Plasmid-Mediated Ampicillin, Quinolone, and Heavy Metal Co-Resistance among ESBL-Producing Isolates from the Yamuna River, New Delhi, India
Abstract
:1. Introduction
2. Materials and Methods
2.1. Features of the Included Bacterial Strains
2.2. Antibiotic Susceptibility Testing and Multiple Antibiotic Resistance (MAR) Index
2.3. Plasmid DNA Isolation and Amplification
2.4. Detection and Characterization of blaCMY and PMQR Genes
2.5. Detection of Heavy Metal Resistance Genes
2.6. Susceptibility towards Heavy Metals
2.7. Conjugation Experiment
2.8. Accession Numbers
3. Results
3.1. Susceptibility Profile of Test Strains
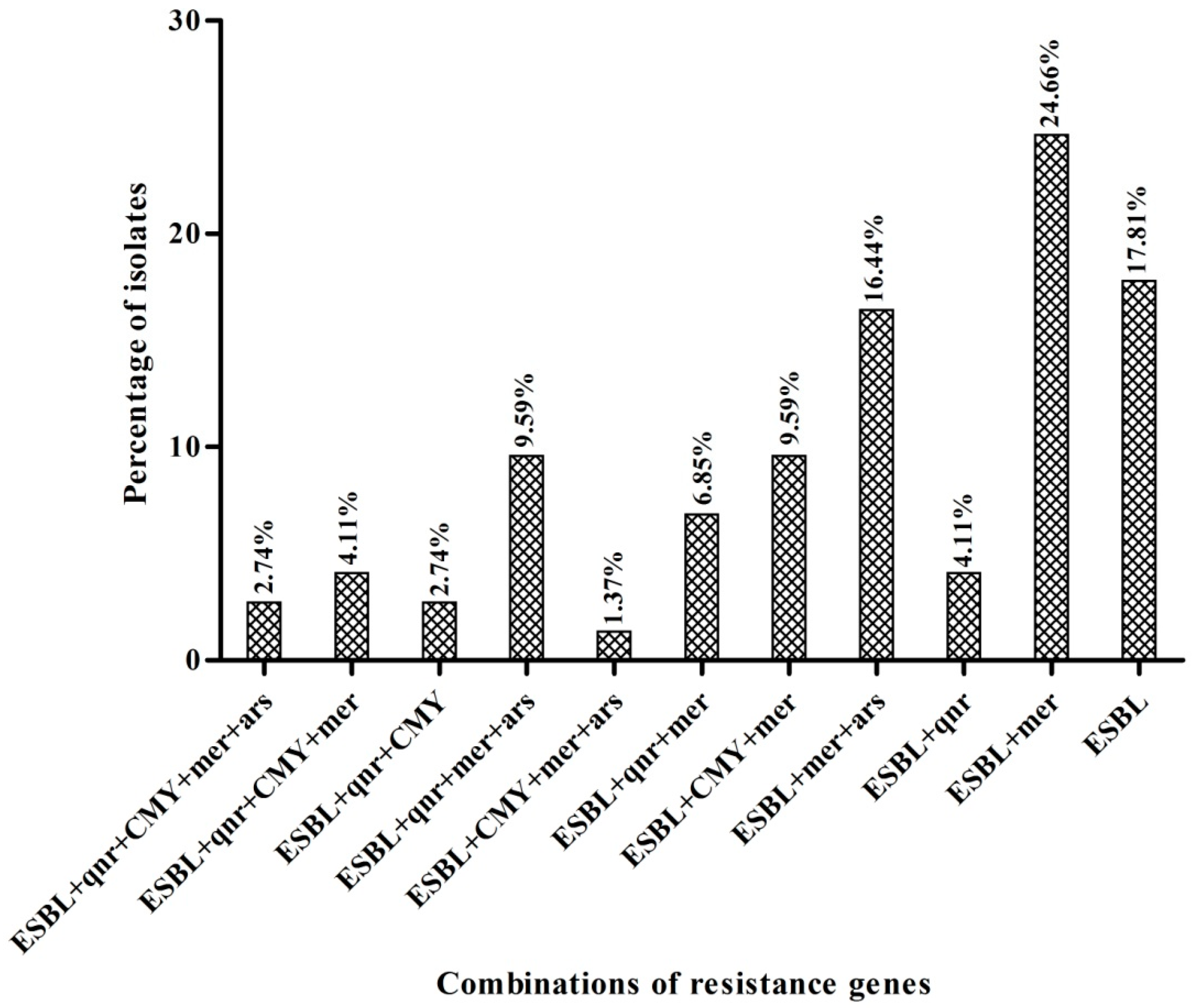
3.2. Detection and Characterization of blaCMY and PMQR Genes
3.3. Detection of Heavy Metal Resistance Genes
3.4. Susceptibility to Heavy Metals
3.5. Conjugation Experiments
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Philippon, A.; Arlet, G.; Jacoby, G.A. Plasmid-determined AmpC-type beta-lactamases. Antimicrob. Agents Chemother. 2002, 46, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Hopkins, K.L.; Davies, R.H.; Threlfall, E.J. Mechanisms of quinolone resistance in Escherichia coli and Salmonella: Recent developments. Int. J. Antimicrob. Agents 2005, 25, 358–373. [Google Scholar] [CrossRef] [PubMed]
- Bradford, P.A. Extended-spectrum beta-lactamases in the 21st century: Characterization, epidemiology, and detection of this important resistance threat. Clin. Microbiol. Rev. 2001, 14, 933–951. [Google Scholar] [CrossRef] [Green Version]
- Fasciana, T.; Gentile, B.; Aquilina, M.; Ciammaruconi, A.; Mascarella, C.; Anselmo, A.; Fortunato, A.; Fillo, S.; Petralito, G.; Lista, F.; et al. Co-existence of virulence factors and antibiotic resistance in new Klebsiella pneumoniae clones emerging in south of Italy. BMC Infect. Dis. 2019, 19, 928. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bauernfeind, A.; Chong, Y.; Schweighart, S. Extended broad spectrum beta-lactamase in Klebsiella pneumoniae including resistance to cephamycins. Infection 1989, 17, 316–321. [Google Scholar] [CrossRef] [PubMed]
- Jacoby, G.A. AmpC beta-lactamases. Clin. Microbiol. Rev. 2009, 22, 161–182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hentschke, M.; Kotsakis, S.D.; Wolters, M.; Heisig, P.; Miriagou, V.; Aepfelbacher, M. CMY-42, a novel plasmid-mediated CMY-2 variant AmpC beta-lactamase. Microb. Drug Resist. 2011, 17, 165–169. [Google Scholar] [CrossRef]
- Zhang, X.; Lou, D.; Xu, Y.; Shang, Y.; Li, D.; Huang, X.; Li, Y.; Hu, L.; Wang, L.; Yu, F. First identification of coexistence of blaNDM-1 and blaCMY-42 among Escherichia coli ST167 clinical isolates. BMC Microbiol. 2013, 13, 282. [Google Scholar] [CrossRef] [Green Version]
- Bajaj, P.; Singh, N.S.; Kanaujia, P.K.; Virdi, J.S. Distribution and molecular characterization of genes encoding CTX-M and AmpC β-lactamases in Escherichia coli isolated from an Indian urban aquatic environment. Sci. Total Environ. 2015, 505, 350–356. [Google Scholar] [CrossRef]
- Hooper, D.C.; Jacoby, G.A. Topoisomerase Inhibitors: Fluoroquinolone mechanisms of action and resistance. Cold Spring Harb. Perspect. Med. 2016, 6. [Google Scholar] [CrossRef] [Green Version]
- Robicsek, A.; Jacoby, G.A.; Hooper, D.C. The worldwide emergence of plasmid-mediated quinolone resistance. Lancet Infect. Dis. 2006, 6, 629–640. [Google Scholar] [CrossRef]
