The Antibacterial Potential of Honeydew Honey Produced by Stingless Bee (Heterotrigona itama) against Antibiotic Resistant Bacteria
Abstract
:1. Introduction
2. Results
2.1. Inhibitory Effects
2.2. Bactericidal Effects
2.3. Antibacterial Factors
2.4. Interactive Effects with Antibiotics
3. Discussion
3.1. Inhibitory and Bactericidal Effects
3.2. Antibacterial Factors
3.3. Interactive Effects with Antibiotics
4. Materials and Methods
4.1. Honey Samples
4.2. Bacterial Samples
4.3. Antibacterial Properties
4.3.1. Agar-Well Diffusion Method
4.3.2. Endotoxin Quantification
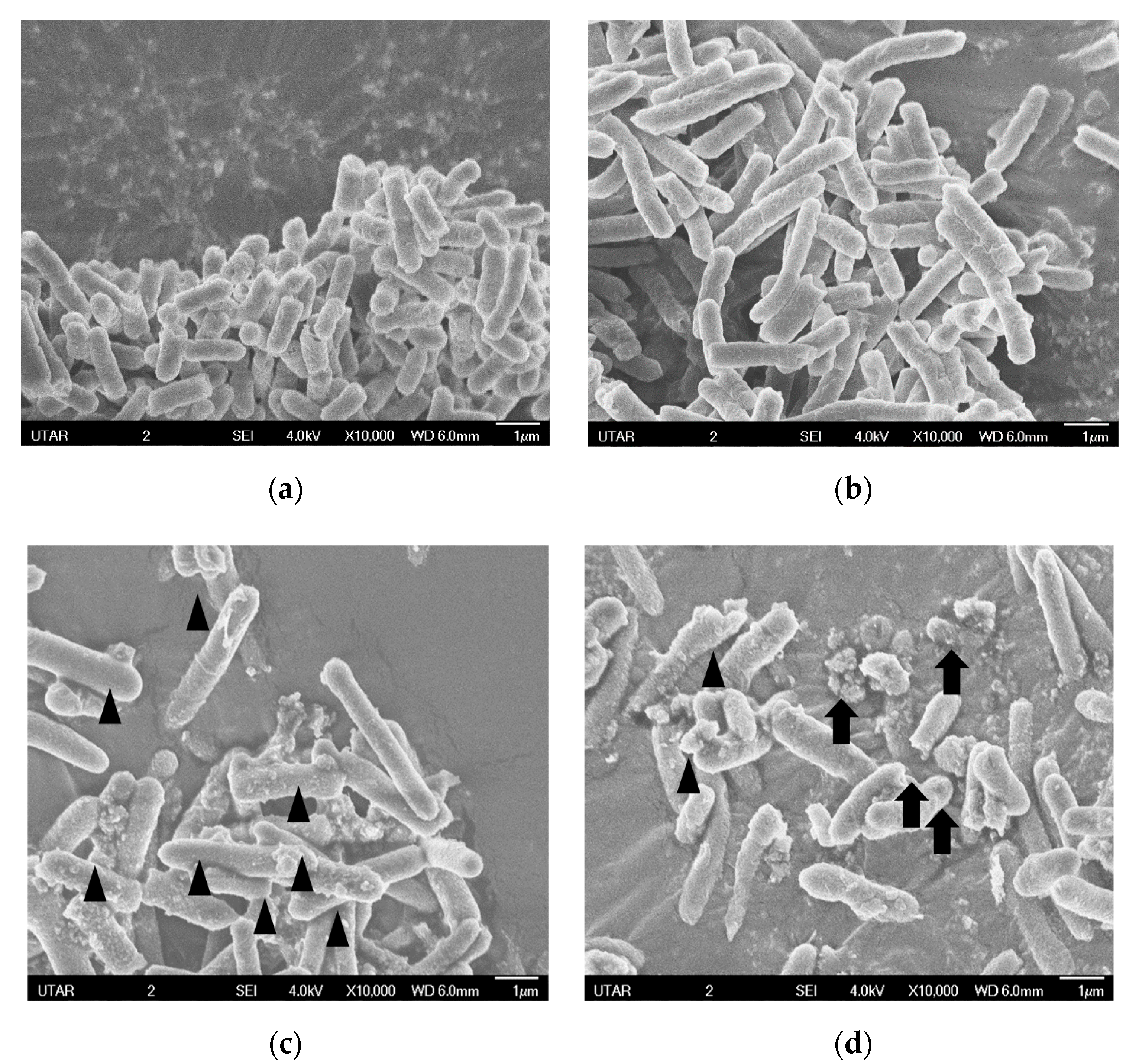
4.3.3. Scanning Electron Microscopy
4.3.4. Determination of Antibacterial Factors
4.3.5. Interactive Effect with Antibiotics
4.3.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kwakman, P.H.; Zaat, S.A. Antibacterial components of honey. IUBMB Life 2012, 64, 48–55. [Google Scholar] [CrossRef] [PubMed]
- Boorn, K.L.; Khor, Y.Y.; Sweetman, E.; Tan, F.; Heard, T.A.; Hammer, K.A. Antimicrobial activity of honey from the stingless bee Trigona carbonaria determined by agar diffusion, agar dilution, broth microdilution and time-kill methodology. J. Appl. Microbiol. 2010, 108, 1534–1543. [Google Scholar] [CrossRef] [PubMed]
- Ewnetu, Y.; Lemma, W.; Birhane, N. Antibacterial effects of Apis mellifera and stingless bees honeys on susceptible and resistant strains of Escherichia coli, Staphylococcus aureus and Klebsiella pneumoniae in Gondar, Northwest Ethiopia. BMC Complement. Altern. Med. 2013, 13, 1–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nishio, E.K.; Ribeiro, J.M.; Oliveira, A.G.; Andrade, C.G.T.J.; Proni, E.A.; Kobayashi, R.K.T.; Nakazato, G. Antibacterial synergic effect of honey from two stingless bees: Scaptotrigona bipunctata Lepeletier, 1836, and S. postica Latreille, 1807. Sci. Rep. 2016, 6, 1–8. [Google Scholar] [CrossRef]
- Kelly, N.; Farisya, M.S.N.; Kumara, T.K.; Marcela, P. Species diversity and external nest characteristics of stingless bees in meliponiculture. Pertanika J. Trop. Agric. Sci. 2014, 37, 293–298. [Google Scholar]
- Saludin, S.F.; Kamarulzaman, N.H.; Ismail, M.M. Measuring consumers’ preferences of stingless bee honey (meliponine honey) based on sensory characteristics. Int. Food Res. J. 2019, 26, 225–235. [Google Scholar]
- Santos, C.G.D.; Megiolaro, F.L.; Serrão, J.E.; Blochtein, B. Morphology of the head salivary and intramandibular glands of the stingless bee Plebeia emerina (Hymenoptera: Meliponini) workers associated with propolis. Ann. Entomol. Soc. Am. 2009, 102, 137–143. [Google Scholar] [CrossRef] [Green Version]
- Simone-Finstrom, M.; Spivak, M. Propolis and bee health: The natural history and significance of resin use by honey bees. Apidologie 2010, 41, 295–311. [Google Scholar] [CrossRef] [Green Version]
