Assessment of the Sensitivity of Some Plant Pathogenic Fungi to 6-Demethylmevinolin, a Putative Natural Sensitizer Able to Help Overcoming the Fungicide Resistance of Plant Pathogens
Abstract
1. Introduction
2. Results
2.1. The Effect of 6-DMM on the Fungal Growth and Spore Germination
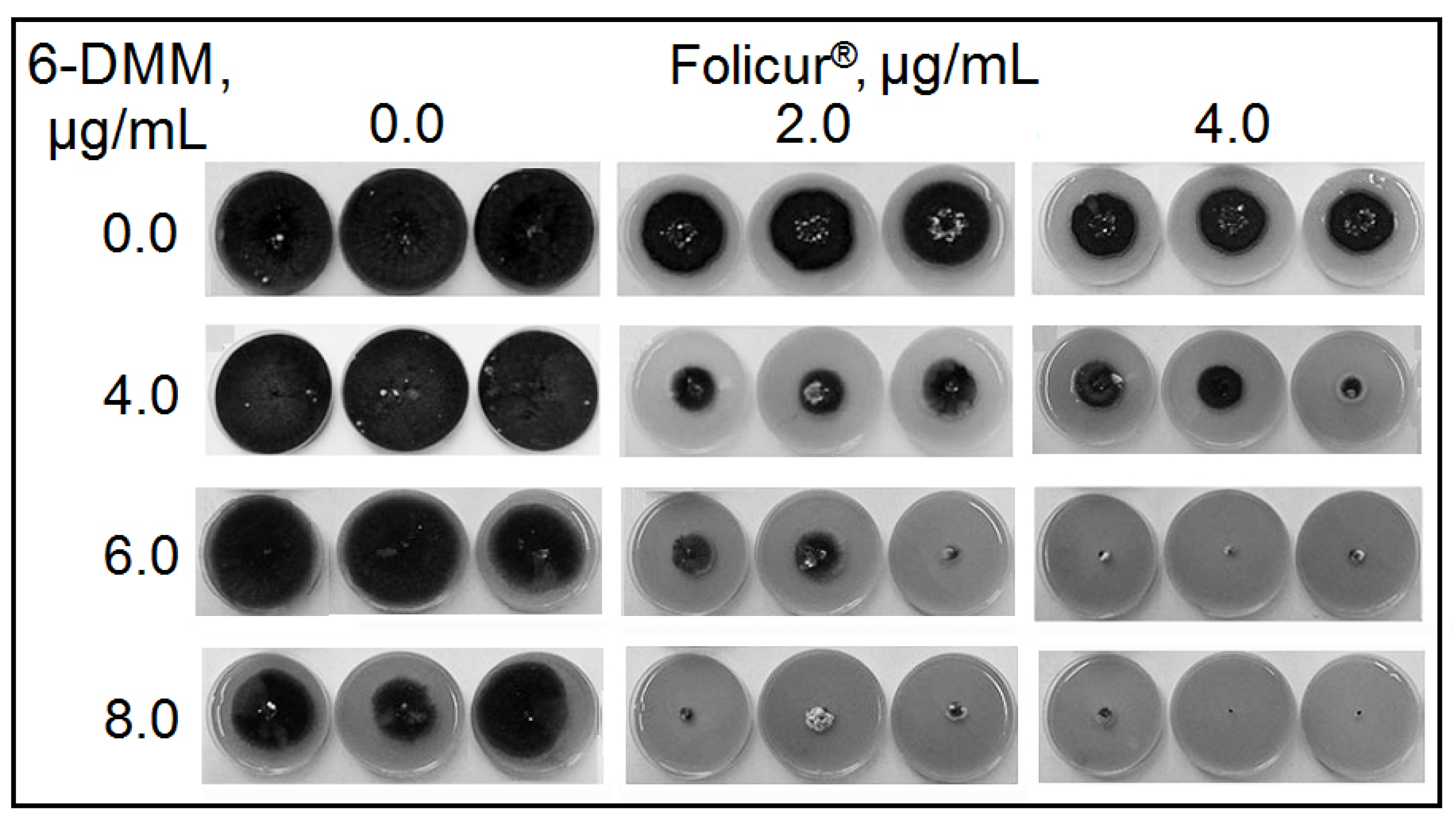
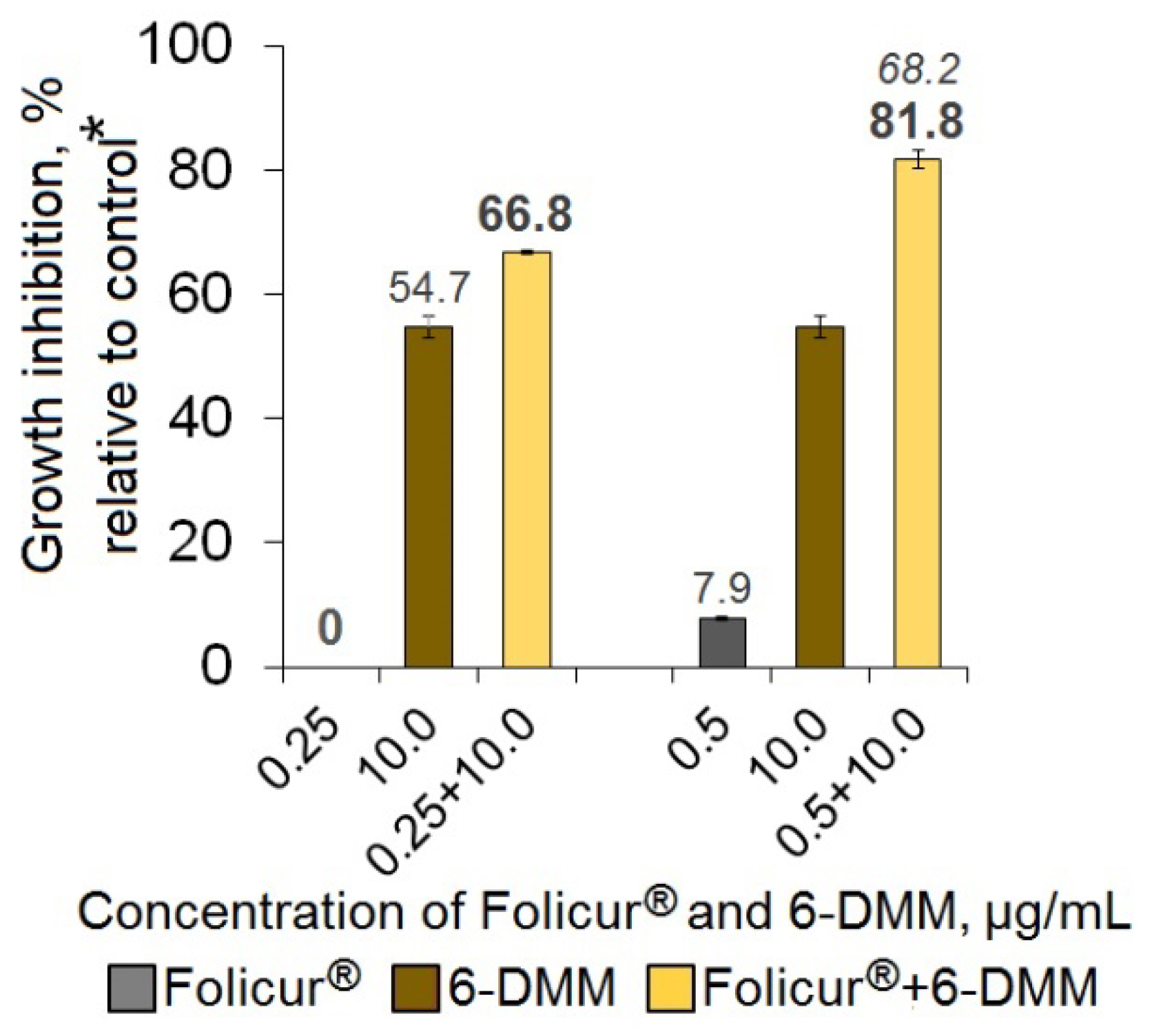
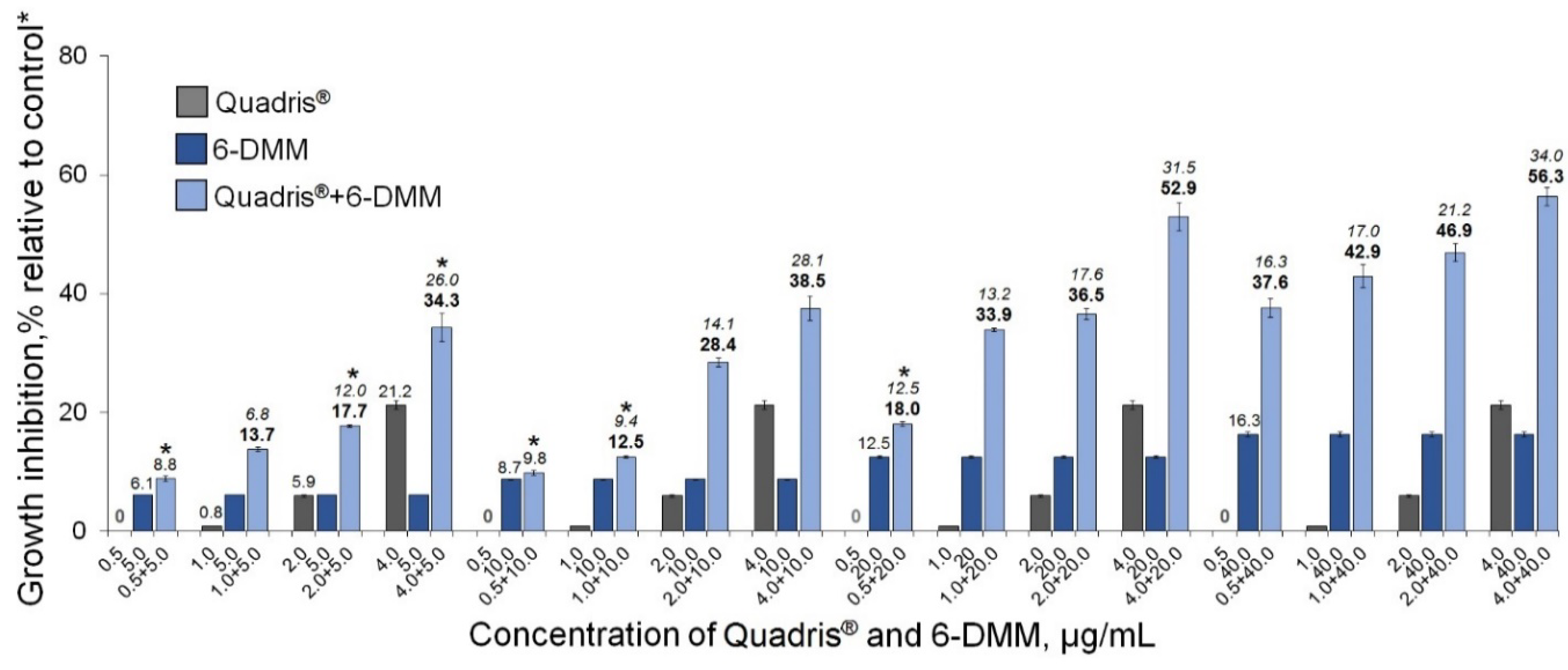
2.2. The Sensitizing Effect of 6-DMM on B. sorokiniana and R. solani
3. Discussion
4. Materials and Methods
4.1. Fungal Strains and Their Culturing
4.2. Preparation of 6-DMM and Fungicide Samples for Microbiological Experiments
4.3. In Vitro Assessment of a 6-DMM Effect on the Fungal Growth and Spore Germination
4.4. In Vitro Assessment of a 6-DMM Sensitizing Effect
4.5. Data Analysis and Statistical Treatment
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Moore, D.; Robson, G.D.; Trinci, A.P. 21st Century Guidebook to Fungi, 2nd ed.; Cambridge University Press: Cambridge, UK, 2020; pp. 408–409. [Google Scholar]
- Termorshuizen, A.J. Fungal and fungus-like pathogens of potato. In Potato Biology and Biotechnology: Advances and Perspectives; Vreugdenhil, D., Ed.; Elsevier: Oxford, UK, 2007; pp. 643–665. [Google Scholar]
- Carson, G.R.; Edwards, N.M. Criteria of wheat and flour quality. In Wheat Chemistry and Technology, 4th ed.; Khan, K., Shewry, P.R., Eds.; AACC International: St. Paul, MN, USA, 2009; pp. 97–118. [Google Scholar]
- Rosentrater, K.A.; Evers, A.D. Introduction to cereals and pseudocereals and their production. In Kent’s Technology of Cereals, 5th ed.; Rosentrater, K.A., Evers, A.D., Eds.; Woodhead Publishing: Duxford, UK, 2018; pp. 3–75. [Google Scholar]
- Hawkins, N.J.; Bass, C.; Dixon, A.; Neve, P. The evolutionary origins of pesticide resistance. Biol. Rev. 2019, 94, 135–155. [Google Scholar] [CrossRef] [PubMed]
