.H.; Kim, J.Y.; Hong, S.P.; Kim, M.H.; Park, B.C. Co-infection of Scedosporium apiospermum and Mycobacterium chelonae in an immunocompetent host. J. Dermatol. 2014, 41, 922–925. [Google Scholar] [CrossRef]
- Furuuchi, K.; Ito, A.; Hashimoto, T.; Kumagai, S.; Ishida, T. Clinical significance of Aspergillus species isolated from respiratory specimens in patients with Mycobacterium avium complex lung disease. Eur. J. Clin. Microbiol. Infect. Dis. 2017, 37, 91–98. [Google Scholar] [CrossRef]
- Zoumot, Z.; Boutou, A.K.; Gill, S.S.; Van Zeller, M.; Hansell, D.M.; Wells, A.U.; Wilson, R.; Loebinger, M.R. Mycobacterium aviumcomplex infection in non-cystic fibrosis bronchiectasis. Respirology 2014, 19, 714–722. [Google Scholar] [CrossRef]
- Bansod, S.; Rai, M. Emerging of Mycotic Infection in Patients Infected with Mycobacterium tuberculosis. World J. Med. Sci. 2008, 7, 74–80. [Google Scholar]
- Kosmidis, C.; Muldoon, E.G. Challenges in the management of chronic pulmonary aspergillosis. Med. Mycol. 2016, 55, 63–68. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Samayoa, B.; Aguirre, L.; Bonilla, O.; Medina, N.; Lau-Bonilla, D.; Mercado, D.; Moller, A.; Perez, J.C.; Alastruey-Izquierdo, A.; Arathoon, E.; et al. The Diagnostic Laboratory Hub: A New Health Care System Reveals the Incidence and Mortality of Tuberculosis, Histoplasmosis, and Cryptococcosis of PWH in Guatemala. Open Forum Infect. Dis. 2020, 7, ofz534. [Google Scholar] [CrossRef]
- Jhun, B.W.; Jung, W.J.; Hwang, N.Y.; Park, H.Y.; Jeon, K.; Kang, E.-S.; Koh, W.-J. Risk factors for the development of chronic pulmonary aspergillosis in patients with nontuberculous mycobacterial lung disease. PLoS ONE 2017, 12, e0188716. [Google Scholar] [CrossRef] [PubMed]
- Amiri, M.R.J.; Siami, R.; Khaledi, A. Tuberculosis Status and Coinfection of Pulmonary Fungal Infections in Patients Referred to Reference Laboratory of Health Centers Ghaemshahr City during 2007–2017. Ethiop. J. Health Sci. 2018, 28, 683–690. [Google Scholar] [CrossRef]
- Johnston, I. Mycobacterium xenopi infection and aspergilloma. Tubercle 1988, 69, 139–143. [Google Scholar] [CrossRef]
- Kobashi, Y.; Fukuda, M.; Yoshida, K.; Miyashita, N.; Niki, Y.; Oka, M. Chronic necrotizing pulmonary aspergillosis as a complication of pulmonary Mycobacterium avium complex disease. Respirology 2006, 11, 809–813. [Google Scholar] [CrossRef]
- Torres-González, P.; Niembro-Ortega, M.D.; Martínez-Gamboa, A.; Ahumada-Topete, V.H.; Andrade-Villanueva, J.F.; Araujo-Meléndez, J.; Chaparro-Sánchez, A.; Crabtree-Ramírez, B.; Cruz-Martínez, S.; Gamboa-Domínguez, A.; et al. Diagnostic accuracy cohort study and clinical value of the Histoplasma urine antigen (ALPHA Histoplasma EIA) for disseminated histoplasmosis among HIV infected patients: A multicenter study. PLoS Negl. Trop. Dis. 2018, 12, e0006872. [Google Scholar] [CrossRef] [Green Version]
- Wickremasinghe, M.; Ozerovitch, L.J.; Davies, G.; Wodehouse, T.; Chadwick, M.V.; Abdallah, S.; Shah, P.; Wilson, R. Non-tuberculous mycobacteria in patients with bronchiectasis. Thorax 2005, 60, 1045–1051. [Google Scholar] [CrossRef] [Green Version]
