Finding the Dose for Ceftolozane-Tazobactam in Critically Ill Children with and without Acute Kidney Injury
Abstract
1. Introduction
2. Methods
2.1. Sampling and Analytical Method
2.2. Data Analysis
2.3. Pharmacodynamics
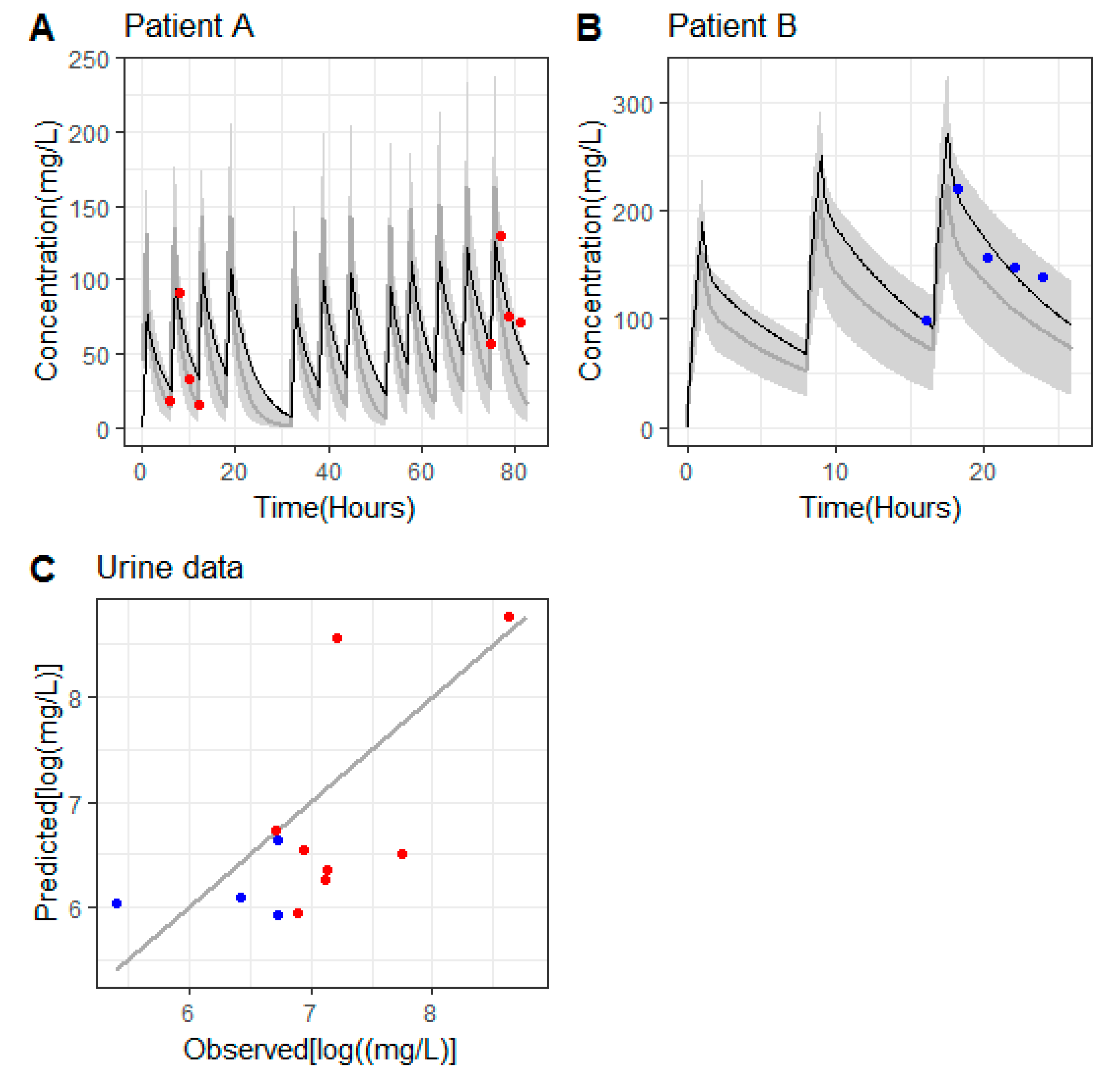
3. Results
3.1. Patient Characteristics, Dosing and Sampling
3.2. Pharmacokinetics of Ceftolozane in Patients without Continuous Renal Replacement Therapy (CRRT)
3.3. Pharmacokinetics of Ceftolozane in the Hemodialyzed Patient
3.4. Pharmacodynamic Target
4. Discussion
4.1. CRRT Clearance
4.2. Pharmacodynamic Target and Area under Curve (AUC)
5. Conclusions
6. Limitations
7. Addendum to the Material and Methods Section
7.1. Population Pharmacokinetics (PK) Modeling Analysis
7.1.1. Patients without Hemodialysis
7.1.2. Patient with CRRT
- (i)
- CPre was considered as the predicted plasma ceftolozane concentrations obtained as A1/V1 using the set of Equations (1)–(7), in which the entire Equation (4) was substituted by the parameter CLRRT.