- Abd Jalil, M.A.; Kasmuri, A.R.; Hadi, H. Stingless bee honey, the natural wound healer: A review. Skin Pharmacol. Physiol. 2017, 30, 66–75. [Google Scholar] [CrossRef]
- Codex Alimentarius Commission. Revised codex standard for honey. Codex Stan. 2001, 12, 1–8. [Google Scholar]
- Kačániová, M.; Vuković, N.; Bobková, A.; Fikselová, M.; Rovná, K.; Haščík, P.; Čuboň, J.; Hleba, L.; Bobko, M. Antimicrobial and antiradical activity of Slovakian honeydew honey samples. J. Microbiol. Biotechnol. Food Sci. 2020, 9, 354–368. [Google Scholar]
- Majtan, J.; Majtanova, L.; Bohova, J.; Majtan, V. Honeydew honey as a potent antibacterial agent in eradication of multi-drug resistant Stenotrophomonas maltophilia isolates from cancer patients. Phytother. Res. 2011, 25, 584–587. [Google Scholar] [CrossRef] [Green Version]
- Grego, E.; Robino, P.; Tramuta, C.; Giusto, G.; Boi, M.; Colombo, R.; Serra, G.; Chiadò-Cutin, S.; Gandini, M.; Nebbia, P. Evaluation of antimicrobial activity of Italian honey for wound healing application in veterinary medicine. Schweiz. Arch. Tierheilkd. 2016, 158, 521–527. [Google Scholar] [CrossRef]
- Bucekova, M.; Buriova, M.; Pekarik, L.; Majtan, V.; Majtan, J. Phytochemicals-mediated production of hydrogen peroxide is crucial for high antibacterial activity of honeydew honey. Sci. Rep. 2018, 8, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Manyi-Loh, C.E.; Clarke, A.M.; Ndip, N. An overview of honey: Therapeutic properties and contribution in nutrition and human health. Afr. J. Microbiol. Res. 2011, 5, 844–852. [Google Scholar] [CrossRef]
- Szweda, P. Antimicrobial activity of honey. In Honey Analysis, 1st ed.; Arnaut de Toledo, V.D.A., Ed.; IntechOpen: London, UK, 2017; pp. 215–232. [Google Scholar] [CrossRef] [Green Version]
- Carter, D.A.; Blair, S.E.; Cokcetin, N.N.; Bouzo, D.; Brooks, P.; Schothauer, R.; Harry, E.J. Therapeutic manuka honey: No longer so alternative. Front. Microbiol. 2016, 7, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Bereket, W.; Hemalatha, K.; Getenet, B.; Wondwossen, T.; Solomon, A.; Zeynudin, A.; Kannan, S. Update on bacterial nosocomial infections. Eur. Rev. Med. Pharmacol. Sci. 2012, 16, 1039–1044. [Google Scholar]
- Brown, E.; O’Brien, M.; Georges, K.; Suepaul, S. Physical characteristics and antimicrobial properties of Apis mellifera, Frieseomelitta nigra and Melipona favosa bee honeys from apiaries in Trinidad and Tobago. BMC Complement. Altern. Med. 2020, 20, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Chanchao, C. Antimicrobial activity by Trigona laeviceps (stingless bee) honey from Thailand. Pak. J. Med. Sci. 2009, 25, 364–369. [Google Scholar]