- Fujita, K.; Ito, Y.; Hirai, T.; Kubo, T.; Togashi, K.; Ichiyama, S.; Mishima, M. Prevalence and risk factors for chronic co-infection in pulmonaryMycobacterium aviumcomplex disease. BMJ Open Respir. Res. 2014, 1, e000050. [Google Scholar] [CrossRef] [Green Version]
- Takeda, K.; Imamura, Y.; Takazono, T.; Yoshida, M.; Ide, S.; Hirano, K.; Tashiro, M.; Saijo, T.; Kosai, K.; Morinaga, Y.; et al. The risk factors for developing of chronic pulmonary aspergillosis in nontuberculous mycobacteria patients and clinical characteristics and outcomes in chronic pulmonary aspergillosis patients coinfected with nontuberculous mycobacteria. Med. Mycol. 2015, 54, 120–127. [Google Scholar] [CrossRef] [Green Version]
- Choudat, D.; Horreard, P.; Pretet, S.; Grosset, J.; Toty, L. Superinfection by Aspergillus fumigatus of a pulmonary lesion caused by Mycobacterium xenopi. Tubercle 1986, 67, 71–74. [Google Scholar] [CrossRef]
- Kimmerling, E.A.; Fedrick, J.A.; Tenholder, M.F. Invasive Aspergillus niger with Fatal Pulmonary Oxalosis in Chronic Obstructive Pulmonary Disease. Chest 1992, 101, 870–872. [Google Scholar] [CrossRef]
- Montaigne, E.; Petit, F.-X.; Gourdier, A.-L.; Urban, T.; Gagnadoux, F. Aspergillose pulmonaire compliquant une infection à mycobactérie atypique chez deux patients atteints de bronchopneumopathie chronique obstructive. Rev. des Mal. Respir. 2012, 29, 79–83. [Google Scholar] [CrossRef] [Green Version]
- Kampitak, T.; Suwanpimolkul, G.; Browne, S.; Suankratay, C. Anti-interferon-γ autoantibody and opportunistic infections: Case series and review of the literature. Infection 2011, 39, 65–71. [Google Scholar] [CrossRef]
- Kampmann, B.; Hemingway, C.; Stephens, A.; Davidson, R.; Goodsall, A.; Anderson, S.; Nicol, M.; Schölvinck, E.; Relman, D.; Waddell, S.; et al. Acquired predisposition to mycobacterial disease due to autoantibodies to IFN-γ. J. Clin. Investig. 2005, 115, 2480–2488. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reischl, U.; Melzl, H.; Kroppenstedt, R.M.; Miethke, T.; Naumann, L.; Mariottini, A.; Mazzarelli, G.; Tortoli, E. Mycobacterium monacense sp. nov. Int. J. Syst. Evol. Microbiol. 2006, 56, 2575–2578. [Google Scholar] [CrossRef] [Green Version]
- Chmiel, J.F.; Aksamit, T.R.; Chotirmall, S.H.; Dasenbrook, E.C.; Elborn, J.S.; Lipuma, J.J.; Ranganathan, S.C.; Waters, V.J.; Ratjen, F.A. Antibiotic Management of Lung Infections in Cystic Fibrosis. II. Nontuberculous Mycobacteria, Anaerobic Bacteria, and Fungi. Ann. Am. Thorac. Soc. 2014, 11, 1298–1306. [Google Scholar] [CrossRef] [Green Version]
- Jones, P.G.; Cohen, R.L.; Batts, D.H.; Jr, J.S. Disseminated Histoplasmosis, Invasive Pulmonary Aspergillosis, and Other Opportunistic Infections in a Homosexual Patient with Acquired Immune Deficiency Syndrome. Sex. Transm. Dis. 1983, 10, 202–204. [Google Scholar] [CrossRef]
- Wheat, L.; Slama, T.G.; Zeckel, M.L. Histoplasmosis in the acquired immune deficiency syndrome. Am. J. Med. 1985, 78, 203–210. [Google Scholar] [CrossRef]
- Maliwan, N.; Zvetina, J.R. Pulmonary Mycetoma Following Mycobacterium kansasii Infection. Arch. Intern. Med. 1985, 145, 2180–2183. [Google Scholar] [CrossRef]