- Fisher, M.C.; Hawkins, N.J.; Sanglard, D.; Gurr, S.J. Worldwide emergence of resistance to antifungal drugs challenges human health and food security. Science 2018, 360, 739–742. [Google Scholar] [CrossRef] [PubMed]
- Takagaki, M.; Kaku, K.; Watanabe, S.; Kawai, K.; Shimizu, T.; Sawada, H.; Kumakura, K.; Nagayama, K. Mechanism of resistance to carpropamid in Magnaporthe grisea. Pest. Manag. Sci. 2004, 60, 921–926. [Google Scholar] [CrossRef] [PubMed]
- Olaya, G.; Buitrago, C.; Pearsaul, D.; Sierotzki, H.; Tally, A. Detection of resistance to QoI fungicides in Rhizoctonia solani isolates from rice. Phytopathology 2012, 102, 88. [Google Scholar]
- Fairchild, K.L.; Miles, T.D.; Wharton, P.S. Assessing fungicide resistance in populations of Alternaria in Idaho potato fields. Crop Protect. 2013, 49, 31–39. [Google Scholar] [CrossRef]
- Cheval, P.; Siah, A.; Bomble, M.; Popper, A.D.; Reignault, P.; Halama, P. Evolution of QoI resistance of the wheat pathogen Zymoseptoria tritici in Northern France. Crop Protect. 2017, 92, 131–133. [Google Scholar] [CrossRef]
- Muzhinji, N.; Woodhall, J.W.; Truter, M.; van der Waals, J.E. Variation in fungicide sensitivity among Rhizoctonia isolates recovered from potatoes in South Africa. Plant Dis. 2018, 102, 1520–1526. [Google Scholar] [CrossRef]
- Liu, S.; Fu, L.; Wang, S.; Chen, J.; Jiang, J.; Che, Z.; Tian, Y.; Chen, G. Carbendazim resistance of Fusarium graminearum from Henan wheat. Plant Dis. 2019, 103, 2536–2540. [Google Scholar] [CrossRef]
- Fernández-Ortuño, D.; Torés, J.A.; de Vicente, A.; Pérez-García, A. Mechanisms of resistance to QoI fungicides in phytopathogenic fungi. Int. Microbiol. 2008, 11, 1–9. [Google Scholar]
- Lucas, J.A.; Hawkins, N.J.; Fraaije, B.A. The evolution of fungicide resistance. Adv. Appl. Microbiol. 2015, 90, 29–92. [Google Scholar]
