Phenolic Content and Antimicrobial and Anti-Inflammatory Effects of Solidago virga-aurea, Phyllanthus niruri, Epilobium angustifolium, Peumus boldus, and Ononis spinosa Extracts
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Phytochemical Analysis
2.3. Artemia salina Lethality Test
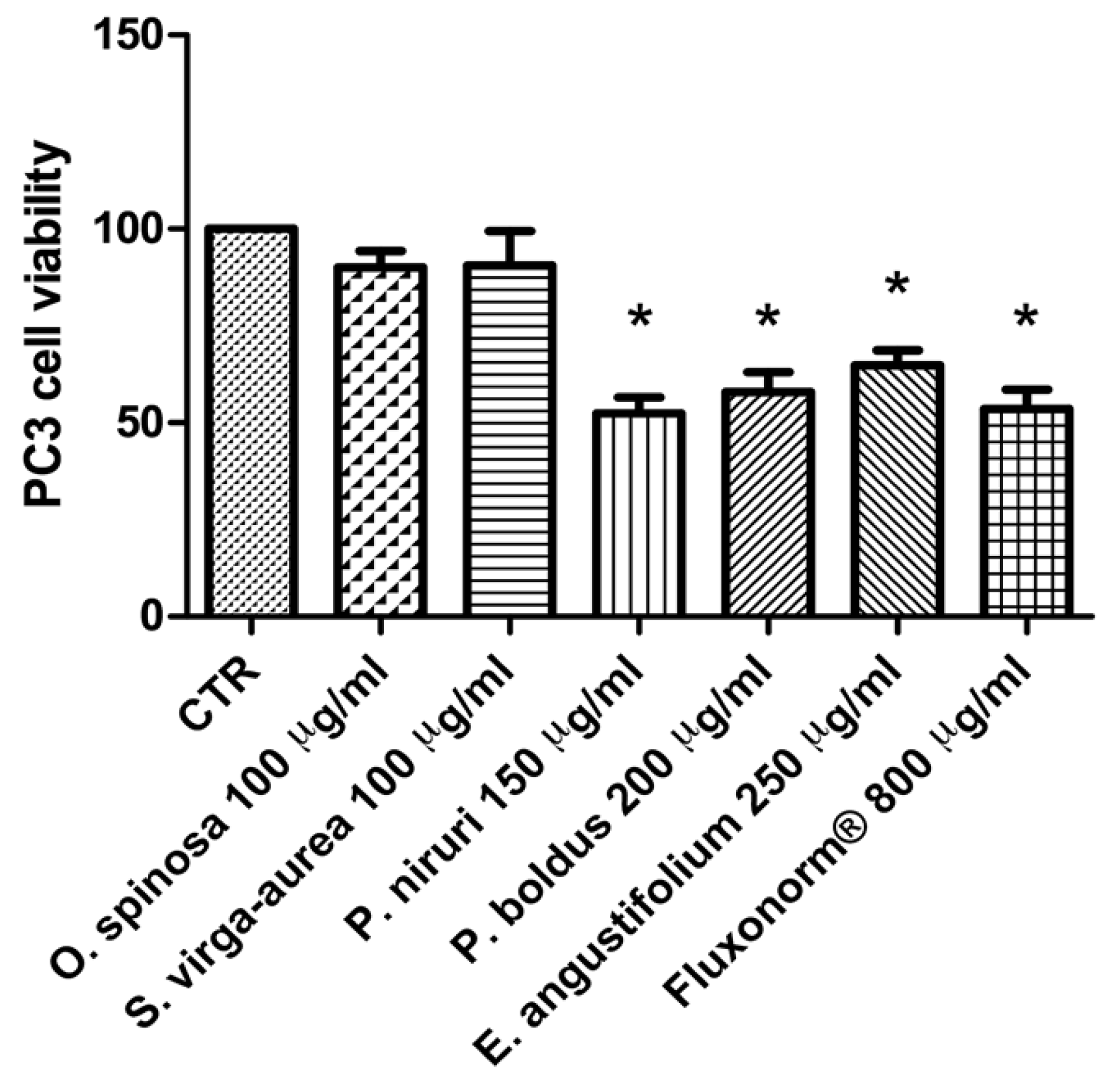
2.4. Cell Cultures and Viability Test
2.5. Ex Vivo Pharmacological Study
2.6. 8-Iso-PGF2α and PGE2 Radioimmunoassay
2.7. Antibacterial and Antimycotic Activities
2.8. Bioinformatics
2.9. Statistical Analysis
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Steenkamp, V.; Gouws, M.C.; Gulumian, M.; Elgorashi, E.E.; van Staden, J. Studies on antibacterial, anti-inflammatory and antioxidant activity of herbal remedies used in the treatment of benign prostatic hyperplasia and prostatitis. J. Ethnopharmacol. 2006, 103, 71–75. [Google Scholar] [CrossRef]
- Delcaru, C.; Podgoreanu, P.; Alexandru, I.; Popescu, N.; Măruţescu, L.; Bleotu, C.; Mogoşanu, G.D.; Chifiriuc, M.C.; Gluck, M.; Lazăr, V. Antibiotic Resistance and Virulence Phenotypes of Recent Bacterial Strains Isolated from Urinary Tract Infections in Elderly Patients with Prostatic Disease. Pathogens 2017, 6, 22. [Google Scholar] [CrossRef]
- Delcaru, C.; Alexandru, I.; Podgoreanu, P.; Grosu, M.; Stavropoulos, E.; Chifiriuc, M.C.; Lazar, V. Microbial Biofilms in Urinary Tract Infections and Prostatitis: Etiology, Pathogenicity, and Combating strategies. Pathogens 2016, 5, 65. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marzano, R.; Dinelli, N.; Ales, V.; Bertozzi, M.A. Effectiveness on urinary symptoms and erectile function of Prostamev Plus® vs only extract Serenoa repens. Arch. Ital. Urol. Androl. 2015, 87, 25–27. [Google Scholar] [CrossRef] [Green Version]
- Bjorling, D.E.; Wang, Z.Y.; Bushman, W. Models of inflammation of the lower urinary tract. Neurourol. Urodyn. 2011, 30, 673–682. [Google Scholar] [CrossRef] [Green Version]
- Nickel, J.C. Alpha-blockers for the treatment of prostatitis-like syndromes. Rev. Urol. 2006, 8 (Suppl. S4), S26–S34. [Google Scholar] [PubMed]
- Stamatiou, K.; Pierris, N. Serenoa repens extract additionally to quinolones in the treatment of chronic bacterial prostatitis. The preliminary results of a long term observational study. Arch. Ital. Urol. Androl. 2013, 85, 190–196. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cai, T.; Morgia, G.; Carrieri, G.; Terrone, C.; Imbimbo, C.; Verze, P.; Mirone, V. IDIProst® Gold Study Group. An improvement in sexual function is related to better quality of life, regardless of urinary function improvement: Results from the IDIProst® Gold Study. Arch. Ital. Urol. Androl. 2013, 85, 184–189. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Ma, H.L.; Liu, B.; Wang, H.B.; Xie, H.; Li, R.D.; Wang, J.F. Pinus massoniana bark extract protects against oxidative damage in L-02 hepatic cells and mice. Am. J. Chin. Med. 2010, 38, 909–919. [Google Scholar] [CrossRef] [PubMed]