- Johnson, P.C.; Khardor, N.; Najjar, A.F.; Butt, F.; Mansell, P.W.; Sarosi, G.A. Progressive disseminated histoplasmosis in patients with acquired immunodeficiency syndrome. Am. J. Med. 1988, 85, 152–158. [Google Scholar] [CrossRef]
- Crothers, K.; Huang, L. Recurrence of Pneumocystis carinii pneumonia in an HIV-infected patient: Apparent selective immune reconstitution after initiation of antiretroviral therapy. HIV Med. 2003, 4, 346–349. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Castelli, A.A.; Rosenthal, D.G.; Ignacio, R.B.; Chu, H.Y. Hemophagocytic Lymphohistiocytosis Secondary to Human Immunodeficiency Virus-Associated Histoplasmosis. Open Forum Infect. Dis. 2015, 2, ofv140. [Google Scholar] [CrossRef]
- Pinheiro, M.V.C.; Ho, Y.-L.; Nicodemo, A.C.; Duarte-Neto, A. The diagnosis of multiple opportunistic infections in advanced stage AIDS: When Ockham’s Razor doesn’t cut it. Autops. Case Rep. 2018, 8, 8. [Google Scholar] [CrossRef]
- Basso, R.P.; Poester, V.R.; Silveira, J.M.; Vieira, R.S.; Da Mota, L.D.; Klafke, G.B.; Müller, J.N.; Penna, C.P.; Vianna, J.S.; Busatto, C.; et al. Histoplasma capsulatum and Mycobacterium avium co-infection in an immunocompromised patient: Case report and literature review. Med. Mycol. Case Rep. 2020, 28, 29–32. [Google Scholar] [CrossRef]
- Choi, J.; Nikoomanesh, K.; Uppal, J.; Wang, S. Progressive disseminated histoplasmosis with concomitant disseminated nontuberculous mycobacterial infection in a patient with AIDS from a nonendemic region (California). BMC Pulm. Med. 2019, 19, 46. [Google Scholar] [CrossRef] [PubMed]
- Tajan, J.; Espasa, M.; Sala, M.; Navarro, M.; Font, B.; González-Martín, J.; Segura, F. Disseminated Infection by Mycobacterium sherrisii and Histoplasma capsulatum in an African HIV-Infected Patient. Am. J. Trop. Med. Hyg. 2013, 88, 914–917. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Souilamas, R.; Danel, C.; Chauffour, X.; Riquet, M. Lung cancer occurring with Mycobacterium xenopi and Aspergillus. Eur. J. Cardio Thoracic Surg. 2001, 20, 211–213. [Google Scholar] [CrossRef] [Green Version]
- Wu, M.-L.; Aziz, D.B.; Dartois, V.; Dick, T. NTM drug discovery: Status, gaps and the way forward. Drug Discov. Today 2018, 23, 1502–1519. [Google Scholar] [CrossRef]
- Daley, C.L.; Iaccarino, J.M.; Lange, C.; Cambau, E.; Wallace, R.J., Jr.; Andrejak, C.; Böttger, E.C.; Brozek, J.; Griffith, E.D.E.; Guglielmetti, L.; et al. Treatment of Nontuberculous Mycobacterial Pulmonary Disease: An Official ATS/ERS/ESCMID/IDSA Clinical Practice Guideline. Clin. Infect. Dis. 2020, 71, 905–913. [Google Scholar] [CrossRef] [PubMed]
- Haworth, C.S.; Banks, J.; Capstick, T.; Fisher, A.J.; Gorsuch, T.; Laurenson, I.F.; Leitch, A.; Loebinger, M.R.; Milburn, H.J.; Nightingale, M.; et al. British Thoracic Society guidelines for the management of non-tuberculous mycobacterial pulmonary disease (NTM-PD). Thorax 2017, 72, ii1–ii64. [Google Scholar] [CrossRef] [Green Version]
- Gopalaswamy, R.; Shanmugam, S.; Mondal, R.; Subbian, S. Of tuberculosis and non-tuberculous mycobacterial infections—A comparative analysis of epidemiology, diagnosis and treatment. J. Biomed. Sci. 2020, 27, 1–17. [Google Scholar] [CrossRef]