- Chen, Z.-F.; Ying, G.-G. Occurrence, fate and ecological risk of five typical azole fungicides as therapeutic and personal care products in the environment: A review. Environ. Int. 2015, 84, 142–153. [Google Scholar] [CrossRef] [PubMed]
- Esteve-Turrillas, F.A.; Agulló, C.; Abad-Somovilla, A.; Mercader, J.V.; Abad-Fuentes, A. Fungicide multiresidue monitoring in international wines by immunoassays. Food Chem. 2016, 196, 1279–1286. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, F.P. Pesticides, environment, and food safety. Food Energy Secur. 2017, 6, 48–60. [Google Scholar] [CrossRef]
- Brent, K.J.; Hollomon, D.W. Fungicide Resistance in Crop Pathogens: How Can It be Managed? Fungicide Resistance Action Committee: Brussels, Belgium, 2007. [Google Scholar]
- Álvarez-Martín, A.; Rodríguez-Cruz, M.S.; Andrades, M.S.; Sánchez-Martín, M.J. Application of a biosorbent to soil: A potential method for controlling water pollution by pesticides. Environ. Sci. Pollut. Res. Int. 2016, 23, 9192–9203. [Google Scholar] [CrossRef]
- Campbell, B.C.; Chan, K.L.; Kim, J.H. Chemosensitization as a means to augment commercial antifungal agents. Front. Microbiol. 2012, 3, 79. [Google Scholar] [CrossRef]
- Dzhavakhiya, V.G.; Shcherbakova, L.A.; Semina, Y.V.; Zhemchuzhina, N.S.; Campbell, B. Chemosensitization of plant pathogenic fungi to agricultural fungicides. Front. Microbiol. 2012, 3, 87. [Google Scholar] [CrossRef]
- Kim, K.; Lee, Y.; Ha, A.; Kim, J.I.; Park, A.R.; Yu, N.H.; Son, H.; Choi, G.J.; Park, H.W.; Lee, C.W.; et al. Chemosensitization of Fusarium graminearum to chemical fungicides using cyclic lipopeptides produced by Bacillus amyloliquefaciens strain JCK-12. Front. Plant Sci. 2017, 8, 2010. [Google Scholar] [CrossRef]
- Shcherbakova, L.A.; Syomina, Y.V.; Arslanova, L.R.; Nazarova, T.A.; Dzhavakhiya, V.G. Metabolites secreted by a nonpathogenic Fusarium sambucinum inhabiting wheat rhizosphere enhance fungicidal effect of some triazoles against Parastagonospora nodorum. AIP Conf. Proc. 2019, 2063, 030018. [Google Scholar]