- Fonseca, E.L.; Vicente, A.C.P. Epidemiology of qnrVC alleles and emergence out of the Vibrionaceae family. J. Med. Microbiol. 2013, 62, 1628–1630. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakaminami, H.; Noguchi, N.; Sasatsu, M. Fluoroquinolone efflux by the plasmid-mediated multidrug efflux pump QacB variant QacBIII in Staphylococcus aureus. Antimicrob. Agents Chemother. 2010, 54, 4107–4111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bajaj, P.; Kanaujia, P.K.; Singh, N.S.; Sharma, S.; Kumar, S.; Virdi, J.S. Quinolone co-resistance in ESBL- or AmpC-producing Escherichia coli from an Indian urban aquatic environment and their public health implications. Environ. Sci. Pollut. Res. Int. 2016, 23, 1954–1959. [Google Scholar] [CrossRef] [PubMed]
- Cattoir, V.; Poirel, L.; Rotimi, V.; Soussy, C.J.; Nordmann, P. Multiplex PCR for detection of plasmid-mediated quinolone resistance qnr genes in ESBL-producing enterobacterial isolates. J. Antimicrob. Chemother. 2007, 60, 394–397. [Google Scholar] [CrossRef] [Green Version]
- Caruso, G.; Giammanco, A.; Cardamone, C.; Oliveri, G.; Mascarella, C.; Capra, G.; Fasciana, T. Extra-intestinal fluoroquinolone-resistant escherichia coli strains isolated from meat. BioMed Res. Int. 2018, 2018, 8714975. [Google Scholar] [CrossRef]
- Alonso, A.; Sánchez, P.; Martínez, J.L. Environmental selection of antibiotic resistance genes. Environ. Microbiol. 2001, 3, 1–9. [Google Scholar] [CrossRef]
- Baker-Austin, C.; Wright, M.S.; Stepanauskas, R.; McArthur, J.V. Co-selection of antibiotic and metal resistance. Trends Microbiol. 2006, 14, 176–182. [Google Scholar] [CrossRef]
- Ji, X.; Shen, Q.; Liu, F.; Ma, J.; Xu, G.; Wang, Y.; Wu, M. Antibiotic resistance gene abundances associated with antibiotics and heavy metals in animal manures and agricultural soils adjacent to feedlots in Shanghai; China. J. Hazard. Mater. 2012, 235, 178–185. [Google Scholar] [CrossRef]
- Sehgal, M.; Garg, A.; Suresh, R.; Dagar, P. Heavy metal contamination in the Delhi segment of Yamuna basin. Environ. Monit. Assess. 2012, 184, 1181–1196. [Google Scholar] [CrossRef]
- Mutiyar, P.K.; Mittal, A.K. Occurrences and fate of selected human antibiotics in influents and effluents of sewage treatment plant and effluent-receiving river Yamuna in Delhi (India). Environ. Monit. Assess. 2014, 186, 541–557. [Google Scholar] [CrossRef] [PubMed]
- Jackson, R.W.; Vinatzer, B.; Arnold, D.L.; Dorus, S.; Murillo, J. The influence of the accessory genome on bacterial pathogen evolution. Mob. Genet. Elem. 2011, 1, 55–65. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Slots, J.; Feik, D.; Rams, T.E. Prevalence and antimicrobial susceptibility of enterobacteriaceae, pseudomonadaceae and acinetobacter in human periodontitis. Oral Microbiol. Immunol. 1990, 5, 149–154. [Google Scholar] [CrossRef] [PubMed]
- Klasen, H.J. A historical review of the use of silver in the treatment of burns. II. Renewed interest for silver. Burn. J. Int. Soc. Burn Inj. 2000, 26, 131–138. [Google Scholar] [CrossRef]
- Sunita, M.S.L.; Prashant, S.; Bramha Chari, P.V.; Nageswara Rao, S.; Balaravi, P.; Kavi Kishor, P.B. Molecular identification of arsenic-resistant estuarine bacteria and characterization of their ars genotype. Ecotoxicology 2012, 21, 202–212. [Google Scholar] [CrossRef]
- Knapp, C.W.; Callan, A.C.; Aitken, B.; Shearn, R.; Koenders, A.; Hinwood, A. Relationship between antibiotic resistance genes and metals in residential soil samples from Western Australia. Environ. Sci. Pollut. Res. 2017, 24, 2484–2494. [Google Scholar] [CrossRef]
- Hobman, J.L.; Crossman, L.C. Bacterial antimicrobial metal ion resistance. J. Med. Microbiol. 2015, 64, 471–497. [Google Scholar] [CrossRef]
- Gupta, A.; Phung, L.T.; Taylor, D.E.; Silver, S. Diversity of silver resistance genes in IncH incompatibility group plasmids. Microbiology 2001, 147, 3393–3402. [Google Scholar] [CrossRef] [Green Version]