- Omar, S.; Mat-Kamir, N.F.; Sanny, M. Antibacterial activity of Malaysian produced stingless-bee honey on wound pathogens. J. Sustain. Sci. Manag. 2019, 14, 67–79. [Google Scholar]
- Zainol, M.I.; Yusoff, K.M.; Yusof, M.Y.M. Antibacterial activity of selected Malaysian honey. BMC Complement. Altern. Med. 2013, 13, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al-Naama, R.T. Evaluation of in-vitro inhibitory effect of honey on some microbial isolate. Afr. J. Microbiol. Res. 2009, 1, 64–67. [Google Scholar] [CrossRef]
- Tuksitha, L.; Chen, Y.L.S.; Chen, Y.L.; Wong, K.Y.; Peng, C.C. Antioxidant and antibacterial capacity of stingless bee honey from Borneo (Sarawak). J. Asia Pac. Entomol. 2018, 21, 563–570. [Google Scholar] [CrossRef]
- Mohapatra, D.P.; Thakur, V.; Brar, S.K. Antibacterial efficacy of raw and processed honey. Biotechnol. Res. Int. 2011, 2011, 917505. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brudzynski, K.; Sjaarda, C. Antibacterial compounds of Canadian honeys target bacterial cell wall inducing phenotype changes, growth inhibition and cell lysis that resemble action of β-lactam antibiotics. PLoS ONE 2014, 9, e106967. [Google Scholar] [CrossRef] [Green Version]
- Tramuta, C.; Nebbia, P.; Robino, P.; Giusto, G.; Gandini, M.; Chiadò-Cutin, S.; Grego, E. Antibacterial activities of manuka and honeydew honey-based membranes against bacteria that cause wound infections in animals. Schweiz. Arch. Tierheilkd. 2017, 159, 117–121. [Google Scholar] [CrossRef] [Green Version]
- Albaridi, N.A. Antibacterial potency of honey. Int. J. Microbiol. 2019, 2019, 2464507. [Google Scholar] [CrossRef]
- Molan, P.C. The antibacterial activity of honey: 1. The nature of the antibacterial activity. Bee World 1992, 73, 5–28. [Google Scholar] [CrossRef]
- Jin, Q.; Kirk, M.F. pH as a primary control in environmental microbiology: 1. thermodynamic perspective. Front. Environ. Sci. Eng. 2018, 6, 1–15. [Google Scholar] [CrossRef]
- Al-Waili, N.S.; Salom, K.; Butler, G.; Al Ghamdi, A.A. Honey and microbial infections: A review supporting the use of honey for microbial control. J. Med. Food 2011, 14, 1079–1096. [Google Scholar] [CrossRef]
- Massaro, C.F.; Shelley, D.; Heard, T.A.; Brooks, P. In vitro antibacterial phenolic extracts from “sugarbag” pot-honeys of Australian stingless bees (Tetragonula carbonaria). J. Agric. Food Chem. 2014, 62, 12209–12217. [Google Scholar] [CrossRef]
- Estevinho, L.; Pereira, A.P.; Moreira, L.; Dias, L.G.; Pereira, E. Antioxidant and antimicrobial effects of phenolic compounds extracts of Northeast Portugal honey. Food Chem. Toxicol. 2008, 46, 3774–3779. [Google Scholar] [CrossRef]