- Kim, J.H.; Chan, K.L. Augmenting the antifungal activity of an oxidizing agent with kojic acid: Control of Penicillium strains infecting crops. Molecules 2014, 19, 18448–18464. [Google Scholar] [CrossRef]
- Kartashov, M.I.; Shcherbakova, L.A.; Dzhavakhiya, V.G. In vitro enhancement of the sensitivity to tebuconazole in Bipolaris sorokiniana, a causative agent of cereal root rots, by a microbial metabolite 6-demethylmevinolin. In Abstract Book of 19th International Reinhardsbrunn Symposium on Modern Fungicides and Antifungal Compounds, Friedrichroda, Germany, 7–11 April 2019; DPG Verlag: Braunschweig, Germany, 2019; p. 48. [Google Scholar]
- Wang, F.; Wang, Z.; Zhang, B.; Zhang, Q. Degradation and adsorption of tebuconazole and tribenuron-methyl in wheat soil, alone and in combination. Chil. J. Agric. Res. 2017, 77, 281–286. [Google Scholar] [CrossRef][Green Version]
- Odintsova, T.; Shcherbakova, L.; Slezina, M.; Pasechnik, T.; Kartabaeva, B.; Istomina, E.; Dzhavakhiya, V. Hevein-like antimicrobial peptides WAMPs: Structure-function relationship in antifungal activity and sensitization of plant pathogenic fungi to tebuconazole by WAMP-2-derived peptides. IJMS 2020, 21, 7912. [Google Scholar] [CrossRef] [PubMed]
- Smiley, R.W.; Gourlie, J.A.; Easley, S.A.; Patterson, L.M. Pathogenicity of fungi associated with the wheat crown rot complex in Oregon and Washington. Plant Dis. 2005, 89, 949–957. [Google Scholar] [CrossRef] [PubMed]
- Gagkaeva, T.Y.; Gavrilova, O.P.; Levitin, M.M.; Novozhilov, K.V. Fusarium diseases of grain crops. Zashchita i Karantin Rastenii 2011, S5, 69–120. [Google Scholar]
- Matin, M.; Faruq, A. Prevalence of pathogenic fungi in soils of wheat field. J. Exp. Biosci. 2013, 4, 63–68. [Google Scholar]
- Richer, D.L. Synergism: A patent view. Pestic. Sci. 1987, 19, 309–315. [Google Scholar] [CrossRef]