- Johnson, T.J.; Siek, K.E.; Johnson, S.J.; Nolan, L.K. DNA sequence and comparative genomics of pAPEC-O2-R, an avian pathogenic Escherichia coli transmissible R plasmid. Antimicrob. Agents Chemother. 2005, 49, 4681–4688. [Google Scholar] [CrossRef] [Green Version]
- Tacão, M.; Moura, A.; Correia, A.; Henriques, I. Co-resistance to different classes of antibiotics among ESBL-producers from aquatic systems. Water Res. 2014, 48, 100–107. [Google Scholar] [CrossRef]
- Zurfluh, K.; Abgottspon, H.; Hächler, H.; Nüesch-Inderbinen, M.; Stephan, R. Quinolone resistance mechanisms among extended-spectrum beta-lactamase (ESBL) producing Escherichia coli isolated from rivers and lakes in Switzerland. PLoS ONE 2014, 9, e95864. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Siddiqui, M.T.; Mondal, A.H.; Sultan, I.; Ali, A.; Haq, Q.M.R. Co-occurrence of ESBLs and silver resistance determinants among bacterial isolates inhabiting polluted stretch of river Yamuna, India. Int. J. Environ. Sci. Technol. 2019, 16, 5611–5622. [Google Scholar] [CrossRef]
- Krumperman, P.H. Multiple antibiotic resistance indexing of Escherichia coli to identify high-risk sources of fecal contamination of foods. Appl. Environ. Microbiol. 1983, 46, 165–170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Birnboim, H.C.; Doly, J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979, 7, 1513–1523. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.B.; Park, C.H.; Kim, C.J.; Kim, E.C.; Jacoby, G.A.; Hooper, D.C. Prevalence of plasmid-mediated quinolone resistance determinants over a 9-year period. Antimicrob. Agents Chemother. 2009, 53, 639–645. [Google Scholar] [CrossRef] [Green Version]
- Wang, M.; Guo, Q.; Xu, X.; Wang, X.; Ye, X.; Wu, S.; Hooper, D.C.; Wang, M. New plasmid-mediated quinolone resistance gene, qnrC, found in a clinical isolate of Proteus mirabilis. Antimicrob. Agents Chemother. 2009, 53, 1892–1897. [Google Scholar] [CrossRef] [Green Version]
- Cavaco, L.M.; Hasman, H.; Xia, S.; Aarestrup, F.M. qnrD, a novel gene conferring transferable quinolone resistance in Salmonella enterica serovar Kentucky and Bovismorbificans strains of human origin. Antimicrob. Agents Chemother. 2009, 53, 603–608. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.; Zhang, W.; Pan, W.; Yin, J.; Pan, Z.; Gao, S.; Jiao, X. Prevalence of qnr, aac(6′)-Ib-cr, qepA, and oqxAB in Escherichia coli isolates from humans, animals, and the environment. Antimicrob. Agents Chemother. 2012, 56, 3423–3427. [Google Scholar] [CrossRef] [Green Version]
- Pérez-Pérez, F.J.; Hanson, N.D. Detection of plasmid-mediated AmpC beta-lactamase genes in clinical isolates by using multiplex PCR. J. Clin. Microbiol. 2002, 40, 2153–2162. [Google Scholar] [CrossRef] [Green Version]
- Azam, M.; Jan, A.T.; Haq, Q.M. blaCTX-M-152, a novel variant of CTX-M-group-25, identified in a study performed on the prevalence of multidrug resistance among natural inhabitants of River Yamuna, India. Front. Microbiol. 2016, 7, 176. [Google Scholar] [CrossRef]
- Figueiredo, N.L.; Canário, J.; Duarte, A.; Serralheiro, M.L.; Carvalho, C. Isolation and characterization of mercury-resistant bacteria from sediments of Tagus Estuary (Portugal): Implications for environmental and human health risk assessment. J. Toxicol. Environ. Health Part A 2014, 77, 155–168. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Shu, W.; Chang, X.; Chen, J.-a.; Guo, Y.; Tan, Y. The profile of antibiotics resistance and integrons of extended-spectrum beta-lactamase producing thermotolerant coliforms isolated from the Yangtze River basin in Chongqing. Environ. Pollut. 2010, 158, 2459–2464. [Google Scholar] [CrossRef] [PubMed]
- di Cesare, A.; Eckert, E.M.; D’Urso, S.; Bertoni, R.; Gillan, D.C.; Wattiez, R.; Corno, G. Co-occurrence of integrase 1, antibiotic and heavy metal resistance genes in municipal wastewater treatment plants. Water Res. 2016, 94, 208–214. [Google Scholar] [CrossRef]