- Iglesias-Gato, D.; Carsten, T.; Vesterlund, M.; Pousette, A.; Schoop, R.; Norstedt, G. Androgen-independent effects of Serenoa repens extract (Prostasan®) on prostatic epithelial cell proliferation and inflammation. Phytother. Res. 2012, 26, 259–264. [Google Scholar] [CrossRef]
- Chiavaroli, A.; Recinella, L.; Ferrante, C.; Locatelli, M.; Carradori, S.; Macchione, N.; Zengin, G.; Leporini, L.; Leone, S.; Martinotti, S.; et al. Crocus sativus, Serenoa repens and Pinus massoniana extracts modulate inflammatory response in isolated rat prostate challenged with LPS. J. Biol. Regul. Homeost. Agents 2017, 31, 531–541. [Google Scholar] [PubMed]
- Menghini, L.; Ferrante, C.; Leporini, L.; Recinella, L.; Chiavaroli, A.; Leone, S.; Pintore, G.; Vacca, M.; Orlando, G.; Brunetti, L. A natural formula containing lactoferrin, Equisetum arvensis, soy isoflavones and vitamin D3 modulates bone remodeling and inflammatory markers in young and aged rats. J. Biol. Regul. Homeost. Agents 2016, 30, 985–996. [Google Scholar]
- Tabatabaei-Malazy, O.; Larijani, B.; Abdollahi, M. Targeting metabolic disorders by natural products. J. Diabetes Metab. Disord. 2015, 14, 57. [Google Scholar] [CrossRef] [Green Version]
- Jones, W.P.; Chin, Y.W.; Kinghorn, A.D. The role of pharmacognosy in modern medicine and pharmacy. Curr. Drug Targets. 2006, 7, 247–264. [Google Scholar] [CrossRef]
- Chichiriccò, G.; Ferrante, C.; Menghini, L.; Recinella, L.; Leone, S.; Chiavaroli, A.; Brunetti, L.; Di Simone, S.; Ronci, M.; Piccone, P.; et al. Crocus sativus by-products as sources of bioactive extracts: Pharmacological and toxicological focus on anthers. Food Chem. Toxicol. 2019, 126, 7–14. [Google Scholar] [CrossRef]
- Ferrante, C.; Recinella, L.; Ronci, M.; Menghini, L.; Brunetti, L.; Chiavaroli, A.; Leone, S.; Di Iorio, L.; Carradori, S.; Tirillini, B.; et al. Multiple pharmacognostic characterization on hemp commercial cultivars: Focus on inflorescence water extract activity. Food Chem. Toxicol. 2019, 125, 452–461. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.Q.; Jaganath, I.; Manikam, R.; Sekaran, S.D. Phyllanthus Suppresses Prostate Cancer Cell, PC-3, Proliferation and Induces Apoptosis through Multiple Signalling Pathways (MAPKs, PI3K/Akt, NFκB, and Hypoxia). Evid. Based. Complement. Alternat. Med. 2013, 2013, 609581. [Google Scholar] [CrossRef] [Green Version]
- Gerhardt, D.; Bertola, G.; Dietrich, F.; Figueiró, F.; Zanotto-Filho, A.; Moreira Fonseca, J.C.; Morrone, F.B.; Barrios, C.H.; Battastini, A.M.; Salbego, C.G. Boldine induces cell cycle arrest and apoptosis in T24 human bladder cancer cell line via regulation of ERK, AKT, and GSK-3β. Urol. Oncol. 2014, 32, 36.e1–36.e9. [Google Scholar] [CrossRef]