- (ii)
- CPost and CEfflu were expressed as a function of CPre and CLRRT as indicated below:where and are the corrected plasma and total effluent flows, respectively. The value of was calculated as , with BPR the blood to plasma concentration ratio, and an additional parameter to be estimated from the model. The total effluent was measured during the study (470 mL/h).
7.1.3. Evaluation of Modeling Results
7.1.4. Derived Pharmacokinetic Parameters and Metrics
7.1.5. CRRT Parameters
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bassetti, M.; Vena, A. How to manage Pseudomonas aeruginosa infections. Drugs Context. 2018, 7, 212527. [Google Scholar] [CrossRef] [PubMed]
- Tamma, P.D.; Hsu, A.J. Defining the Role of Novel β-Lactam Agents That Target Carbapenem-Resistant Gram-Negative Organisms. J. Pediatric. Infect. Dis. Soc. 2019, 8, 251–260. [Google Scholar] [CrossRef] [PubMed]
- Gallagher, J.C.; Satlin, M.J. Ceftolozane-Tazobactam for the Treatment of Multidrug-Resistant Pseudomonas aeruginosa Infections: A Multicenter Study. Open Forum Infect. Dis. 2018, 5, ofy280. [Google Scholar] [CrossRef] [PubMed]
- Zerbaxa® Ceftolozane and Tazobactam for Injection [Package Insert]. Available online: https://www.merck.com/product/usa/pi_circulars/z/zerbaxa/zerbaxa_pi.pdf (accessed on 9 September 2020).
- Roberts, J.A.; Abdul-Aziz, M.H.; Lipman, J. International Society of Anti-Infective Pharmacology and the Pharmacokinetics and Pharmacodynamics Study Group of the European Society of Clinical Microbiology and Infectious Diseases. Individualised antibiotic dosing for patients who are critically ill: Challenges and potential solutions. Lancet Infect. Dis. 2014, 14, 498–509. [Google Scholar] [PubMed]
- Basu, R.K.; Kaddourah, A. AWARE Study Investigators. Assessment of a renal angina index for prediction of severe acute kidney injury in critically ill children: A multicentre, multinational, prospective observational study. Lancet Child Adolesc. Health 2018, 2, 112–120. [Google Scholar] [CrossRef]
- Seyler, L.; Cotton, F. Recommended β-lactam regimens are inadequate in septic patients treated with continuous renal replacement therapy. Crit. Care 2011, 15, R137. [Google Scholar] [CrossRef]
- Ruiz, J.; Favieres, C. Individualised antimicrobial dosing in critically ill patients undergoing continuous renal replacement therapy: Focus on total drug clearance. Eur. J. Hosp. Pharm. 2018, 25, 123–126. [Google Scholar] [CrossRef]
- Larson, K.B.; Patel, Y.T.; Willavize, S.; Bradley, J.S.; Rhee, E.G.; Caro, L.; Rizk, M.L. Ceftolozane-Tazobactam Population Pharmacokinetics and Dose Selection for Further Clinical Evaluation in Pediatric Patients with Complicated Urinary Tract or Complicated Intra-abdominal Infections. Antimicrob. Agents Chemother. 2019, 63, e02578-18. [Google Scholar] [CrossRef]