- Kannan, S.K.; Krishnamoorthy, R. Isolation of mercury resistant bacteria and influence of abiotic factors on bioavailability of mercury—A case study in Pulicat Lake North of Chennai, South East India. Sci. Total Environ. 2006, 367, 341–353. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.Y.; Zhang, Y.L.; Geng, S.N.; Li, T.Y.; Ye, Z.M.; Zhang, D.S.; Zou, F.; Zhou, H.W. High diversity of extended-spectrum beta-lactamase-producing bacteria in an urban river sediment habitat. Appl. Environ. Microbiol. 2010, 76, 5972–5976. [Google Scholar] [CrossRef] [Green Version]
- Siddiqui, K.; Mondal, A.; Siddiqui, M.T.; Azam, M.; Rizwanul, Q. Prevalence and molecular characterization of ESBL producing enterobacteriaceae from highly polluted stretch of River Yamuna, India. Microbiol. Biotechnol. Lett. 2018, 46. [Google Scholar] [CrossRef] [Green Version]
- Mondal, A.H.; Siddiqui, M.T.; Sultan, I.; Haq, Q.M.R. Prevalence and diversity of blaTEM, blaSHV and blaCTX-M variants among multidrug resistant Klebsiella spp. from an urban riverine environment in India. Int. J. Environ. Health Res. 2019, 29, 117–129. [Google Scholar] [CrossRef]
- Gogry, F.A.; Siddiqui, M.T.; Haq, Q.M.R. Emergence of mcr-1 conferred colistin resistance among bacterial isolates from urban sewage water in India. Environ. Sci. Pollut. Res. Int. 2019, 26, 33715–33717. [Google Scholar] [CrossRef]
- Harada, K.; Shimizu, T.; Mukai, Y.; Kuwajima, K.; Sato, T.; Usui, M.; Tamura, Y.; Kimura, Y.; Miyamoto, T.; Tsuyuki, Y.; et al. Phenotypic and molecular characterization of antimicrobial resistance in klebsiella spp. isolates from companion animals in Japan: Clonal dissemination of multidrug-resistant extended-spectrum β-lactamase-producing klebsiella pneumoniae. Front. Microbiol. 2016, 7, 1021. [Google Scholar] [CrossRef] [Green Version]
- Ojer-Usoz, E.; González, D.; Vitas, A.I.; Leiva, J.; García-Jalón, I.; Febles-Casquero, A.; Escolano, M.D.L.S. Prevalence of extended-spectrum β-lactamase-producing Enterobacteriaceae in meat products sold in Navarra, Spain. Meat Sci. 2013, 93, 316–321. [Google Scholar] [CrossRef]
- Lenart-Boroń, A. Antimicrobial resistance and prevalence of extended-spectrum beta-lactamase genes in Escherichia coli from major rivers in Podhale, southern Poland. Int. J. Environ. Sci. Technol. 2017, 14, 241–250. [Google Scholar] [CrossRef] [Green Version]
- Martinez-Medina, M.; Strozzi, F.; Castillo, B.R.D.; Serrano-Morillas, N.; Bustins, N.F.; Martínez-Martínez, L. Antimicrobial resistance profiles of adherent invasive escherichia coli show increased resistance to β-lactams. Antibiotics 2020, 9, 251. [Google Scholar] [CrossRef] [PubMed]
- Lien, T.Q.; Lan, P.T.; Chuc, N.T.K.; Hoa, N.Q.; Nhung, P.H.; Thoa, N.T.M.; Diwan, V.; Tamhankar, A.J.; Lundborg, C.S. Antibiotic resistance and antibiotic resistance genes in escherichia coli isolates from hospital wastewater in vietnam. Int. J. Environ. Res. Public Health 2017, 14, 699. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chitanand, M.P.; Kadam, T.A.; Gyananath, G.; Totewad, N.D.; Balhal, D.K. Multiple antibiotic resistance indexing of coliforms to identify high risk contamination sites in aquatic environment. Indian J. Microbiol. 2010, 50, 216–220. [Google Scholar] [CrossRef] [Green Version]
- Loncaric, I.; Misic, D.; Szostak, M.P.; Künzel, F.; Schäfer-Somi, S.; Spergser, J. Broad-spectrum cephalosporin-resistant and/or fluoroquinolone-resistant enterobacterales associated with canine and feline urogenital infections. Antibiotics 2020, 9, 387. [Google Scholar] [CrossRef]