- Paul, P.A.; Lipps, P.E.; Hershman, D.E.; McMullen, M.P.; Draper, M.A.; Madden, L.V. Efficacy of triazole-based fungicides for Fusarium head blight and deoxynivalenol control in wheat. Phytopathology 2008, 98, 999–1011. [Google Scholar] [CrossRef]
- Klix, M.B.; Verreet, J.A.; Beyer, M. Comparison of the declining triazole sensitivity of Gibberella zeae and increased sensitivity achieved by advances in triazole fungicide development. Crop Prot. 2007, 26, 683–690. [Google Scholar] [CrossRef]
- Masiello, M.; Somma, S.; Ghionna, V.; Logrieco, A.F.; Moretti, A. In vitro and in field response of different fungicides against Aspergillus flavus and Fusarium species causing ear rot disease of maize. Toxins 2019, 11, 11. [Google Scholar] [CrossRef]
- Mullenborn, C.; Steiner, U.; Ludwig, M.; Oerke, E.C. Effect of fungicides on the complex of Fusarium species and saprophytic fungi colonizing wheat kernels. Eur. J. Plant Pathol. 2008, 120, 157–166. [Google Scholar] [CrossRef]
- Ivic, D.; Sever, Z.; Kuzmanovska, B. In vitro sensitivity of Fusarium graminearum, F. avenaceum and F. verticillioides to carbendazim, tebuconazole, flutriafol, metconazole and prochloraz. Pestic. Fitomedicina 2011, 26, 35–42. [Google Scholar] [CrossRef]
- Deising, H.B.; Reimann, S.; Pascholati, S.F. Mechanisms and significance of fungicide resistance. Br. J. Microbiol. 2008, 39, 286–295. [Google Scholar] [CrossRef]
- Becher, R.; Hettwer, U.; Karlovsky, P.; Deising, H.B.; Wirsel, S.G.R. Adaptation of Fusarium graminearum to tebuconazole yielded descendants diverging for levels of fitness, fungicide resistance, virulence, and mycotoxin production. Phytopathology 2010, 100, 444–453. [Google Scholar] [CrossRef] [PubMed]
- Avozani, A.; Tonin, R.B.; Reis, E.M.; Camera, J.; Ranzi, C. In vitro sensitivity of Fusarium graminearum isolates to fungicides. Summa Phytopathol. 2014, 40, 231–247. [Google Scholar] [CrossRef][Green Version]