- Bradley, J.S.; Ang, J.Y.; Arrieta, A.C.; Larson, K.B.; Rizk, M.L.; Caro, L.; Yang, S.; Yu, B.; Johnson, M.G.; Rhee, E.G. Pharmacokinetics and Safety of Single Intravenous Doses of Ceftolozane/Tazobactam in Children With Proven or Suspected Gram-Negative Infection. Pediatr. Infect. Dis. J. 2018, 37, 1130–1136. [Google Scholar] [CrossRef] [PubMed]
- Bauer, R.J. NONMEM Tutorial Part I: Description of Commands and Options, with Simple Examples of Population Analysis. CPT Pharmacomet. Syst. Pharmacol. 2019, 8, 525–537. [Google Scholar] [CrossRef]
- Broeker, A.; Vossen, M.G.; Thalhammer, F.; Wallis, S.C.; Lipman, J.; Roberts, J.A.; Wicha, S.G. An Integrated Dialysis Pharmacometric (IDP) Model to Evaluate the Pharmacokinetics in Patients Undergoing Renal Replacement Therapy. Pharm. Res. 2020, 37, 96. [Google Scholar] [CrossRef] [PubMed]
- European Medicines Agency. Guideline on the Use of Pharmacokinetics and Pharmacodynamics in the Development of Antimicrobial Medicinal Products; European Medicines Agency: London, UK, 2016; Available online: https://www.ema.europa.eu/en/use-pharmacokinetics-pharmacodynamics-development-antibacterial-medicinal-products (accessed on 9 September 2020).
- Aitken, S.L.; Kontoyiannis, D.P. Use of Ceftolozane/Tazobactam in the Treatment of Multidrug-resistant Pseudomonas aeruginosa Bloodstream Infection in a Pediatric Leukemia Patient. Pediatr. Infect. Dis. J. 2016, 35, 1040–1042. [Google Scholar] [CrossRef]
- Martín-Cazaña, M.; Grau, S. Successful ceftolozane-tazobactam rescue therapy in a child with endocarditis caused by multidrug-resistant Pseudomonas aeruginosa. J. Paediatr. Child Health 2019, 55, 985–987. [Google Scholar] [CrossRef]
- KDIGO AKI Work Group. KDIGO clinical practice guideline for acute kidney injury. Kidney Int. 2012, 2 (Suppl. 2), 1–138. [Google Scholar]
- Yu, B.; Adedoyin, A.; Hershberger, E.; Caro, L.; Xiao, A.; Rhee, E.G.; Huntington, J.A. Safety, Tolerability, and Pharmacokinetics of 3 g of Ceftolozane/Tazobactam in Healthy Adults: A Randomized, Placebo-Controlled, Multiple-Dose Study. Clin. Pharmacol. Drug Dev. 2018, 7, 382–391. [Google Scholar] [CrossRef]
- Wooley, M.; Miller, B.; Krishna, G.; Hershberger, E.; Chandorkar, G. Impact of renal function on the pharmacokinetics and safety of ceftolozane-tazobactam. Antimicrob. Agents Chemother. 2014, 58, 2249–2255. [Google Scholar] [CrossRef] [PubMed]
- Kakara, M.; Larson, K.; Feng, H.P.; Shiomi, M.; Yoshitsugu, H.; Rizk, M.L. Population pharmacokinetics of tazobactam/ceftolozane in Japanese patients with complicated urinary tract infection and complicated intra-abdominal infection. J. Infect. Chemother. 2019, 25, 182–191. [Google Scholar] [CrossRef]