- Deshpande, L.M.; Jones, R.N.; Fritsche, T.R.; Sader, H.S. Occurrence of plasmidic AmpC type beta-lactamase-mediated resistance in Escherichia coli: Report from the SENTRY Antimicrobial Surveillance Program (North America, 2004). Int. J. Antimicrob. Agents 2006, 28, 578–581. [Google Scholar] [CrossRef]
- Oteo, J.; Pérez-Vázquez, M.; Campos, J. Extended-spectrum [beta]-lactamase producing Escherichia coli: Changing epidemiology and clinical impact. Curr. Opin. Infect. Dis. 2010, 23, 320–326. [Google Scholar] [CrossRef]
- Li, L.; Wang, B.; Feng, S.; Li, J.; Wu, C.; Wang, Y.; Ruan, X.; Zeng, M. Prevalence and characteristics of extended-spectrum β-lactamase and plasmid-mediated fluoroquinolone resistance genes in Escherichia coli isolated from chickens in Anhui province, China. PLoS ONE 2014, 9, e104356. [Google Scholar] [CrossRef] [Green Version]
- Mood, E.H.; Meshkat, Z.; Izadi, N.; Rezaei, M.; Amel Jamehdar, S.; Nasab, M.N. Prevalence of quinolone resistance genes among extended-spectrum B-lactamase-producing escherichia coli in Mashhad, Iran. Jundishapur J. Microbiol. 2015, 8, e16217. [Google Scholar] [CrossRef] [Green Version]
- Poirel, L.; Pitout, J.D.; Calvo, L.; Rodriguez-Martinez, J.M.; Church, D.; Nordmann, P. In vivo selection of fluoroquinolone-resistant Escherichia coli isolates expressing plasmid-mediated quinolone resistance and expanded-spectrum beta-lactamase. Antimicrob. Agents Chemother. 2006, 50, 1525–1527. [Google Scholar] [CrossRef] [Green Version]
- Rodríguez-Martínez, J.M.; Velasco, C.; García, I.; Cano, M.E.; Martínez-Martínez, L.; Pascual, A. Mutant Prevention Concentrations of Fluoroquinolones for Enterobacteriaceae Expressing the Plasmid-Carried Quinolone Resistance Determinant qnrA1. Antimicrob. Agents Chemother. 2007, 51, 2236. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boening, D.W. Ecological effects, transport, and fate of mercury: A general review. Chemosphere 2000, 40, 1335–1351. [Google Scholar] [CrossRef]
- Kothari, A.; Soneja, D.; Tang, A.; Carlson, H.K.; Deutschbauer, A.M.; Mukhopadhyay, A. Native plasmid-encoded mercury resistance genes are functional and demonstrate natural transformation in environmental bacterial isolates. mSystems 2019, 4, e00588-19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Luca Rebello, R.C.; Machado Gomes, K.; Duarte, R.S.; Tavora Coelho da Costa Rachid, C.; Rosado, A.S.; Regua-Mangia, A.H. Diversity of mercury resistant Escherichia coli strains isolated from aquatic systems in Rio de Janeiro, Brazil. Int. J. Biodivers. 2013, 2013, 265356. [Google Scholar] [CrossRef]
- Skippington, E.; Ragan, M. Lateral genetic transfer and the construction of genetic exchange communities. FEMS Microbiol. Rev. 2011, 35, 707–735. [Google Scholar] [CrossRef]
- Rosen, B.P. Biochemistry of arsenic detoxification. FEBS Lett. 2002, 529, 86–92. [Google Scholar] [CrossRef] [Green Version]
- Kaur, S.; Kamli, M.R.; Ali, A. Diversity of arsenate reductase genes (arsC Genes) from arsenic-resistant environmental isolates of E. coli. Curr. Microbiol. 2009, 59, 288–294. [Google Scholar] [CrossRef]
- Selvankumar, T.; Radhika, R.; Mythili, R.; Arunprakash, S.; Srinivasan, P.; Govarthanan, M.; Kim, H. Isolation, identification and characterization of arsenic transforming exogenous endophytic Citrobacter sp. RPT from roots of Pteris vittata. 3 Biotech 2017, 7, 264. [Google Scholar] [CrossRef] [Green Version]
- Titah, H.S.; Abdullah, S.R.S.; Idris, M.; Anuar, N.; Basri, H.; Mukhlisin, M.; Tangahu, B.V.; Purwanti, I.F.; Kurniawan, S.B. Arsenic Resistance and Biosorption by Isolated Rhizobacteria from the Roots of Ludwigia octovalvis. Int. J. Microbiol. 2018, 2018, 3101498. [Google Scholar] [CrossRef] [Green Version]