- Pawar, P.; Bhosale, A.; Lolage, Y.; Arts, S.; Commerce, D. Alternaria blight of tomato (Lycopersicon esculentum Mill). Int. J. Adv. Technol. Innov. Res. 2016, 8, 1727–1728. [Google Scholar]
- Arabiat, S.; Khan, M.F.R. Sensitivity of Rhizoctonia solani AG-2-2 from sugar beet to fungicides. Plant Dis. 2016, 100, 2427–2433. [Google Scholar] [CrossRef]
- Castroagudin, V.L.; Fiser, S.; Cartwright, R.D.; Wamishe, Y.; Correll, J.C. Evaluation of Rhizoctonia solani AG 1-IA and Rhizoctonia species for resistance to QoI fungicides. Phytopathology 2013, 103 (Suppl. 2), S2–S24. [Google Scholar]
- Broquist, H. Slaframine and swainsonine, mycotoxins from Rhizoctonia leguminicola. Toxin Rev. 2008, 5, 241–252. [Google Scholar] [CrossRef]
- Fraeyman, S.; Croubels, S.; Devreese, M.; Antonissen, G. Emerging Fusarium and Alternaria mycotoxins: Occurrence, toxicity and toxicokinetics. Toxins 2017, 9, 228. [Google Scholar] [CrossRef]
- Dzhavakhiya, V.G.; Voinova, T.M.; Popletaeva, S.B.; Statsyuk, N.V.; Limantseva, L.A.; Shcherbakova, L.A. Effect of various compounds blocking the colony pigmentation on the aflatoxin B1 production by Aspergillus flavus. Toxins 2016, 8, 313. [Google Scholar] [CrossRef]
- Fourtouni, A.; Manetas, Y.; Christias, C. Effects of UV-B radiation on growth, pigmentation, and spore production in the phytopathogenic fungus Alternaria solani. Can. J. Bot. 1998, 76, 2093–2099. [Google Scholar]
- Alan, A.; Earle, E. Sensitivity of bacterial and fungal plant pathogens to the lytic peptides, MSI-99, Magainin II, and Cecropin B. Mol. Plant Microbe Interact. 2002, 15, 701–708. [Google Scholar] [CrossRef] [PubMed]
- Ukraintseva, S.N.; Voinova, T.M.; Dzhavakhiya, V.G. Penicillium citrinum strain improvement for compactin production by induced-mutagenesis and optimization of obtained mutant cultivation conditions. In Biotechnology and Medicine; Zaikov, G.E., Ed.; Nova Science Publishers: New York, NY, USA, 2004; pp. 71–78. [Google Scholar]