- Gampe, N.; Darcsi, A.; Kursinszki, L.; Béni, S. Separation and characterization of homopipecolic acid isoflavonoid ester derivatives isolated from Ononis spinosa L. root. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2018, 1091, 21–28. [Google Scholar] [CrossRef]
- Choi, S.Z.; Choi, S.U.; Lee, K.R. Phytochemical constituents of the aerial parts from Solidago virga-aurea var. gigantea. Arch. Pharm. Res. 2004, 27, 164–168. [Google Scholar] [CrossRef]
- Choi, S.Z.; Choi, S.U.; Bae, S.Y.; Pyo, S.; Lee, K.R. Immunobiological [correction of Immunobioloical] activity of a new benzyl benzoate from the aerial parts of Solidago virga-aurea var. gigantea. Arch. Pharm. Res. 2005, 28, 49–54. [Google Scholar] [CrossRef] [PubMed]
- Deng, L.; Zong, W.; Tao, X.; Liu, S.; Feng, Z.; Lin, Y.; Liao, Z.; Chen, M. Evaluation of the therapeutic effect against benign prostatic hyperplasia and the active constituents from Epilobium angustifolium L. J. Ethnopharmacol. 2019, 232, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Schepetkin, I.A.; Ramstead, A.G.; Kirpotina, L.N.; Voyich, J.M.; Jutila, M.A.; Quinn, M.T. Therapeutic Potential of Polyphenols from Epilobium Angustifolium (Fireweed). Phytother. Res. 2016, 30, 1287–1297. [Google Scholar] [CrossRef] [Green Version]
- Locatelli, M.; Ferrante, C.; Carradori, S.; Secci, D.; Leporini, L.; Chiavaroli, A.; Leone, S.; Recinella, L.; Orlando, G.; Martinotti, S.; et al. Optimization of Aqueous Extraction and Biological Activity of Harpagophytum procumbens Root on Ex Vivo Rat Colon Inflammatory Model. Phytother. Res. 2017, 31, 937–944. [Google Scholar] [CrossRef]
- Recinella, L.; Chiavaroli, A.; Ronci, M.; Menghini, L.; Brunetti, L.; Leone, S.; Tirillini, B.; Angelini, P.; Covino, S.; Venanzoni, R.; et al. Multidirectional Pharma-Toxicological Study on Harpagophytum procumbens DC. ex Meisn.: An IBD-Focused Investigation. Antioxidants (Basel) 2020, 9, 168. [Google Scholar] [CrossRef] [Green Version]
- Mishra, P.P.; Prakash, V.; Singh, K.; Mog, H.; Agarwal, S. Bacteriological Profile of Isolates From Urine Samples in Patients of Benign Prostatic Hyperplasia and or Prostatitis Showing Lower Urinary Tract Symptoms. J. Clin. Diagn. Res. 2016, 10, 16–18. [Google Scholar] [CrossRef]
- Jain, S.; Samal, A.G.; Das, B.; Pradhan, B.; Sahu, N.; Mohapatra, D.; Behera, P.K.; Satpathi, P.S.; Mohanty, A.K.; Satpathi, S.; et al. Escherichia coli, a common constituent of benign prostate hyperplasia-associated microbiota induces inflammation and DNA damage in prostate epithelial cells. Prostate 2020. [Google Scholar] [CrossRef]
- Odabasi, Z.; Mert, A. Candida urinary tract infections in adults. World J. Urol. 2019. [Google Scholar] [CrossRef] [PubMed]
- Irimie, M.; Tătaru, A.; Oantă, A.; Moga, M. In vitro susceptibility of dermatophytes isolated from patients with end-stage renal disease: A case-control study. Mycoses 2014, 57, 129–134. [Google Scholar] [CrossRef]