- Miklasińska-Majdanik, M.; Kępa, M.; Wojtyczka, R.D.; Idzik, D.; Wąsik, T.J. Phenolic compounds diminish antibiotic resistance of Staphylococcus aureus clinical strains. Int. J. Environ. Res. Public Health 2018, 15, 2321. [Google Scholar] [CrossRef] [Green Version]
- Hegazi, A.; AbdEl-Moez, S.; Abdou, A.M.; Abd Allah, F. Synergistic antibacterial activity of Egyptian honey and common antibiotics against Clostridium reference strains. Int. J. Curr. Microbiol. App. Sci. 2014, 3, 312–325. [Google Scholar]
- Liu, M.; Lu, J.; Müller, P.; Turnbull, L.; Burke, C.M.; Schlothauer, R.C.; Carter, D.A.; Whitchurch, C.B.; Harry, E.J. Antibiotic-specific differences in the response of Staphylococcus aureus to treatment with antimicrobials combined with manuka honey. Front. Microbiol. 2015, 5, 779. [Google Scholar] [CrossRef] [Green Version]
- Jenkins, R.; Cooper, R. Improving antibiotic activity against wound pathogens with manuka honey in vitro. PLoS ONE 2012, 7, e45600. [Google Scholar] [CrossRef] [Green Version]
- Ng, W.J.; Lye, P.Y.; Chan, Y.J.; Lau, Z.K.; Ee, K.Y. Synergistic effect of Trigona honey and ampicillin on Staphylococcus aureus isolated from infected wound. Int. J. Pharmacol. 2017, 13, 403–407. [Google Scholar] [CrossRef] [Green Version]
- Kalan, L.; Wright, G.D. Antibiotic adjuvants: Multicomponent anti-infective strategies. Expert Rev. Mol. Med. 2011, 13, 511–517. [Google Scholar] [CrossRef]
- Brudzynski, K.; Abubaker, K.; Castle, A. Re-examining the role of hydrogen peroxide in bacteriostatic and bactericidal activities of honey. Front. Microbiol. 2011, 2, 213. [Google Scholar] [CrossRef] [Green Version]
- Jantakee, K.; Tragoolpua, Y. Activities of different types of Thai honey on pathogenic bacteria causing skin diseases, tyrosinase enzyme and generating free radicals. Biol. Res. 2015, 48, 4. [Google Scholar] [CrossRef] [Green Version]
- Schlunzen, F.; Zarivach, R.; Harms, J.; Bashan, A.; Tocilj, A.; Albrecht, R.; Yonath, A.; Franceschi, F. Structural basis for the interaction of antibiotics with the peptidyl transferase centre in eubacteria. Nature 2001, 413, 814–821. [Google Scholar] [CrossRef]
- Blair, S.E.; Cokcetin, N.N.; Harry, E.J.; Carter, D.A. The unusual antibacterial activity of medical-grade Leptospermum honey: Antibacterial spectrum, resistance and transcriptome analysis. Eur. J. Clin. Microbiol. Infect. Dis. 2009, 28, 1199–1208. [Google Scholar] [CrossRef]
- Packer, J.M.; Irish, J.; Herbert, B.R.; Hill, C.; Padula, M.; Blair, S.E.; Carter, D.A.; Harry, E.J. Specific non-peroxide antibacterial effect of manuka honey on the Staphylococcus aureus proteome. Int. J. Antimicrob. Agents 2012, 40, 43–50. [Google Scholar] [CrossRef]
- Bowler, P.G.; Duerden, B.I.; Armstrong, D.G. Wound microbiology and associated approaches to wound management. Clin. Microbiol. Rev. 2001, 14, 244–269. [Google Scholar] [CrossRef] [Green Version]