- Firrincieli, A.; Presentato, A.; Favoino, G.; Marabottini, R.; Allevato, E.; Stazi, S.R.; Mugnozza, G.S.; Harfouche, A.; Petruccioli, M.; Turner, R.J.; et al. Identification of Resistance Genes and Response to Arsenic in Rhodococcus aetherivorans BCP1. Front. Microbiol. 2019, 10. [Google Scholar] [CrossRef] [Green Version]
- Martinez, J.L. Environmental pollution by antibiotics and by antibiotic resistance determinants. Environ. Pollut. 2009, 157, 2893–2902. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Thungrat, K.; Boothe, D.M. Occurrence of OXA-48 carbapenemase and other β-Lactamase genes in ESBL-producing multidrug resistant escherichia coli from dogs and cats in the United States, 2009–2013. Front. Microbiol. 2016, 7, 1057. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Gene | Primer Sequence | Product Size | References |
|---|---|---|---|
| merB | 5′-ATGAAGCTCGCCCCATATATTTTA-3′ 5′-TCACGGTGTCCTAGATGACATGGT-3′ | 640 bp | This study |
| merP | 5′-ATGAAGAAACTGTTTGCCTCCCTT-3′ 5′-TCACTGCTTGACGCTGGACG GA-3′ | 272 bp | This study |
| merT | 5′-TTAATAGAAAAATGGAACGACATA-3′ 5′-ATGTCTGAACCACAAAACGGG CG3′ | 355 bp | This study |
| arsC | 5′-GTAATACGCTGGAGATGATCCG-3 5′-TTTTCCTGCTTCATCAACGAC-3′ | 409 bp | [25] |
| qnrA | 5′-TGTATTTCGCTGTTGCTGGGGAG-3′ 5′-AAGTGACCAGAATAAGCGGC-3′ | 580 bp | [15] |
| qnrB | 5′-ATG GTG ACA AAG AGA GTG CA-3′ 5′-GTT CTG TTG CGG CTG GGT AA -3′ | 476 bp | [35] |
| qnrC | 5′-GGGTTGTACATTTATTGAATC-3′ 5′-TCCACTTTACGAGGTTCT-3′ | 447 bp | [36] |
| qnrD | 5′-CGAGATCAATTTACGGGGAATA-3′ 5′-AACAAGCTGAAGCGCCTG-3′ | 582 bp | [37] |
| qnrS | 5′-GCCTGTGTTTCGCTGCTGTT-3′ 5′-AGTAAGTCACCAGAACGAGC-3′ | 428 bp | [15] |
| aac(6′)-Ib | 5′-TTGCGATGCTCTATGAGTGGCTA-3′ 5′-CTCGAATGCCTGGCGTGTTT-3′ | 482 bp | [38] |
| blaCMY | 5′-ATGAGTATTCAACATTTCCG-3′ 5′-CCAATGCTTAATCAGTGAGG-3′ | 1226 bp | [39] |
| Strains | ESBLs Genes (from Previous Study) | MAR Index | PMQR Gene | blaCMY Gene | Heavy Metal Resistance Genes | MIC | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| merB | merP | merT | arsC | HgCl2 (mg/L) | Na2HAsO4 (mg/L) | |||||
| Acinetobacter calcoaceticus SRT58 | CTX-M-15 | 0.588 | qnrS | - | + | - | - | <1 | - | |
| Acinetobacter calcoaceticus CCT66 | CTX-M-15 | 0.647 | - | - | - | - | - | - | ||
| Acinetobacter johnsonii BOT41 | CTX-M-152 | 0.411 | - | - | - | - | - | - | ||
| Acinetobacter schindleri BOT46 | CTX-M-15 | 0.235 | + | + | + | - | <1 | - | ||
| Acinetobacter schindleri CCT65 | CTX-M-15, CTX-M-152, TEM-116 | 0.294 | CMY-2 | - | + | + | - | 2 | - | |
| Acinetobacter sp. BOT44 | CTX-M-152, TEM-116 | 0.352 | - | - | - | - | - | - | ||
| Acinetobacter sp. BOT40 | CTX-M-15, TEM-116 | 0.529 | CMY-2 | + | - | + | + | 2 | >512 | |
| Acinetobacter sp. CCT53 | CTX-M-15, CTX-M-152, TEM-116 | 0.176 | + | + | + | - | 8 | - | ||
| Acinetobacter sp. CCT54 | CTX-M-15, TEM-116 | 0.058 | + | + | + | + | 4 | >512 | ||
| Acinetobacter sp. CCT59 | CTX-M-15, CTX-M-152 ‘TEM-181 | 0.235 | - | - | - | - | - | - | ||
| Acinetobacter sp. MKT43 | CTX-M-15, TEM-116 | 0.588 | qnrS | + | + | + | - | 8 | - | |
| Acinetobacter sp. MKT45 | CTX-M-15, CTX-M-152, SHV-12 | 0.529 | qnrS | - | + | - | - | <1 | - | |
| Acinetobacter sp. MKT46 | CTX-M-15 | 0.235 | - | + | + | - | 1 | - | ||
| Acinetobacter sp. MKT48 | CTX-M-15, CTX-M-152, SHV-42 | 0.411 | CMY-2 | + | + | + | - | 8 | - | |
| Acinetobacter sp. SRT63 | CTX-M-152, TEM-116 | 0.117 | + | - | + | - | <1 | - | ||
| Acinetobacter sp. SRT64 | CTX-M-3 | 0.529 | CMY-42 | - | + | - | - | <1 | - | |
| Acinetobacter sp. SRT65 | CTX-M-15 | 0.529 | qnrS | + | - | + | - | 1 | - | |
| Acinetobacter sp. RBT26 | CTX-M-15, CTX-M-152, TEM-116 | 0.470 | CMY-2 | + | + | + | - | <1 | - | |
| Acinetobacter junii IBT27 | CTX-M-15 | 0.294 | + | + | + | + | 4 | 256 | ||