- Magagnin, C.M.; Stopiglia, C.D.; Vieira, F.J.; Heidrich, D.; Machado, M.; Vetoratto, G.; Lamb, F.M.; Scroferneker, M.L. Antifungal susceptibility of dermatophytes isolated from patients with chronic renal failure. An. Bras. Dermatol. 2011, 86, 694–701. [Google Scholar] [CrossRef] [PubMed]
- Casamiquela, K.M.; Cohen, P.R. Radiation port dermatophytosis: Tinea corporis occurring at the site of irradiated skin. Dermatol. Online J. 2012, 18, 5. [Google Scholar]
- Sousa, V.; Luís, Â.; Oleastro, M.; Domingues, F.; Ferreira, S. Polyphenols as resistance modulators in Arcobacter butzleri. Folia Microbiol. (Praha) 2019, 64, 547–554. [Google Scholar] [CrossRef]
- Koudoufio, M.; Desjardins, Y.; Feldman, F.; Spahis, S.; Delvin, E.; Levy, E. Insight into Polyphenol and Gut Microbiota Crosstalk: Are Their Metabolites the Key to Understand Protective Effects against Metabolic Disorders? Antioxidants (Basel) 2020, 9, 982. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Delgado, M.A.; Malovaná, S.; Pérez, J.P.; Borges, T.; García Montelongo, F.J. Separation of phenolic compounds by high-performance liquid chromatography with absorbance and fluorimetric detection. J. Chromatogr. A 2001, 912, 249–257. [Google Scholar] [CrossRef]
- Orlando, G.; Leone, S.; Ferrante, C.; Chiavaroli, A.; Mollica, A.; Stefanucci, A.; Macedonio, G.; Dimmito, M.P.; Leporini, L.; Menghini, L.; et al. Effects of Kisspeptin-10 on Hypothalamic Neuropeptides and Neurotransmitters Involved in Appetite Control. Molecules 2018, 23, 3071. [Google Scholar] [CrossRef] [Green Version]
- Chiavaroli, A.; Brunetti, L.; Orlando, G.; Recinella, L.; Ferrante, C.; Leone, S.; Di Michele, P.; Di Nisio, C.; Vacca, M. Resveratrol inhibits isoprostane production in young and aged rat brain. J. Biol. Regul. Homeost. Agents 2010, 24, 441–446. [Google Scholar]
- Ferrante, C.; Angelini, P.; Venanzoni, R.; Angeles Flores, G.; Tirillini, B.; Recinella, L.; Chiavaroli., A.; Brunetti, L.; Leone, S.; Di Simone, S.C.; et al. Antimicrobial, Antioxidant, and Antiproliferative Effects of Coronilla minima: An Unexplored Botanical Species. Antibiotics (Basel) 2020, 9, 611. [Google Scholar] [CrossRef]
- Angelini, P.; Venanzoni, R.; Angeles Flores, G.; Tirillini, B.; Orlando, G.; Recinella, L.; Chiavaroli, A.; Brunetti, L.; Leone, S.; Di Simone, S.C.; et al. Evaluation of Antioxidant, Antimicrobial and Tyrosinase Inhibitory Activities of Extracts from Trichophyton goniospermum, an Edible Wild Mushroom. Antibiotics (Basel) 2020, 9, 513. [Google Scholar] [CrossRef]
- Gu, L.; Lu, J.; Li, Q.; Wu, N.; Zhang, L.; Li, H.; Xing, W.; Zhang, X. A network-based analysis of key pharmacological pathways of Andrographis paniculata acting on Alzheimer’s disease and experimental validation. J. Ethnopharmacol. 2019, 251, 112488. [Google Scholar] [CrossRef]