- Bessa, L.J.; Fazii, P.; Di Giulio, M.; Cellini, L. Bacterial isolates from infected wounds and their antibiotic susceptibility pattern: Some remarks about wound infection. Int. Wound J. 2015, 12, 47–52. [Google Scholar] [CrossRef]
- Cheng, M.Z.S.Z.; Ismail, M.; Chan, K.W.; Ooi, D.J.; Ismail, N.; Zawawi, N.; Mohd Esa, N. Comparison of sugar content, mineral elements and antioxidant properties of Heterotrigona itama honey from suburban and forest in Malaysia. Malaysian J. Med. Health Sci. 2019, 15, 104–112. [Google Scholar]

| Sample | S. aureus (ATCC 25923) * | S. aureus (ATCC 33591) | E. coli (ATCC 25922) *^ | E. coli (ATCC 35218) *^ |
|---|---|---|---|---|
| A1 | Nil | Nil | 1.0 ± 0.2 | 0.9 ± 0 |
| A2 | Nil | Nil | 0.9 ± 0.1 | 0.9 ± 0 |
| A3 | Nil | Nil | 0.8 ± 0.1 | 0.8 ± 0 |
| A4 | 0.8 ± 0 | Nil | 0.9 ± 0 | 0.8 ± 0 |
| A5 | 0.9 ± 0.1 | 0.9 ± 0 | 0.9 ± 0 | 0.8 ± 0 |
| A6 | 0.8 ± 0.1 | 0.8 ± 0 | 0.9 ± 0.1 | 0.8 ± 0 |
| A7 | Nil | Nil | 1.0 ± 0.2 | 0.8 ± 0 |
| A8 | Nil | Nil | 0.9 ± 0.1 | 0.8 ± 0.1 |
| A9 | Nil | Nil | 0.7 ± 0.1 | 0.7 ± 0.1 |
| A10 | Nil | Nil | 0.7 ± 0 | 0.7 ± 0 |
| A11 | Nil | Nil | 0.9 ± 0.1 | 0.8 ± 0.1 |
| A12 | Nil | Nil | 1.0 ± 0.1 | 0.8 ± 0.1 |
| A13 | Nil | Nil | Nil | Nil |
| A14 | Nil | Nil | Nil | Nil |
| A15 | Nil | Nil | Nil | 0.8 ± 0.1 |
| A16 | Nil | Nil | 0.8 ± 0.1 | Nil |
| A17 | Nil | Nil | 0.8 ± 0.1 | Nil |
| A18 | Nil | Nil | 0.8 ± 0 | Nil |
| A19 | Nil | Nil | 0.7 ± 0 | Nil |
| A20 | Nil | Nil | 0.8 ± 0 | Nil |
| A21 | 0.9 ± 0.1 | 0.8 ± 0 | 1.0 ± 0 | 0.8 ± 0.1 |
| A22 | 0.8 ± 0.1 | Nil | 1.1 ± 0.1 | 0.8 ± 0.1 |
| A23 | 0.8 ± 0.1 | 0.8 ± 0 | 0.9 ± 0.1 | 0.7 ± 0 |
| Average | 0.8 ± 0.1 | 0.8 ± 0.1 | 0.9 ± 0.1 | 0.8 ± 0.1 |
| A. cerana honeydew (A1–A9) | 0.8 ± 0.1 | 0.9 ± 0.1 | 0.9 ± 0.1 | 0.8 ± 0.1 |
| A. cerana blossom (A10–A23) | 0.8 ± 0.1 | 0.8 ± 0 | 0.9 ± 0.1 | 0.8 ± 0 |
| S1 | 1.2 ± 0.1 | 1.0 ± 0.1 | 1.5 ± 0.1 | 1.4 ± 0.1 |
| S2 | 1.1 ± 0 | 0.9 ± 0.2 | 1.4 ± 0.1 | 1.3 ± 0.1 |
| S3 | 1.1 ± 0 | 0.9 ± 0 | 1.6 ± 0 | 1.3 ± 0.1 |
| S4 | 1.4 ± 0.1 | 0.9 ± 0.1 | 1.7 ± 0.1 | 1.5 ± 0 |
| S5 | 1.0 ± 0.1 | 0.9 ± 0 | 1.1 ± 0.1 | 1.2 ± 0 |
| S6 | 0.9 ± 0.1 | 0.9 ± 0.1 | 0.9 ± 0.1 | 1.0 ± 0 |
| S7 | 0.9 ± 0.1 | 0.8 ± 0 | 1.2 ± 0.1 | 1.2 ± 0 |
| S8 | 0.9 ± 0.1 | 0.8 ± 0 | 1.0 ± 0 | 1.1 ± 0 |
| S9 | 0.7 ± 0.1 | 0.8 ± 0.1 | 1.1 ± 0 | 0.9 ± 0 |
| S10 | 0.8 ± 0.1 | 0.8 ± 0 | 1.0 ± 0.1 | 0.9 ± 0.1 |
| S11 | 0.9 ± 0.1 | 0.8 ± 0 | 1.1 ± 0.1 | 0.9 ± 0 |
| S12 | 0.9 ± 0.1 | 0.7 ± 0 | 0.9 ± 0.1 | 0.7 ± 0 |
| S13 | 0.8 ± 0.1 | 0.7 ± 0 | 1.0 ± 0.1 | 0.7 ± 0.1 |
| S14 | 0.8 ± 0.1 | 0.8 ± 0 | 1.0 ± 0.1 | 0.7 ± 0.2 |
| S15 | 0.8 ± 0.1 | 0.8 ± 0.1 | 1.0 ± 0.1 | 0.8 ± 0.1 |
| S16 | 0.8 ± 0.1 | 0.8 ± 0 | 0.9 ± 0.1 | 0.7 ± 0 |
| S17 | 1.0 ± 0.1 | 0.8 ± 0.1 | 1.3 ± 0.1 | 1.0 ± 0 |