- Ang, J.Y.; Arrieta, A.; Bradley, J.S.; Zhang, Z.; Yu, B.; Rizk, M.L.; Johnson, M.G.; Rhee, E.G. Ceftolozane/Tazobactam in Neonates and Young Infants: The Challenges of Collecting Pharmacokinetics and Safety Data in This Vulnerable Patient Population. Am. J. Perinatol. 2020. [Google Scholar] [CrossRef]
- Sime, F.B.; Lassig-Smith, M.; Starr, T.; Stuart, J.; Pandey, S.; Parker, S.L.; Wallis, S.C.; Lipman, J.; Roberts, J.A. Population Pharmacokinetics of Unbound Ceftolozane and Tazobactam in Critically Ill Patients without Renal Dysfunction. Antimicrob. Agents Chemother. 2019, 63, e01265-19. [Google Scholar] [CrossRef]
- Piepsz, A.; Tondeur, M.; Ham, H. Revisi-ting normal (51)Cr-ethylene diamine-te-tra-acetic acid clearance values in children. Eur. J. Nucl. Med. Mol. Imaging 2006, 33, 1477–1482. [Google Scholar] [CrossRef]
- Chaijamorn, W.; Shaw, A.R.; Lewis, S.J.; Mueller, B.A. Ex vivo Ceftolozane/Tazobactam Clearance during Continuous Renal Replacement Therapy. Blood Purif. 2017, 44, 16–23. [Google Scholar] [CrossRef] [PubMed]
- Fernández, S.N.; Santiago, M.J.; López-Herce, J.; García, M.; Del Castillo, J.; Alcaraz, A.J.; Bellón, J.M. Citrate anticoagulation for CRRT in children: Comparison with heparin. Biomed. Res. Int. 2014, 2014, 786301. [Google Scholar] [CrossRef] [PubMed]
- Sime, F.B.; Lassig-Smith, M.; Starr, T.; Stuart, J.; Pandey, S.; Parker, S.L.; Wallis, S.C.; Lipman, J.; Roberts, J.A. A Population Pharmacokinetic Model-Guided Evaluation of Ceftolozane-Tazobactam Dosing in Critically Ill Patients Undergoing Continuous Venovenous Hemodiafiltration. Antimicrob. Agents Chemother. 2019, 64. [Google Scholar] [CrossRef] [PubMed]
- Oliver, W.D.; Heil, E.L.; Gonzales, J.P.; Mehrotra, S.; Robinett, K.; Saleeb, P.; Nicolau, D.P. Ceftolozane-Tazobactam Pharmacokinetics in a Critically Ill Patient on Continuous Venovenous Hemofiltration. Antimicrob. Agents Chemother. 2015, 60, 1899–1901. [Google Scholar] [CrossRef]
- Rawlins, M.; Cheng, V.; Raby, E.; Dyer, J.; Regli, A.; Ingram, P.; McWhinney, B.C.; Ungerer, J.P.J.; Roberts, J.A. Pharmacokinetics of Ceftolozane-Tazobactam during Prolonged Intermittent Renal Replacement Therapy. Chemotherapy 2018, 63, 203–206. [Google Scholar] [CrossRef]
- Mian, A.N.; Schwartz, G.J. Measurement and Estimation of Glomerular Filtration Rate in Children. Adv. Chronic Kidney Dis. 2017, 24, 348–356. [Google Scholar] [CrossRef]

| Patient | Age (Months) | Weight (kg) | Diagnosis | Infection |
|---|---|---|---|---|
| A | 8 | 8.7 | Hypoplastic left heart syndrome (Norwood stage) | Sepsis and pneumonia due to MDR P. aeruginosa |
| B | 19 | 11 | Hypoplastic left heart syndrome (Glenn stage) | Pneumonia due to MDR P. aeruginosa |