- Daglia, M.; Di Lorenzo, A.; Nabavi, S.F.; Talas, Z.S.; Nabavi, S.M. Polyphenols: Well beyond the antioxidant capacity: Gallic acid and related compounds as neuroprotective agents: You are what you eat! Curr. Pharm. Biotechnol. 2014, 15, 362–372. [Google Scholar] [CrossRef] [PubMed]
- Özdemir, Z.; Utku, S.; Mathew, B.; Carradori, S.; Orlando, G.; Di Simone, S.; Alagöz, M.A.; Özçelik, A.B.; Uysal, M.; Ferrante, C. Synthesis and biological evaluation of new 3(2H)-pyridazinone derivatives as non-toxic anti-proliferative compounds against human colon carcinoma HCT116 cells. J. Enzym. Inhib. Med. Chem. 2020, 35, 1100–1109. [Google Scholar] [CrossRef] [Green Version]
- Huang, E.; Yang, X.; Zhang, L.; Moon, S.H.; Yousef, A.E. New Paenibacillus strain produces a family of linear and cyclic antimicrobial lipopeptides: Cyclization is not essential for their antimicrobial activity. FEMS Microbiol. Lett. 2017, 364. [Google Scholar] [CrossRef] [Green Version]
- Guo, Y.; Huang, E.; Yang, X.; Zhang, L.; Yousef, A.E.; Zhong, J. Isolation and characterization of a Bacillus atrophaeus strain and its potential use in food preservation. Food Control. 2016, 60, 511–518. [Google Scholar] [CrossRef]
- Yang, X.; Huang, E.; Yuan, C.; Zhang, L.; Yousef, A.E. Isolation and Structural Elucidation of Brevibacillin, an Antimicrobial Lipopeptide from Brevibacillus laterosporus That Combats Drug-Resistant Gram-Positive Bacteria. Appl. Environ. Microbiol. 2016, 82, 2763–2772. [Google Scholar] [CrossRef] [Green Version]
- Orlando, G.; Ferrante, C.; Zengin, G.; Sinan, K.I.; Bene, K.; Diuzheva, A.; Jekő, J.; Cziáky, Z.; Simone, S.D.; Recinella, L.; et al. Qualitative Chemical Characterization and Multidirectional Biological Investigation of Leaves and Bark Extracts of Anogeissus leiocarpus (DC.) Guill. & Perr. (Combretaceae). Antioxidants (Basel) 2019, 8, 343. [Google Scholar] [CrossRef] [Green Version]
- di Giacomo, V.; Ferrante, C.; Ronci, M.; Cataldi, A.; Di Valerio, V.; Rapino, M.; Recinella, L.; Chiavaroli, A.; Leone, S.; Vladimir-Knežević, S.; et al. Multiple pharmacological and toxicological investigations on Tanacetum parthenium and Salix alba extracts: Focus on potential application as anti-migraine agents. Food Chem. Toxicol. 2019, 133, 110783. [Google Scholar] [CrossRef]
- Abnosi, M.H.; Yari, S. The toxic effect of gallic acid on biochemical factors, viability and proliferation of rat bone marrow mesenchymal stem cells was compensated by boric acid. J. Trace Elem. Med. Biol. 2018, 48, 246–253. [Google Scholar] [CrossRef]
- Locatelli, M.; Macchione, N.; Ferrante, C.; Chiavaroli, A.; Recinella, L.; Carradori, S.; Zengin, G.; Cesa, S.; Leporini, L.; Leone, S.; et al. Graminex Pollen: Phenolic Pattern, Colorimetric Analysis and Protective Effects in Immortalized Prostate Cells (PC3) and Rat Prostate Challenged with LPS. Molecules 2018, 23, 1145. [Google Scholar] [CrossRef] [Green Version]