| S18 | 1.0 ± 0.1 | 0.8 ± 0 | 1.3 ± 0.1 | 1.0 ± 0.1 |
| S19 | 1.2 ± 0.1 | 0.9 ± 0 | 1.3 ± 0.1 | 1.0 ± 0 |
| S20 | 0.9 ± 0 | 0.9 ± 0 | 1.1 ± 0.1 | 0.8 ± 0.1 |
| S21 | 1.0 ± 0.1 | 0.9 ± 0.1 | 1.0 ± 0 | 0.8 ± 0.1 |
| S22 | 1.0 ± 0.1 | 0.8 ± 0 | 1.3 ± 0.1 | 1.0 ± 0.1 |
| S23 | 1.0 ± 0.1 | 0.9 ± 0.1 | 1.2 ± 0.1 | 1.1 ± 0 |
| Average | 1.0 ± 0.2 | 0.8 ± 0.1 | 1.2 ± 0.2 | 1.0 ± 0.2 |
| H. itama honeydew (S1–S8) | 1.1 ± 0.2 | 0.9 ± 0.1 | 1.3 ± 0.3 | 1.3 ± 0.2 |
| H. itama blossom (S9–S16) | 0.8 ± 0.1 a | 0.8 ± 0 a | 1.0 ± 0.1 a | 0.8 ± 0.1 a |
| G. thoracica blossom (S17–S23) | 1.0 ± 0.1 | 0.9 ± 0.1 | 1.2 ± 0.1 | 1.0 ± 0.1 b |
| E. coli (ATCC 25922) | E. coli (ATCC 35218) | |||
|---|---|---|---|---|
| Sample | 0-h | 24-h ^ | 0-h | 24-h ^ |
| S1 | 1.77 ± 0 | 2.32 ± 0 | 1.68 ± 0 | 2.32 ± 0 |
| S2 | 1.58 ± 0 | 2.22 ± 0 | 1.51 ± 0 | 2.21 ± 0 |
| S3 | 1.88 ± 0 | 2.30 ± 0 | 1.58 ± 0 | 2.20 ± 0 |
| S4 | 1.56 ± 0 | 2.21 ± 0 | 1.56 ± 0 | 2.21 ± 0 |
| S5 | 1.72 ± 0 | 2.29 ± 0 | 1.62 ± 0 | 2.25 ± 0 |
| S6 | 1.89 ± 0 | 2.35 ± 0 | 1.69 ± 0 | 2.30 ± 0 |
| S7 | 1.97 ± 0 | 2.38 ± 0 | 1.67 ± 0 | 2.30 ± 0 |
| S8 | 1.88 ± 0 | 2.32 ± 0 | 1.88 ± 0 | 2.32 ± 0 |
| S9 | 1.33 ± 0 | 2.12 ± 0 | 1.31 ± 0 | 2.10 ± 0 |
| S10 | 1.38 ± 0 | 2.12 ± 0 | 1.35 ± 0 | 2.11 ± 0 |
| S11 | 1.27 ± 0 | 2.02 ± 0 | 1.37 ± 0 | 2.09 ± 0 |
| S12 | 1.38 ± 0 | 2.05 ± 0 | 1.38 ± 0 | 2.05 ± 0 |
| S13 | 1.27 ± 0 | 2.00 ± 0 | 1.25 ± 0 | 2.00 ± 0 |
| S14 | 1.38 ± 0 | 2.15 ± 0 | 1.30 ± 0 | 2.05 ± 0 |
| S15 | 1.37 ± 0 | 2.13 ± 0 | 1.35 ± 0 | 2.10 ± 0 |
| S16 | 1.38 ± 0 | 2.12 ± 0 | 1.33 ± 0 | 2.12 ± 0 |
| S17 | 1.67 ± 0 | 2.22 ± 0 | 1.56 ± 0 | 2.20 ± 0 |
| S18 | 1.68 ± 0 | 2.31 ± 0 | 1.58 ± 0 | 2.22 ± 0 |
| S19 | 1.57 ± 0 | 2.22 ± 0 | 1.50 ± 0 | 2.23 ± 0 |
| S20 | 1.57 ± 0 | 2.19 ± 0 | 1.57 ± 0 | 2.18 ± 0 |
| S21 | 1.57 ± 0 | 2.17 ± 0 | 1.57 ± 0 | 2.16 ± 0 |
| S22 | 1.50 ± 0 | 2.15 ± 0 | 1.48 ± 0 | 2.14 ± 0 |
| S23 | 1.57 ± 0 | 2.19 ± 0 | 1.58 ± 0 | 2.29 ± 0 |
| Average | 1.57 ± 0.21 | 2.20 ± 0.10 | 1.51 ± 0.16 | 2.18 ± 0.09 |
| H. itama honeydew (S1–S8) | 1.78 ± 0.15 * | 2.30 ± 0.06 * | 1.65 ± 0.11 * | 2.26 ± 0.05 * |
| H. itama blossom (S9–S16) | 1.35 ± 0.05 | 2.09 ± 0.06 | 1.33 ± 0.04 | 2.08 ± 0.04 |
| G. thoracica blossom (S17–S23) | 1.59 ± 0.06 | 2.21 ± 0.05 | 1.55 ± 0.04 | 2.20 ± 0.05 |
| Sample | Zone of Inhibition | Endotoxin Level | ||||
|---|---|---|---|---|---|---|
| E. coli (ATCC 25922) | E. coli (ATCC 35218) | E. coli (ATCC 25922) | E. coli (ATCC 35218) | |||
| 0-h | 24-h | 0-h | 24-h | |||
| Sugar solution | Nil | Nil | 1.20 ± 0 | 1.25 ± 0 | 1.07 ± 0 | 1.10 ± 0 |
| Hydrogen peroxide solution | Nil | Nil | 1.41 ± 0 | 1.52 ± 0 | 1.22 ± 0 | 1.35 ± 0 |
| Acid solution | Nil | Nil | 1.54 ± 0 | 1.58 ± 0 | 1.29 ± 0 | 1.34 ± 0 |