- Lu, M.; Fitzgerald, D.; Karpelowsky, J.; Selvadurai, H.; Pandit, C.; Robinson, P.; Marais, B.J. Surgery in nontuberculous mycobacteria pulmonary disease. Breathe 2018, 14, 288–301. [Google Scholar] [CrossRef] [Green Version]
- A Brown-Elliott, B.; Mann, L.B.; Hail, D.; Whitney, C.; Wallace, R.J. Antimicrobial Susceptibility of Nontuberculous Mycobacteria From Eye Infections. Cornea 2012, 31, 900–906. [Google Scholar] [CrossRef]
- Ganapathy, U.S.; Dartois, V.; Dick, T. Repositioning rifamycins for Mycobacterium abscessus lung disease. Expert Opin. Drug Discov. 2019, 14, 867–878. [Google Scholar] [CrossRef] [Green Version]
- Griffith, D.E. Treatment of Mycobacterium avium Complex (MAC). Semin. Respir. Crit. Care Med. 2018, 39, 351–361. [Google Scholar] [CrossRef]
- Koh, W.-J.; Jeon, K.; Lee, N.Y.; Kim, B.-J.; Kook, Y.-H.; Lee, S.; Jo, E.-K.; Kim, C.K.; Shin, S.J.; Huitt, G.A.; et al. Clinical Significance of Differentiation ofMycobacterium massiliensefromMycobacterium abscessus. Am. J. Respir. Crit. Care Med. 2011, 183, 405–410. [Google Scholar] [CrossRef]
- Kim, H.-Y.; Kim, B.J.; Kook, Y.; Yun, Y.-J.; Shin, J.H.; Kim, B.-J.; Kook, Y.-H. Mycobacterium massiliense is differentiated from Mycobacterium abscessus and Mycobacterium bolletii by erythromycin ribosome methyltransferase gene (erm) and clarithromycin susceptibility patterns. Microbiol. Immunol. 2010, 54, 347–353. [Google Scholar] [CrossRef]
- Philley, J.V.; Griffith, D.E. Medical Management of Pulmonary Nontuberculous Mycobacterial Disease. Thorac. Surg. Clin. 2019, 29, 65–76. [Google Scholar] [CrossRef]
- Martiniano, S.L.; Wagner, B.D.; Levin, A.; Nick, J.A.; Sagel, S.D.; Daley, C.L. Safety and Effectiveness of Clofazimine for Primary and Refractory Nontuberculous Mycobacterial Infection. Chest 2017, 152, 800–809. [Google Scholar] [CrossRef]
- Ellis, D. Amphotericin B: Spectrum and resistance. J. Antimicrob. Chemother. 2002, 49, 7–10. [Google Scholar] [CrossRef] [Green Version]
- Sangalli-Leite, F.; Scorzoni, L.; Mesa-Arango, A.C.; Casas, C.; Herrero, E.; Gianinni, M.J.S.M.; Rodriguez-Tudela, J.; Cuenca-Estrella, M.; Zaragoza, O. Amphotericin B mediates killing in Cryptococcus neoformans through the induction of a strong oxidative burst. Microbes Infect. 2011, 13, 457–467. [Google Scholar] [CrossRef] [Green Version]
- Sanglard, D. Resistance of human fungal pathogens to antifungal drugs. Curr. Opin. Microbiol. 2002, 5, 379–385. [Google Scholar] [CrossRef]
- Hamill, R.J. Amphotericin B Formulations: A Comparative Review of Efficacy and Toxicity. Drugs 2013, 73, 919–934. [Google Scholar] [CrossRef]
- Kathiravan, M.K.; Salake, A.B.; Chothe, A.S.; Dudhe, P.B.; Watode, R.P.; Mukta, M.S.; Gadhwe, S. The biology and chemistry of antifungal agents: A review. Bioorganic Med. Chem. 2012, 20, 5678–5698. [Google Scholar] [CrossRef]
- Polak, A.; Scholer, H. Mode of Action of 5-Fluorocytosine and Mechanisms of Resistance. Chemother. 1975, 21, 113–130. [Google Scholar] [CrossRef]
- Perfect, J.R.; Dismukes, W.E.; Dromer, F.; Goldman, D.L.; Graybill, J.R.; Hamill, R.J.; Harrison, T.S.; Larsen, R.A.; Lortholary, O.; Nguyen, M.; et al. Clinical Practice Guidelines for the Management of Cryptococcal Disease: 2010 Update by the Infectious Diseases Society of America. Clin. Infect. Dis. 2010, 50, 291–322. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chastain, D.B.; Henao-Martínez, A.F.; Franco-Paredes, C. Opportunistic Invasive Mycoses in AIDS: Cryptococcosis, Histoplasmosis, Coccidiodomycosis, and Talaromycosis. Curr. Infect. Dis. Rep. 2017, 19, 36. [Google Scholar] [CrossRef]