- Koeberle, A.; Werz, O. Inhibitors of the microsomal prostaglandin E(2) synthase-1 as alternative to non steroidal anti-inflammatory drugs (NSAIDs)-a critical review. Curr. Med. Chem. 2009, 16, 4274–4296. [Google Scholar] [CrossRef] [PubMed]
- Brunetti, L.; Leone, S.; Chiavaroli, A.; Orlando, G.; Recinella, L.; Ferrante, C.; Di Nisio, C.; Verratti, V.; Vacca, M. Cafeteria diet increases prostaglandin E2 levels in rat prostate, kidney and testis. Int. J. Immunopathol. Pharmacol. 2010, 23, 1073–1078. [Google Scholar] [CrossRef] [Green Version]
- Verratti, V.; Brunetti, L.; Ferrante, C.; Orlando, G.; Recinella, L.; Chiavaroli, A.; Leone, S.; Wang, R.; Berardinelli, F. Physiological and pathological levels of prostaglandin E2 in renal parenchyma and neoplastic renal tissue. Prostaglandins Other Lipid Mediat. 2019, 141, 11–13. [Google Scholar] [CrossRef] [PubMed]
- Uttara, B.; Singh, A.V.; Zamboni, P.; Mahajan, R.T. Oxidative stress and neurodegenerative diseases: A review of upstream and downstream antioxidant therapeutic options. Curr. Neuropharmacol. 2009, 7, 65–74. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Praticò, D. Alzheimer’s disease and oxygen radicals: New insights. Biochem. Pharmacol. 2002, 63, 563–567. [Google Scholar] [CrossRef]
- Verratti, V.; Brunetti, L.; Tenaglia, R.; Chiavaroli, A.; Ferrante, C.; Leone, S.; Orlando, G.; Berardinelli, F.; Di Giulio, C.; Vacca, M. Physiological analysis of 8-ISO-PGF2 alpha: A homeostatic agent in superficial bladder cancer. J. Biol. Regul. Homeost. Agents 2011, 25, 71–76. [Google Scholar]
- Al-Sayed, E.; Abdel-Daim, M.M. Analgesic and anti-inflammatory activities of epicatechin gallate from Bauhinia hookeri. Drug Dev. Res. 2018, 79, 157–164. [Google Scholar] [CrossRef]
- Micali, S.; Territo, A.; Pirola, G.M.; Ferrari, N.; Sighinolfi, M.C.; Martorana, E.; Navarra, M.; Bianchi, G. Effect of green tea catechins in patients with high-grade prostatic intraepithelial neoplasia: Results of a short-term double-blind placebo controlled phase II clinical trial. Arch. Ital. Urol. Androl. 2017, 89, 197–202. [Google Scholar] [CrossRef] [Green Version]
- Hayakawa, S.; Ohishi, T.; Miyoshi, N.; Oishi, Y.; Nakamura, Y.; Isemura, M. Anti-Cancer Effects of Green Tea Epigallocatchin-3-Gallate and Coffee Chlorogenic Acid. Molecules 2020, 25, 4553. [Google Scholar] [CrossRef]
- Reddivari, L.; Vanamala, J.; Safe, S.H.; Miller, J.C., Jr. The bioactive compounds alpha-chaconine and gallic acid in potato extracts decrease survival and induce apoptosis in LNCaP and PC3 prostate cancer cells. Nutr. Cancer 2010, 62, 601–610. [Google Scholar] [CrossRef]