| Gallic acid solution | Nil | Nil | 1.22 ± 0 | 1.23 ± 0 | 1.11 ± 0 | 1.10 ± 0 |
| Antibiotic | E. coli 1 | E. coli 2 | E. coli 3 | E. coli 4 |
|---|---|---|---|---|
| Ampicillin (10 µg) | R | R | R | R |
| Chloramphenicol (30 µg) | S | S | R | S |
| Gentamicin (10 µg) | S | S | S | S |
| Tetracycline (30 µg) | S | R | R | R |
| Sample | E. coli 1 | E. coli 2 | E. coli 3 | E. coli 4 | E. coli (ATCC 25922) | E. coli (ATCC 35218) |
|---|---|---|---|---|---|---|
| Honey | 0.7 ± 0.1 | 0.7 ± 0 | 1.0 ± 0.1 | 0.7 ± 0.1 | 1.2 ± 0 | 1.0 ± 0 |
| Ampicillin | Nil | 0.7 ± 0.1 | Nil | Nil | 1.0 ± 0.1 | Nil |
| Honey + Ampicillin | 0.9 ± 0 (S) | 1.3 ± 0 (S) | 1.4 ± 0.1 (S) | 0.7 ± 0.1 | 1.7 ± 0 (S) | 1.0 ± 0.1 |
| Gentamicin | 1.3 ± 0 | 1.0 ± 0 | Nil | 1.3 ± 0 | 2.0 ± 0 | 1.6 ± 0 |
| Honey + Gentamicin | 1.3 ± 0.1 | 1.3 ± 0.1 (S) | 1.4 ± 0 (S) | 0.9 ± 0.1 | 2.2 ± 0 (S) | 1.3 ± 0.1 |
| Sample | Bee Type | Bee Species | Origin | Collection |
|---|---|---|---|---|
| A1 | Honey bee | Apis cerana | Honeydew | November 2016 |
| A2 | Honey bee | Apis cerana | Honeydew | May 2017 |
| A3 | Honey bee | Apis cerana | Honeydew | June 2017 |
| A4 | Honey bee | Apis cerana | Honeydew | April 2018 |
| A5 | Honey bee | Apis cerana | Honeydew | July 2018 |
| A6 | Honey bee | Apis cerana | Honeydew | September 2018 |
| A7 | Honey bee | Apis cerana | Honeydew | November 2016 |
| A8 | Honey bee | Apis cerana | Honeydew | April 2017 |
| A9 | Honey bee | Apis cerana | Honeydew | June 2017 |
| A10 | Honey bee | Apis cerana | Blossom | October 2016 |
| A11 | Honey bee | Apis cerana | Blossom | May 2017 |
| A12 | Honey bee | Apis cerana | Blossom | July 2017 |
| A13 | Honey bee | Apis cerana | Blossom | March 2018 |
| A14 | Honey bee | Apis cerana | Blossom | June 2018 |
| A15 | Honey bee | Apis cerana | Blossom | October 2018 |
| A16 | Honey bee | Apis cerana | Blossom | November 2016 |
| A17 | Honey bee | Apis cerana | Blossom | April 2017 |
| A18 | Honey bee | Apis cerana | Blossom | July 2017 |
| A19 | Honey bee | Apis cerana | Blossom | November 2016 |
| A20 | Honey bee | Apis cerana | Blossom | April 2017 |
| A21 | Honey bee | Apis cerana | Blossom | March 2018 |
| A22 | Honey bee | Apis cerana | Blossom | June 2018 |
| A23 | Honey bee | Apis cerana | Blossom | October 2018 |
| S1 | Stingless bee | Heterotrigona itama | Honeydew | August 2016 |
| S2 | Stingless bee | Heterotrigona itama | Honeydew | November 2016 |
| S3 | Stingless bee | Heterotrigona itama | Honeydew | April 2017 |
| S4 | Stingless bee | Heterotrigona itama | Honeydew | July 2017 |
| S5 | Stingless bee | Heterotrigona itama | Honeydew | September 2017 |
| S6 | Stingless bee | Heterotrigona itama | Honeydew | April 2018 |
| S7 | Stingless bee | Heterotrigona itama | Honeydew | July 2018 |