| Acinetobacter junii IBT29 | CTX-M-15, TEM-116 | 0.705 | qnrS | CMY-42 | - | + | + | - | 4 | - |
| Acinetobacter junii IBT30 | CTX-M-15, CTX-M-152, TEM-116 | 0.294 | - | + | - | - | <1 | - | ||
| Acinetobacter junii IBT31 | CTX-M-15, CTX-M-152 | 0.235 | - | + | - | - | <1 | - | ||
| Acinetobacter junii IBT33 | CTX-M-15, CTX-M-152, TEM-141, SHV-12 | 0.294 | qnrS | + | + | + | + | 1 | 64 | |
| Acinetobacter junii IBT36 | CTX-M-15, TEM-116 | 0.117 | + | + | + | - | 2 | - | ||
| Acinetobacter junii IBT39 | CTX-M-15 | 0.529 | CMY-2 | + | + | + | - | 2 | - | |
| Acinetobacter junii IBT40 | CTX-M-15, CTX-M-152, TEM-116 | 0.117 | - | - | - | - | - | - | ||
| Acinetobacter junii RBT31 | CTX-M-15, CTX-M-152, SHV-12 | 0.352 | + | + | + | - | 4 | - | ||
| Bacillus altitudinis BOT30 | CTX-M-15, TEM-116 | 0.176 | + | - | + | - | <1 | - | ||
| Bacillus firmus BOT39 | CTX-M-15, CTX-M-152 | 0.235 | - | - | + | - | <1 | - | ||
| Bacillus firmus MBT57 | CTX-M-15, TEM-116 | 0.352 | - | + | - | - | 1 | - | ||
| Bacillus firmus WBT16 | CTX-M15, TEM-116 | 0.235 | + | - | + | - | <1 | - | ||
| Bacillus safensis MKT35 | CTX-M-15, TEM-116 | 0.235 | + | + | + | - | 8 | - | ||
| Bacillus safensis BVT32 | CTX-M-152, TEM-116 | 0.295 | + | + | + | + | 4 | >512 | ||
| Brachymonas chironomi MKT44 | CTX-M-15, CTX-M-152, TEM-116, SHV-12 | 0.529 | qnrS | CMY-42 | + | + | + | - | 8 | - |
| E. coli MKT3 | CTX-M-15, TEM-116, SHV-12 | 0.411 | qnrS | CMY-42 | - | + | - | + | 8 | >512 |
| E. coli MKT25 | CTX-M-15, TEM-116 | 0.588 | qnrS | - | + | - | + | 4 | >512 | |
| E. coli BVT8 | CTX-M-15, TEM-116 | 0.529 | qnrS | - | - | - | - | - | - | |
| E. coli BVT20 | CTX-M-15, SHV-12 | 0.880 | qnrS | + | - | - | + | <1 | 128 | |
| E. coli RBT1 | CTX-M-15 | 0.705 | + | + | + | - | 2 | - | ||
| E. coli IBT13 | CTX-M-15, TEM-116, SHV-12 | 0.941 | qnrS | + | - | + | + | 2 | 64 | |
| E. coli SRT41 | CTX-M-15, TEM-116 | 0.470 | CMY-2 | + | - | + | - | 1 | - | |
| E. coli MBT16 | CTX-M-15 | 0.529 | qnrS | CMY-2 | - | - | - | - | - | - |
| E. coli MBT29 | CTX-M-15 | 0.880 | qnrS | CMY-42 | + | + | - | + | 2 | 32 |
| E. coli MBT42 | CTX-M-15, TEM-116, SHV-12 | 0.176 | + | - | - | + | <1 | 64 | ||
| E. coli CCT7 | CTX-M-15, TEM-116 | 0.880 | qnrS | - | - | - | - | - | - | |
| E. coli CCT42 | CTX-M-15, TEM-116 | 0.823 | qnrS | + | - | + | + | 4 | 512 | |
| E. coli CCT43 | CTX-M-15, TEM-116 | 0.176 | - | - | - | - | - | - | ||
| E. coli CCT50 | CTX-M-15 | 0.529 | - | + | + | + | 8 | >512 | ||
| E. coli CCT64 | CTX-M-15, CTX-M-152, TEM-116 | 0.176 | + | + | + | + | 4 | 128 | ||
| E. coli BOT1 | CTX-M-15 | 0.235 | - | - | - | - | - | - | ||
| Enterobacter cloacae BVT22 | CTX-M-15, TEM-116, SHV-12 | 0.352 | - | - | - | - | - | - | ||
| Enterobacter cloacae BVT29 | CTX-M-15, CTX-M-152, TEM-116, SHV-12 | 0.705 | qnrS | + | + | + | + | 8 | >512 | |
| Enterobacter cloacae BVT34 | CT-M-15, TEM-116 | 0.235 | - | - | - | - | - | - | ||
| Enterobacter cloacae GCT36 | CTX-M-15 | 0.647 | CMY-42 | - | - | - | + | - | 256 | |
| Enterobacter cloacae MBT49 | CTX-M-15, CTX-M-152, TEM-116 | 0.294 | - | - | - | - | - | - | ||
| Enterobacter cloacae CCT13 | CTX-M-15 | 0.764 | qnrS | - | - | - | - | - | - | |
| Enterobacter sp. BVT30 | CTX-M-5 | 0.235 | + | + | + | + | 8 | 512 | ||
| Enterobacter sp. BOT45 | CTX-M-15, CTX-M-152 | 0.176 | + | + | + | + | 1 | >512 | ||
| K. pneumoniae RBT40 | CTX-M-15, CTX-M-152 | 0.294 | + | + | + | + | 8 | >512 | ||
| K. pneumoniae MBT43 | CTX-M-15, TEM-116 | 0.235 | + | - | + | - | 4 | - | ||
| K. pneumoniae MBT50 | CTX-M-15 | 0.705 | qnrS | CMY-42 | + | - | + | - | 2 | - |
| K. pneumoniae MBT51 | CTX-M-15, TEM-116 | 0.235 | + | + | + | + | 4 | >512 | ||
| K. pneumoniae MBT52 | CTX-M-15, SHV-12 | 0.294 | + | + | + | - | 8 | - | ||