- Afsar, T.; Trembley, J.H.; Salomon, C.E.; Razak, S.; Khan, M.R.; Ahmed, K. Growth inhibition and apoptosis in cancer cells induced by polyphenolic compounds of Acacia hydaspica: Involvement of multiple signal transduction pathways. Sci. Rep. 2016, 6, 23077. [Google Scholar] [CrossRef]















| Time (min) | Flow | %A | %B |
|---|---|---|---|
| 0–0.50 | 1 mL/min | 93 | 7 |
| 25 | 72 | 28 | |
| 30 | 72 | 28 | |
| 38 | 75 | 25 | |
| 45 | 2 | 98 | |
| 48 | 93 | 7 | |
| 58 | 93 | 7 |
| Dermatophyte Strains (ID) | Minimum Inhibitory Concentration (MIC) * | |||||
|---|---|---|---|---|---|---|
| P. boldus (µg/mL) | E. angustifolium (µg/mL) | O. spinosa (µg/mL) | P. niruri (µg/mL) | S. virga-aurea (µg/mL) | Griseofulvin (µg/mL) | |
| T. mentagrophytes (CCF 4823) | 78.74 (62.5–125) | 99.21 (62.25–125 | >250 | 198.42 (125–250) | >250 | 2.52 (2–4) |
| T. tonsurans (CCF 4834) | 9.84 (7.81–15.62) | 12.4 (7.81–15.62) | 78.74 (62.5–125) | 49.60 (31.25–62.5) | 49.6 (31.25–62.5) | 0.198 (0.125–0.25) |
| T. rubrum (CCF 4879) | >250 | 78.74 (62.5–125) | 99.21 (62.5–125) | 78.74 (62.5–125) | 99.21 (62.5–125) | 3.175 (2–4) |
| T. rubrum (CCF 4933) | 24.80 (15.62–31.25) | 24.80 (15.62–31.25) | 49.6 (31.25–62.5) | 49.6 (31.25–62.5) | 99.21 (62.5–125) | 1.26 (1–2) |
| A. crocatum (CCF 5300) | 19.68 (15.62–31.25) | 19.68 (15.62–31.25) | 24.80 (15.62–31.25) | 39.37 (31.25–62.5) | 78.74 (62.5–125) | >8 |
| A. quadrifidum (CCF 5792) | 39.37 (31.25–62.25) | 78.74 (62.5–125) | 198.42 (125–250) | >250 | 198.42 (125–250) | >8 |
| T. erinacei (CCF 5930) | >250 | 198.42 (125–250) | >250 | >250 | >250 | 3.174 (2–4) |
| A. gypseum (CCF 6261) | 157.49 (125–250) | 157.49 (125–250) | >250 | >250 | >250 | 1.587 (1–2) |
| A. currey (CCF 5207) | 24.80 (15.62–31.25) | 39.37 (31.25–62.5) | 78.74 (62.5–125) | 49.6 (31.25–62.5) | 78.74 (62.5–125) | >8 |
| A. insingulare (CCF 5417) | 39.37 (31.25–62.25) | 49.61 (31.25–62.5) | 99.21 (62.5–125) | 78.74 (62.5–125) | 99.21 (62.5–125) | >8 |
| Yeast Strains (ID) | Minimum Inhibitory Concentration (MIC) * | |||||
|---|---|---|---|---|---|---|
| P. boldus (µg/mL) | E. angustifolium (µg/mL) | O. spinosa (µg/mL) | P. niruri (µg/mL) | S. virga-aurea (µg/mL) | Fluconazole (µg/mL) | |
| C. tropicalis (DBVPG 6184) | 157.49 (125–250) | 49.60 (31.25–62.5) | 78.74 (62.5–125) | 99.21 (62.5–125) | 49.60 (31.25–62.5) | 2 |
| C. albicans (DBVPG 6379) | >250 | 198.42 (125–250) | 198.42 (125–250) | (≥250) | >250 | 1 |
| C. parapsilosis (DBVPG 6551) | 198.42 (125–250) | (≥250) | >250 | >250 | 198.42 (125–250) | 4 |
| C. albicans (DBVPG 6183) | 99.21 (62.5–125) | 157.49 (125–250) | >250 | >250 | >250 | 2 |
| Bacterial Strains (ID) | Minimum Inhibitory Concentration (MIC) * | |||||
|---|---|---|---|---|---|---|
| P. boldus (µg/mL) | E. angustifolium (µg/mL) | O. spinosa (µg/mL) | P. niruri (µg/mL) | S. virga-aurea (µg/mL) | Ciprofloxacin (µg/mL) | |
| Gram− | ||||||