| C | 9 | 5.8 | Heart transplant | Pneumonia due to MDR P. aeruginosa and Bacteremia due to ESBL Escherichia Coli |
| Patient | Renal Function (eGFR) | Ceftolozane Dosing Regimen | Dose Number Measured | Ceftolozane Concentrations (g/mL) | |||||
|---|---|---|---|---|---|---|---|---|---|
| T0 | T1 | T2 | T4 | T6 | T8 | ||||
| A | 75 mL/min/1.73 m2 | 40 mg/kg q6h | 2 | a 17.8 | 91.5 | 33.4 | 16.1 | ||
| A (2nd profile) | 75 mL/min/1.73 m2 | 40 mg/kg q6h | 12 | a 57.3 | 129.3 | 74.9 | 71.2 | ||
| B | Acute kidney injury (22 mL/min/1.73 m2) | 36 mg/kg q8h | 3 | a 99.1 | 220.2 | 156.1 | 148.2 | 138.1 | |
| C | Anuria. CRRT. | 30 mg/kg q8h | 3 | b 18.8 | 89.5 | 72.5 | 24.1 | ||
| c 15.5 | 75.8 | 62.0 | 18.1 | ||||||
| d 17.5 | 92.3 | 66.5 | 24.4 | ||||||
| Non-Hemodialyzed | Hemodialyzed | ||
|---|---|---|---|
| Parameter | Patient A | Patient B | Patient C |
| CLRenal (or CLRRT) (L/h) | 0.88 | 0.27 | 0.39 |
| Vd1 (L) | 3.45 | 1.13 | 0.74 |
| Vd2 (L) | 0.942 | 1.36 | 1.17 |
| CLD (L/h) 1 | 2.54 | 2.54 | 2.54 |
| BPR (unitless) 2 | - | - | 1.28 |
| t1/2, (h) | 3.51 | 6.62 | 3.51 |
| AUCτ,SSC (mg × h × L−1) 3 | 397.73 | 1481.48 | 448.72 |
| AUC06,SSmicrog/h/mL Median (Range) | t1/2 (h) | Vd (L/kg) | CLRenal (L/kg/h) | |
|---|---|---|---|---|
| Patient A (dose: 40 mg/kg) | 397.73 | 3.51 | 0.505 | 0.101 |
| Bradley Group 4 (dose: 30 mg/kg) Mean (CV%) (SD lower-upper limits) | 202 (158–259) | 1.63 (69%) (0.51–2.75) | 0.34 (21.1%) (0.27–0.41) | 0.149 (43.2%) (0.085–0.213) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Butragueño-Laiseca, L.; Troconiz, I.F.; Grau, S.; Campillo, N.; García, X.; Padilla, B.; Fernández, S.N.; Santiago, M.J. Finding the Dose for Ceftolozane-Tazobactam in Critically Ill Children with and without Acute Kidney Injury. Antibiotics 2020, 9, 887. https://doi.org/10.3390/antibiotics9120887
Butragueño-Laiseca L, Troconiz IF, Grau S, Campillo N, García X, Padilla B, Fernández SN, Santiago MJ. Finding the Dose for Ceftolozane-Tazobactam in Critically Ill Children with and without Acute Kidney Injury. Antibiotics. 2020; 9(12):887. https://doi.org/10.3390/antibiotics9120887
Chicago/Turabian StyleButragueño-Laiseca, Laura, Iñaki F. Troconiz, Santiago Grau, Nuria Campillo, Xandra García, Belén Padilla, Sarah N. Fernández, and María José Santiago. 2020. "Finding the Dose for Ceftolozane-Tazobactam in Critically Ill Children with and without Acute Kidney Injury" Antibiotics 9, no. 12: 887. https://doi.org/10.3390/antibiotics9120887
APA StyleButragueño-Laiseca, L., Troconiz, I. F., Grau, S., Campillo, N., García, X., Padilla, B., Fernández, S. N., & Santiago, M. J. (2020). Finding the Dose for Ceftolozane-Tazobactam in Critically Ill Children with and without Acute Kidney Injury. Antibiotics, 9(12), 887. https://doi.org/10.3390/antibiotics9120887