- Aver’yanov, A.A.; Lapikova, V.P.; Pasechnik, T.D.; Abramova, O.S.; Gaivoronskaya, L.M.; Kuznetsov, V.V.; Baker, C.J. Pre-illumination of rice blast conidia induces tolerance to subsequent oxidative stress. Fungal Biol. 2014, 118, 743–753. [Google Scholar] [CrossRef] [PubMed]
- Canton, E.; Peman, J.; Gobernado, M.; Viudes, A.; Espinel, I.A. Synergistic activities of fluconazole and voriconazole with terbinafine against four Candida species determined by checkerboard, time-kill, and E-test methods. Antimicrob. Agent Chemother. 2005, 49, 1593–1596. [Google Scholar] [CrossRef] [PubMed]
- Orhan, G.; Bayram, A.; Zer, Y.; Balci, I. Synergy tests by E test and checkerboard methods of antimicrobial combinations against Brucella melitensis. J. Clin. Microbiol. 2005, 43, 140–143. [Google Scholar] [CrossRef]
- Odds, F.C. Synergy, antagonism, and what the chequerboard puts between them. J. Antimicrob. Chemother. 2003, 52, 1. [Google Scholar] [CrossRef]
- Sparling, D.W. Modeling in ecotoxicology. In Ecotoxicology Essentials, 1st ed.; Donald, W., Ed.; Academic Press: London, UK, 2016; pp. 361–390. [Google Scholar]
- Espinel-Ingroff, A.; Cantón, E.; Pemán, J. Antifungal susceptibility testing of filamentous fungi. Curr. Fungal Infect. Rep. 2012, 6, 41–50. [Google Scholar] [CrossRef]
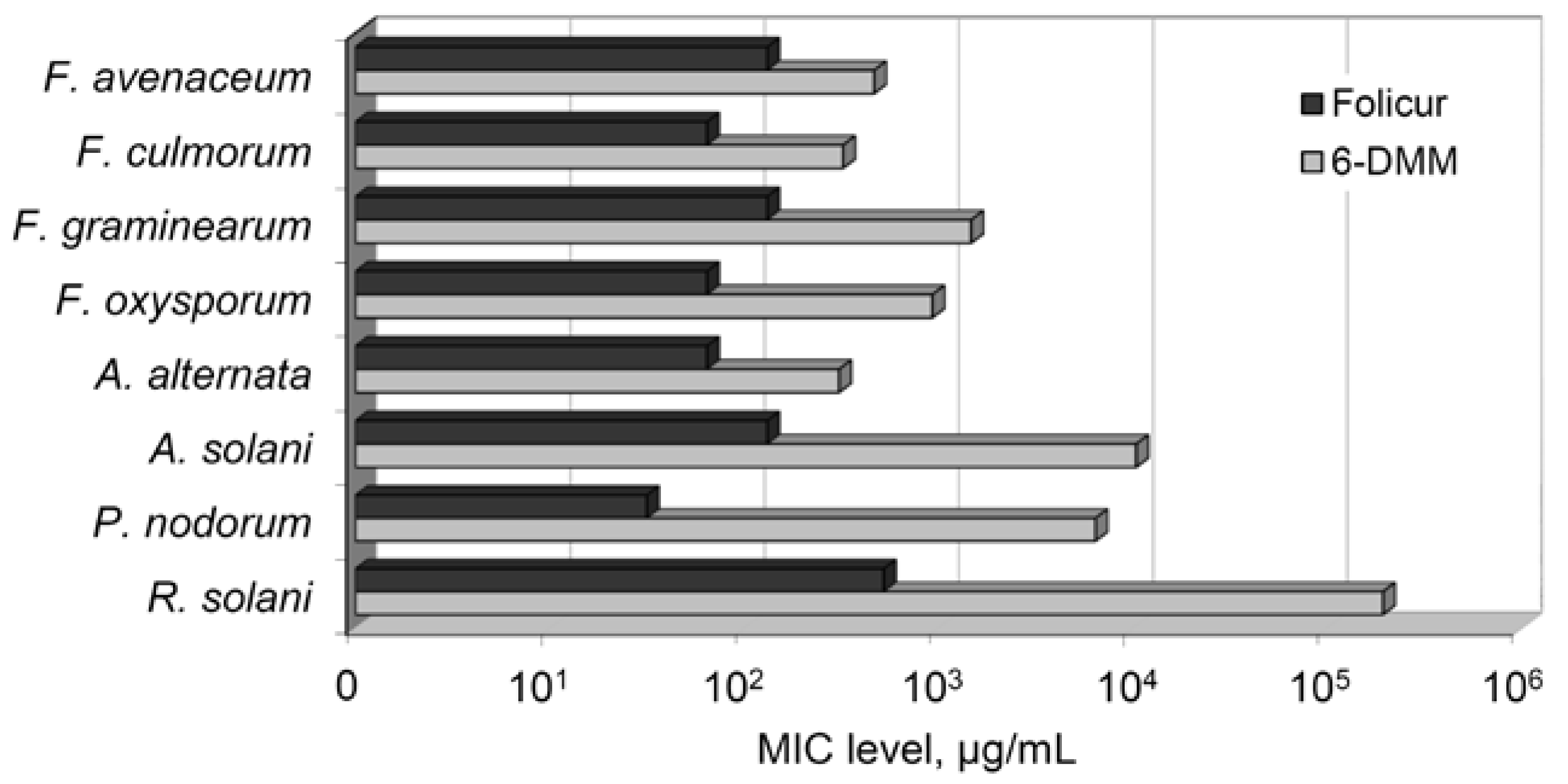


| Pathogen | Inhibitory Concentrations of 6-DMM * | ||||||
|---|---|---|---|---|---|---|---|
| Colony Growth | Spore Germination | ||||||
| ED10, µg/mL | ED50, µg/mL | ED95, mg/mL | R2 | ED50, µg/mL | ED95, mg/mL | R2 | |
| B. sorokiniana ** | 6.0 a | 9.5 a | 0.08 a | 0.977 | 1.30 a | 4.8 a | 0.939 |
| F. culmorum | 1.0 b | 38.0 b | 0.17 a | 0.923 | 0.35 b | 3.2 b | 0.917 |
| F. avenaceum | 1.3 b | 57.5 c | 0.26 b | 0.940 | 0.40 b | 4.1 a | 0.986 |