- Johansen, H.K.; Gøtzsche, P.C. Amphotericin B versus fluconazole for controlling fungal infections in neutropenic cancer patients. Cochrane Database Syst. Rev. 2014, 2014, CD000239. [Google Scholar] [CrossRef] [PubMed]
- Wheat, L.J.; Freifeld, A.G.; Kleiman, M.B.; Baddley, J.W.; McKinsey, D.S.; Loyd, J.E.; Kauffman, C.A. Clinical Practice Guidelines for the Management of Patients with Histoplasmosis: 2007 Update by the Infectious Diseases Society of America. Clin. Infect. Dis. 2007, 45, 807–825. [Google Scholar] [CrossRef] [Green Version]
- Denning, D.W. Echinocandin antifungal drugs. Lancet 2003, 362, 1142–1151. [Google Scholar] [CrossRef]
- Cornely, O.; Bassetti, M.; Calandra, T.; Garbino, J.; Kullberg, B.; Lortholary, O.; Meersseman, W.; Akova, M.; Arendrup, M.; Arikan-Akdagli, S.; et al. ESCMID* *This guideline was presented in part at ECCMID 2011. European Society for Clinical Microbiology and Infectious Diseases. guideline for the diagnosis and management of Candida diseases 2012: Non-neutropenic adult patients. Clin. Microbiol. Infect. 2012, 18, 19–37. [Google Scholar] [CrossRef] [Green Version]
- Vazquez, J.A. Anidulafungin: A new echinocandin with a novel profile. Clin. Ther. 2005, 27, 657–673. [Google Scholar] [CrossRef]
- LeDoux, M.-P.; Guffroy, B.; Nivoix, Y.; Simand, C.; Herbrecht, R. Invasive Pulmonary Aspergillosis. Semin. Respir. Crit. Care Med. 2020, 41, 80–98. [Google Scholar] [CrossRef]
- Malki, M.A.; Pearson, E. Drug–drug–gene interactions and adverse drug reactions. Pharm. J. 2019, 20, 355–366. [Google Scholar] [CrossRef] [Green Version]
- Philley, J.V.; Wallace, R.J.; Benwill, J.L.; Taskar, V.; Brown-Elliott, B.A.; Thakkar, F.; Aksamit, T.R.; Griffith, D.E. Preliminary Results of Bedaquiline as Salvage Therapy for Patients With Nontuberculous Mycobacterial Lung Disease. Chest 2015, 148, 499–506. [Google Scholar] [CrossRef] [Green Version]
- Alexander, D.C.; Vasireddy, R.; Vasireddy, S.; Philley, J.V.; Brown-Elliott, B.A.; Perry, B.J.; Griffith, D.E.; Benwill, J.L.; Cameron, A.D.S.; Wallace, R.J. Emergence ofmmpT5Variants during Bedaquiline Treatment of Mycobacterium intracellulare Lung Disease. J. Clin. Microbiol. 2016, 55, 574–584. [Google Scholar] [CrossRef] [Green Version]
- Pavić, K.; Perković, I.; Pospisilova, S.; Machado, M.; Fontinha, D.; Prudêncio, M.; Jampilek, J.; Coffey, A.; Endersen, L.; Rimac, H.; et al. Primaquine hybrids as promising antimycobacterial and antimalarial agents. Eur. J. Med. Chem. 2018, 143, 769–779. [Google Scholar] [CrossRef] [PubMed]
- Boelaert, J.R.; Appelberg, R.; Gomes, M.S.; Blasi, E.; Mazzolla, R.; Grosset, J.; Lounis, N.; Soteriadou, K.; Thiakaki, M.; Taramelli, D.; et al. Experimental Results on Chloroquine and AIDS-Related Opportunistic Infections. JAIDS J. Acquir. Immune Defic. Syndr. 2001, 26, 300–301. [Google Scholar] [CrossRef]