| S8 | Stingless bee | Heterotrigona itama | Honeydew | September 2018 |
| S9 | Stingless bee | Heterotrigona itama | Blossom | August 2016 |
| S10 | Stingless bee | Heterotrigona itama | Blossom | November 2016 |
| S11 | Stingless bee | Heterotrigona itama | Blossom | May 2017 |
| S12 | Stingless bee | Heterotrigona itama | Blossom | July 2017 |
| S13 | Stingless bee | Heterotrigona itama | Blossom | September 2017 |
| S14 | Stingless bee | Heterotrigona itama | Blossom | April 2018 |
| S15 | Stingless bee | Heterotrigona itama | Blossom | May 2018 |
| S16 | Stingless bee | Heterotrigona itama | Blossom | July 2018 |
| S17 | Stingless bee | Geniotrigona thoracica | Blossom | October 2016 |
| S18 | Stingless bee | Geniotrigona thoracica | Blossom | December 2016 |
| S19 | Stingless bee | Geniotrigona thoracica | Blossom | April 2017 |
| S20 | Stingless bee | Geniotrigona thoracica | Blossom | July 2017 |
| S21 | Stingless bee | Geniotrigona thoracica | Blossom | March 2018 |
| S22 | Stingless bee | Geniotrigona thoracica | Blossom | June 2018 |
| S23 | Stingless bee | Geniotrigona thoracica | Blossom | October 2018 |
| Bacteria Sample | Origin of Isolate |
|---|---|
| S. aureus | Reference strain, ATCC 25923 |
| S. aureus | Reference strain, ATCC 33591 |
| E. coli | Reference strain, ATCC 25922 |
| E. coli | Reference strain, ATCC 35218 |
| E. coli 1 | Clinical strain isolated from urine sample |
| E. coli 2 | Clinical strain isolated from urine sample |
| E. coli 3 | Clinical strain isolated from urine sample |
| E. coli 4 | Clinical strain isolated from ascitic fluid |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ng, W.-J.; Sit, N.-W.; Ooi, P.A.-C.; Ee, K.-Y.; Lim, T.-M. The Antibacterial Potential of Honeydew Honey Produced by Stingless Bee (Heterotrigona itama) against Antibiotic Resistant Bacteria. Antibiotics 2020, 9, 871. https://doi.org/10.3390/antibiotics9120871
Ng W-J, Sit N-W, Ooi PA-C, Ee K-Y, Lim T-M. The Antibacterial Potential of Honeydew Honey Produced by Stingless Bee (Heterotrigona itama) against Antibiotic Resistant Bacteria. Antibiotics. 2020; 9(12):871. https://doi.org/10.3390/antibiotics9120871
Chicago/Turabian StyleNg, Wen-Jie, Nam-Weng Sit, Peter Aun-Chuan Ooi, Kah-Yaw Ee, and Tuck-Meng Lim. 2020. "The Antibacterial Potential of Honeydew Honey Produced by Stingless Bee (Heterotrigona itama) against Antibiotic Resistant Bacteria" Antibiotics 9, no. 12: 871. https://doi.org/10.3390/antibiotics9120871
APA StyleNg, W. -J., Sit, N. -W., Ooi, P. A. -C., Ee, K. -Y., & Lim, T. -M. (2020). The Antibacterial Potential of Honeydew Honey Produced by Stingless Bee (Heterotrigona itama) against Antibiotic Resistant Bacteria. Antibiotics, 9(12), 871. https://doi.org/10.3390/antibiotics9120871