| E. coli (ATCC 10536) | 24.80 (7.81–15.625) | 78.74 (62.5–125) | 49.60 (31.25–62.5) | 39.37 (31.25–62.5) | 78.74 (62.5–125) | <0.12 |
| E. coli (PeruMycA 2) | 39.37 (31.25–62.5) | 157.49 (125–250) | 99.21 (62.5–125) | 49.60 (31.25–62.5) | 49.60 (31.25–62.5) | 1.23 (1.95–0.98) |
| E. coli (PeruMycA 3) | 99.21 (62.5–125) | 198.42 (125–250) | 157.49 (125–250) | (≥250) | >250 | 0.62 (0.98–0.49) |
| P. aeruginosa (PeruMycA 5) | 78.74 (62.5–125) | 99.21 (62.5–125) | 49.60 (31.25–62.5) | 24.8 (15.62–31.25) | 39.37 (31.25–62.5) | 1.23 (1.95–0.98) |
| S. typhy (PeruMycA 7) | 157.49 (125–250) | 198.42 (125–250) | (≥250) | 198.42 (125–250) | (≥250) | 0.38 (0.49–0.24) |
| Gram+ | ||||||
| B. cereus (PeruMycA 4) | 78.74 (62.5–125) | 99.21 (62.5–125) | 198.42 (125–250) | 49.60 (31.25–62.5) | 78.74 (62.5–125) | <0.12 |
| B. subtilis (PeruMycA 6) | 157.49 (125–250) | 198.42 (125–250) | (≥250) | 99.21 (62.5–125) | 157.49 (125–250) | <0.12 |
| S. aureus (ATCC 6538) | 198.42 (125–250) | 198.42 (125–250) | 99.21 (62.5–125) | 78.74 (62.5–125) | 99.21 (62.5–125) | 0.62 (0.98–0.49) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferrante, C.; Chiavaroli, A.; Angelini, P.; Venanzoni, R.; Angeles Flores, G.; Brunetti, L.; Petrucci, M.; Politi, M.; Menghini, L.; Leone, S.; et al. Phenolic Content and Antimicrobial and Anti-Inflammatory Effects of Solidago virga-aurea, Phyllanthus niruri, Epilobium angustifolium, Peumus boldus, and Ononis spinosa Extracts. Antibiotics 2020, 9, 783. https://doi.org/10.3390/antibiotics9110783
Ferrante C, Chiavaroli A, Angelini P, Venanzoni R, Angeles Flores G, Brunetti L, Petrucci M, Politi M, Menghini L, Leone S, et al. Phenolic Content and Antimicrobial and Anti-Inflammatory Effects of Solidago virga-aurea, Phyllanthus niruri, Epilobium angustifolium, Peumus boldus, and Ononis spinosa Extracts. Antibiotics. 2020; 9(11):783. https://doi.org/10.3390/antibiotics9110783
Chicago/Turabian StyleFerrante, Claudio, Annalisa Chiavaroli, Paola Angelini, Roberto Venanzoni, Giancarlo Angeles Flores, Luigi Brunetti, Massimiliano Petrucci, Matteo Politi, Luigi Menghini, Sheila Leone, and et al. 2020. "Phenolic Content and Antimicrobial and Anti-Inflammatory Effects of Solidago virga-aurea, Phyllanthus niruri, Epilobium angustifolium, Peumus boldus, and Ononis spinosa Extracts" Antibiotics 9, no. 11: 783. https://doi.org/10.3390/antibiotics9110783
APA StyleFerrante, C., Chiavaroli, A., Angelini, P., Venanzoni, R., Angeles Flores, G., Brunetti, L., Petrucci, M., Politi, M., Menghini, L., Leone, S., Recinella, L., Zengin, G., Ak, G., Di Mascio, M., Bacchin, F., & Orlando, G. (2020). Phenolic Content and Antimicrobial and Anti-Inflammatory Effects of Solidago virga-aurea, Phyllanthus niruri, Epilobium angustifolium, Peumus boldus, and Ononis spinosa Extracts. Antibiotics, 9(11), 783. https://doi.org/10.3390/antibiotics9110783