| F. oxysporum | 1.1 b | 63.1 c | 0.55 c | 0.932 | 0.80 c | 4.7 a | 0.956 |
| F. graminearum | 1.6 c | 141.3 d | 0.74 d | 0.973 | 1.25 d | 7.1 c | 0.913 |
| A. alternata | 4.8 a | 30.9 b | 0.17 a | 0.979 | 3.60 e | 5.2 d | 0.964 |
| A. solani | 85.8 d | 401.2 e | 13.16 d | 0.891 | 3.85 e | 5.4 d | 0.991 |
| P. nodorum | 7.1 a | 36.3 b | 7.59 e | 0.946 | nd *** | nd | nd |
| R. solani | 50.0 d | 398.0 e | 50.12 f | 0.883 | nd | nd | nd |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shcherbakova, L.; Kartashov, M.; Statsyuk, N.; Pasechnik, T.; Dzhavakhiya, V. Assessment of the Sensitivity of Some Plant Pathogenic Fungi to 6-Demethylmevinolin, a Putative Natural Sensitizer Able to Help Overcoming the Fungicide Resistance of Plant Pathogens. Antibiotics 2020, 9, 842. https://doi.org/10.3390/antibiotics9120842
Shcherbakova L, Kartashov M, Statsyuk N, Pasechnik T, Dzhavakhiya V. Assessment of the Sensitivity of Some Plant Pathogenic Fungi to 6-Demethylmevinolin, a Putative Natural Sensitizer Able to Help Overcoming the Fungicide Resistance of Plant Pathogens. Antibiotics. 2020; 9(12):842. https://doi.org/10.3390/antibiotics9120842
Chicago/Turabian StyleShcherbakova, Larisa, Maksim Kartashov, Natalia Statsyuk, Tatyana Pasechnik, and Vitaly Dzhavakhiya. 2020. "Assessment of the Sensitivity of Some Plant Pathogenic Fungi to 6-Demethylmevinolin, a Putative Natural Sensitizer Able to Help Overcoming the Fungicide Resistance of Plant Pathogens" Antibiotics 9, no. 12: 842. https://doi.org/10.3390/antibiotics9120842
APA StyleShcherbakova, L., Kartashov, M., Statsyuk, N., Pasechnik, T., & Dzhavakhiya, V. (2020). Assessment of the Sensitivity of Some Plant Pathogenic Fungi to 6-Demethylmevinolin, a Putative Natural Sensitizer Able to Help Overcoming the Fungicide Resistance of Plant Pathogens. Antibiotics, 9(12), 842. https://doi.org/10.3390/antibiotics9120842