| K. pneumoniae MBT59 | CTX-M-15, CTX-M-152, TEM-116 | 0.117 | qnrS | + | + | + | - | 2 | - | |
| K. quasipneumoniae MKT39 | CTX-M-3, TEM-116 | 0.470 | + | - | + | - | <1 | - | ||
| Kluyvera georgiana CCT51 | CTX-M-15 | 0.235 | + | + | + | + | 8 | >512 | ||
| Kluyvera georgiana CCT69 | CT-M-152, TEM-116 | 0.588 | - | - | - | - | - | - | ||
| Shigella flexneri RBT20 | CTX-M-15, TEM-116 | 0.705 | qnrS | - | + | + | + | 1 | 16 | |
| Shigella flexneri IBT7 | CTX-M-15, TEM-116 | 0.705 | qnrS | CMY-2 | - | - | - | - | - | - |
| Shigella sonnei MKT34 | CTX-M-15, TEM-116 | 0.176 | - | - | - | + | - | 64 | ||
| Shigella flexneri CCT27 | CX-M-15, TEM-116, SHV-12 | 0.235 | - | - | - | - | - | - | ||
| Shigella flexneri CCT52 | CTX-M-15, CTX-M-152 | 0.235 | + | + | + | - | 8 | - | ||
| Serratia marcescens RBT37 | CTX-M-15, TEM-116 | 0.294 | + | + | + | + | 8 | >512 | ||
| Bacterial Strains | Resistance Genes | MIC | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CTX-M | TEM | SHV | qnrS | CMY | merB | merP | merT | arsC | Ampicillin (µg/mL) | Ciprofloxacin (µg/mL) | Cefotaxime (µg/mL) | HgCl2 (mg/L) | Na2HAsO4 (mg/L) | |
| E. coli MKT3 | + | + | + | + | + | − | + | − | + | >1024 | 64 | >1024 | 8 | >512 |
| Transconjugant (J53AZR) | + | + | − | + | − | − | + | − | − | 512 | 18 | 1024 | 2 | 32 |
| Acinetobacter junii IBT29 | + | + | − | + | + | − | + | + | − | 128 | 32 | 256 | 4 | 64 |
| Transconjugant (J53AZR) | + | + | − | + | + | − | + | − | − | 32 | 16 | 256 | 2 | 32 |
| Enterobacter cloacae BVT29 | + | + | + | + | − | + | + | + | + | 256 | 32 | 256 | 8 | >512 |
| Transconjugant (J53AZR) | + | + | + | + | − | + | + | + | − | 256 | 8 | 32 | 8 | 32 |
| E. coli MBT29 | + | − | − | + | + | + | + | − | + | >1024 | >1024 | >1024 | 2 | 32 |
| Transconjugant (J53AZR) | + | − | − | + | + | − | − | − | − | 256 | 256 | 512 | 1 | 32 |
| K. pneumoniae MBT50 | + | − | − | + | + | + | − | + | − | 128 | 16 | 512 | 2 | 32 |
| Transconjugant (J53AZR) | + | − | − | + | + | − | − | + | − | 128 | 8 | 512 | 1 | 32 |
| E. coli CCT42 | + | + | − | + | − | + | − | + | + | >1024 | 256 | >1024 | 4 | 512 |
| Transconjugant (J53AZR) | + | + | − | − | − | + | − | − | − | 512 | 64 | 256 | 2 | 32 |
| Acinetobacter sp. BOT40 | + | + | − | − | + | + | − | + | + | 32 | 8 | 128 | 2 | >512 |
| Transconjugant (J53AZR) | + | + | − | − | + | − | − | + | − | 32 | 4 | 128 | 2 | 32 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Siddiqui, M.T.; Mondal, A.H.; Gogry, F.A.; Husain, F.M.; Alsalme, A.; Haq, Q.M.R. Plasmid-Mediated Ampicillin, Quinolone, and Heavy Metal Co-Resistance among ESBL-Producing Isolates from the Yamuna River, New Delhi, India. Antibiotics 2020, 9, 826. https://doi.org/10.3390/antibiotics9110826
Siddiqui MT, Mondal AH, Gogry FA, Husain FM, Alsalme A, Haq QMR. Plasmid-Mediated Ampicillin, Quinolone, and Heavy Metal Co-Resistance among ESBL-Producing Isolates from the Yamuna River, New Delhi, India. Antibiotics. 2020; 9(11):826. https://doi.org/10.3390/antibiotics9110826
Chicago/Turabian StyleSiddiqui, Mohammad Tahir, Aftab Hossain Mondal, Firdoos Ahmad Gogry, Fohad Mabood Husain, Ali Alsalme, and Qazi Mohd. Rizwanul Haq. 2020. "Plasmid-Mediated Ampicillin, Quinolone, and Heavy Metal Co-Resistance among ESBL-Producing Isolates from the Yamuna River, New Delhi, India" Antibiotics 9, no. 11: 826. https://doi.org/10.3390/antibiotics9110826
APA StyleSiddiqui, M. T., Mondal, A. H., Gogry, F. A., Husain, F. M., Alsalme, A., & Haq, Q. M. R. (2020). Plasmid-Mediated Ampicillin, Quinolone, and Heavy Metal Co-Resistance among ESBL-Producing Isolates from the Yamuna River, New Delhi, India. Antibiotics, 9(11), 826. https://doi.org/10.3390/antibiotics9110826