- Banaschewski, B.; Verma, D.; Pennings, L.J.; Zimmerman, M.; Ye, Q.; Gadawa, J.; Dartois, V.; Ordway, D.; Van Ingen, J.; Ufer, S.; et al. Clofazimine inhalation suspension for the aerosol treatment of pulmonary nontuberculous mycobacterial infections. J. Cyst. Fibros. 2019, 18, 714–720. [Google Scholar] [CrossRef] [PubMed]
- Thanukrishnan, H.; Corcoran, T.E.; Iasella, C.J.; Moore, C.A.; Nero, J.A.; Morrell, M.R.; McDyer, J.F.; Hussain, S.; Nguyen, M.H.; Venkataramanan, R.; et al. Aerosolization of Second-generation Triazoles. Transplantion 2019, 103, 2608–2613. [Google Scholar] [CrossRef]
- Godet, C.; Goudet, V.; Laurent, F.; Le Moal, G.; Gounant, V.; Frat, J.-P.; Cateau, E.; Cazenave-Roblot, F.; Cadranel, J. Nebulised liposomal amphotericin B for Aspergillus lung diseases: Case series and literature review. Mycoses 2015, 58, 173–180. [Google Scholar] [CrossRef] [PubMed]
- Portell-Buj, E.; Vergara, A.; Alejo, I.; López-Gavín, A.; Monté, M.R.; Nicolás, L.S.; González-Martín, J.; Tudó, G. In vitro activity of 12 antimicrobial peptides against Mycobacterium tuberculosis and Mycobacterium avium clinical isolates. J. Med. Microbiol. 2019, 68, 211–215. [Google Scholar] [CrossRef]
- Azimi, T.; Mosadegh, M.; Nasiri, M.J.; Sabour, S.; Karimaei, S.; Nasser, A. Phage therapy as a renewed therapeutic approach to mycobacterial infections: A comprehensive review. Infect. Drug Resist. 2019, 12, 2943–2959. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Millar, B.C.; Moore, J.E. Antimycobacterial strategies to evade antimicrobial resistance in the nontuberculous mycobacteria. Int. J. Mycobacteriology 2019, 8, 7–21. [Google Scholar] [CrossRef]
- Simões, M.F.; Ottoni, C.A.; Antunes, A. Mycogenic Metal Nanoparticles for the Treatment of Mycobacterioses. Antibiotics 2020, 9, 569. [Google Scholar] [CrossRef]
| Underlying Diseases * | Aetiological Agents | Reference | Observations | |
|---|---|---|---|---|
| NTM | Fungi | |||
| Immunosuppression (steroids treatment) | MAC | A. niger | [117] | Case report. |
| HIV infection | MAC | Cryptococcus | [118] | Case report. |
| Cancer | MAC | Aspergillus sp. | [119] | Case report. |
| Chronic pulmonary infection | MAC | Aspergillus sp. | [120] | Case report. |
| Multiple diseases (oral and colon cancer, hematologic malignancies, connective tissue disease, diabetes, corticosteroid immunosuppression) | M. kansasii | T. marneffei** | [121] | Case report. |
| Autoimmune disease (anti IFN-γ ab) | M. abscessus | Cryptococcus sp. | [122] | Case report of triple infection. |
| AIDS (Kaposi sarcoma) | M. avium intracellulare | C. neoformans | [123] | Case report. |
| Pulmonary MAC disease | MAC (M. avium and M. intracellulare) | Aspergillus spp. (A. fumigatus and A. niger) | [135] | Chronic necrotizing pulmonary aspergillosis (CNPA) as a complication of pulmonary MAC disease. |
| AIDS | M. intracellulare | T. marneffei | [124] | Case report. |
| AIDS | M. avium | H. capsulatum | [154] | Case report. |
| Pneumonia | M. monacense | C. glabrata | [125] | Case report of M. monacense was isolated from blood whereas C. glabrata and K. pneumoniae were isolate from bronchoalveolar lavage fluid. |
| Not reported | M. chelonae | S. apiospermum | [126] | Case report in an immunocompetent patient. |
| anti-IFN-γ ab | M. intermedium | T. marneffei | [143] | Case report. |
| Bronchiectasis, anti-IFN-γ ab | M. fortuitum | Aspergillus sp. | [144] | Case report. |
| Alcoholics, COPD | M. avium, M. xenopi | Aspergillus sp. | [142] | Case report. |
| Miscellaneous conditions (previous M. xenopi infection, bronchectasia, cancer) | M. xenopi | Aspergillus sp. | [157] | Case report. |
| AIDS | MAC | H. capsulatum | [151] | Case report.Disseminated Histoplasma and MAC with recurrence of Pneumocystis carinii pneumonia. |
| AIDS | MAC | H. capsulatum | [152] | Case report of disseminated infection (E. coli bacteremia). |
| AIDS | NTM (probably M. avium-M. intracellulare) | H. capsulatum | [153] | Autopsy Case Report. |
| Bronchiestasis | MAC | C. albicans, A. fumigatus | [137] | |
| Several including MAC pulmonary infection, COPD, bronchiestasis, autoimmune disease, rheumatoid arthritis, corticosteroids use. | MAC | Aspergillus sp. | [138] | Patients with pulmonary MAC disease frequently had chronic coinfection with MSSA, P. aeruginosa and Aspergillus. |
| Lung disease, corticosteroid use | MAC | A. fumigatus | [71] | Retrospective monocentric study. |
| HIV infection | Not specified | Cryptococcus | [131] | Prospective study. |
| Several including COPD and cancer | M. avium and M. intracellulare (most frequent) M. abscessus and M. gordonae | Aspergillus sp. | [139] | Retrospective study. |
| Pulmonary disease (e.g., tuberculosis, COPD) and steroid treatment | MAC and MABC | Aspergillus spp. (A fumigatus, A niger, A. terreus, A. flavus) | [132] | Retrospective study. Only the group infected with NTM that developed chronic pulmonary aspergillosis (CPA) was considered. |
| Non-cystic fibrosis bronchiectasis with different etiologies, immunosuppression and MAC disease | MAC | Aspergillus sp. | [128] | Retrospective study. |
| Pulmonary MAC disease and several comorbidities (rheumatoid arthritis, steroid use) | MAC | A. fumigatus | [127] | Retrospective cohort study. |
| AIDS | MAC | H. capsulatum | [136] | Multicenter Study. |
| AIDS | NTM | H. capsulatum | [155] | Case report. |
| HIV infection | M. sherrisii | H. capsulatum | [156] | Case report. |
| COPD | M. avium | A. fumigatus | [130] | Review including case report. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Joao, I.; Bujdáková, H.; Jordao, L. Opportunist Coinfections by Nontuberculous Mycobacteria and Fungi in Immunocompromised Patients. Antibiotics 2020, 9, 771. https://doi.org/10.3390/antibiotics9110771
Joao I, Bujdáková H, Jordao L. Opportunist Coinfections by Nontuberculous Mycobacteria and Fungi in Immunocompromised Patients. Antibiotics. 2020; 9(11):771. https://doi.org/10.3390/antibiotics9110771
Chicago/Turabian StyleJoao, Ines, Helena Bujdáková, and Luisa Jordao. 2020. "Opportunist Coinfections by Nontuberculous Mycobacteria and Fungi in Immunocompromised Patients" Antibiotics 9, no. 11: 771. https://doi.org/10.3390/antibiotics9110771
APA StyleJoao, I., Bujdáková, H., & Jordao, L. (2020). Opportunist Coinfections by Nontuberculous Mycobacteria and Fungi in Immunocompromised Patients. Antibiotics, 9(11), 771. https://doi.org/10.3390/antibiotics9110771






