Poly-ε-caprolactone Nanoparticles Loaded with 4-Nerolidylcatechol (4-NC) for Growth Inhibition of Microsporum canis
Abstract
1. Introduction
2. Materials and Methods
2.1. 4-NC Isolation
2.2. Development of Nanoparticles
2.3. Encapsulation Efficiency
2.4. Dynamic Light Scattering (DLS) and Zeta Potential (Zeta)
2.5. Nano Tracking Analysis (NTA)
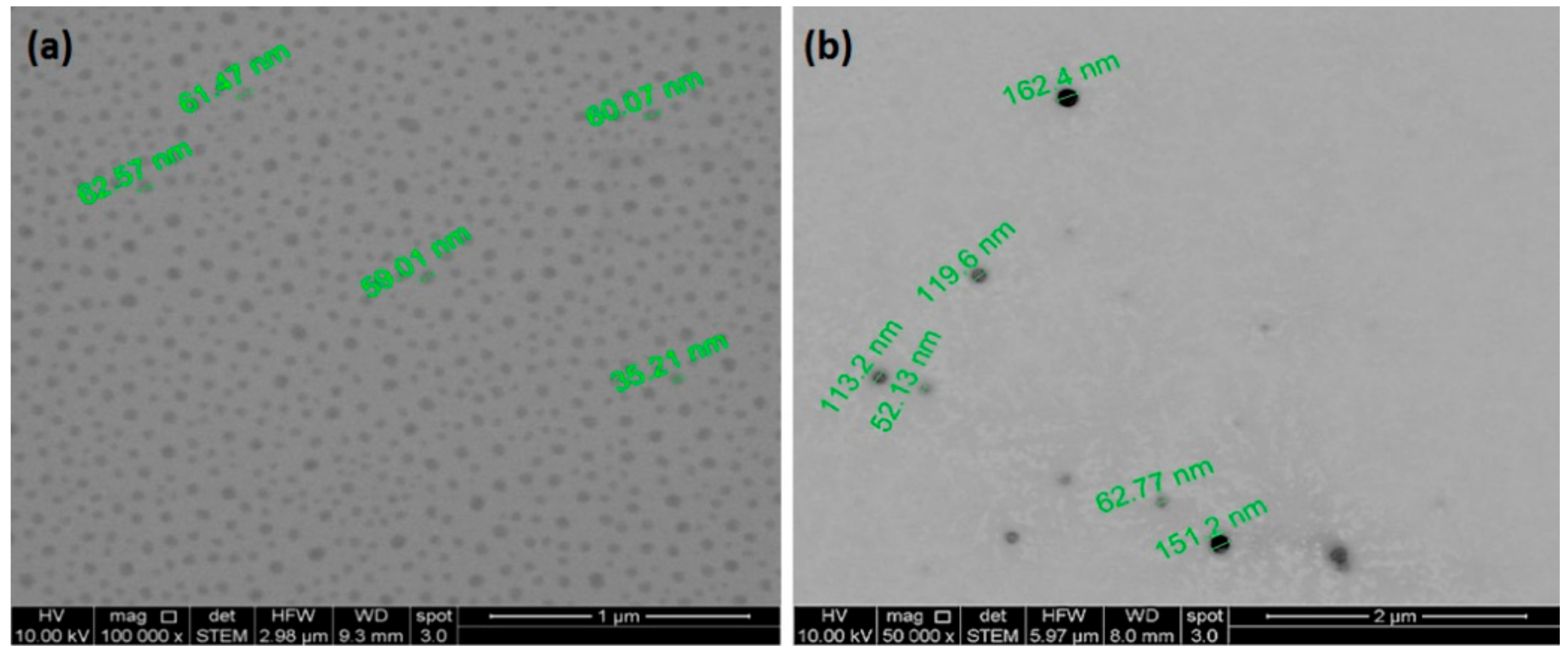
2.6. Scanning Electron Microscopy (SEM)
2.7. Fourier Transformed Infrared Spectroscopy (FTIR)
2.8. In Vitro Evaluation of 4-NC and 4-NCNP Antifungal Activity
2.9. Preparation of Gel Formulations for Use in In Vivo Study
2.10. In Vivo Antimycotic Evaluation in SWISS MICE
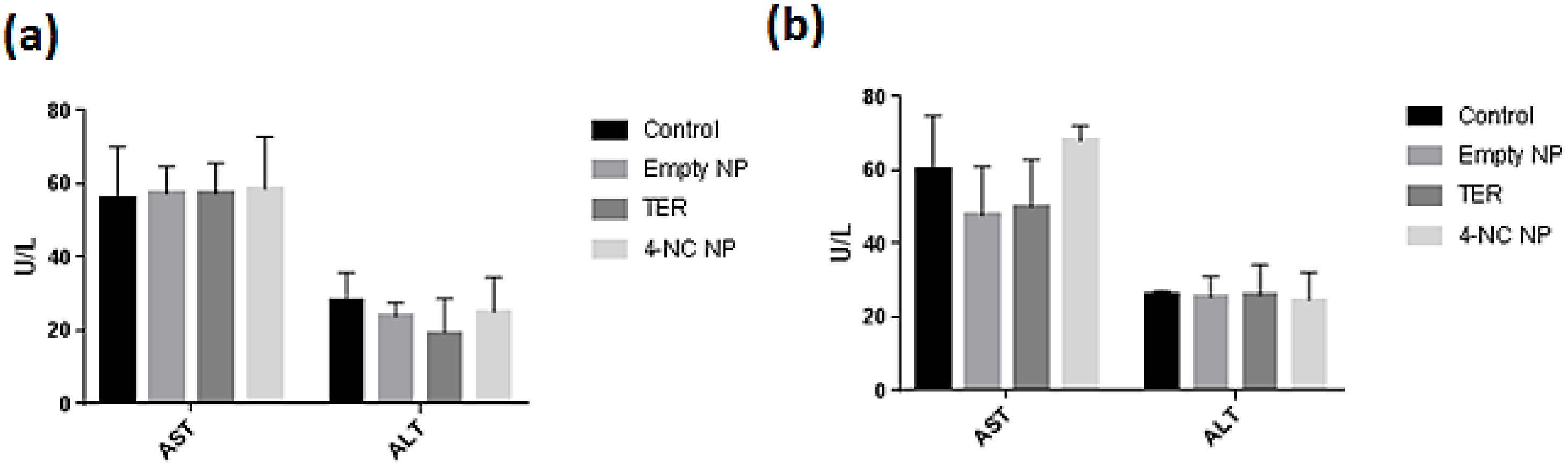
2.11. Analysis of the Hepatic Profile of the Animals
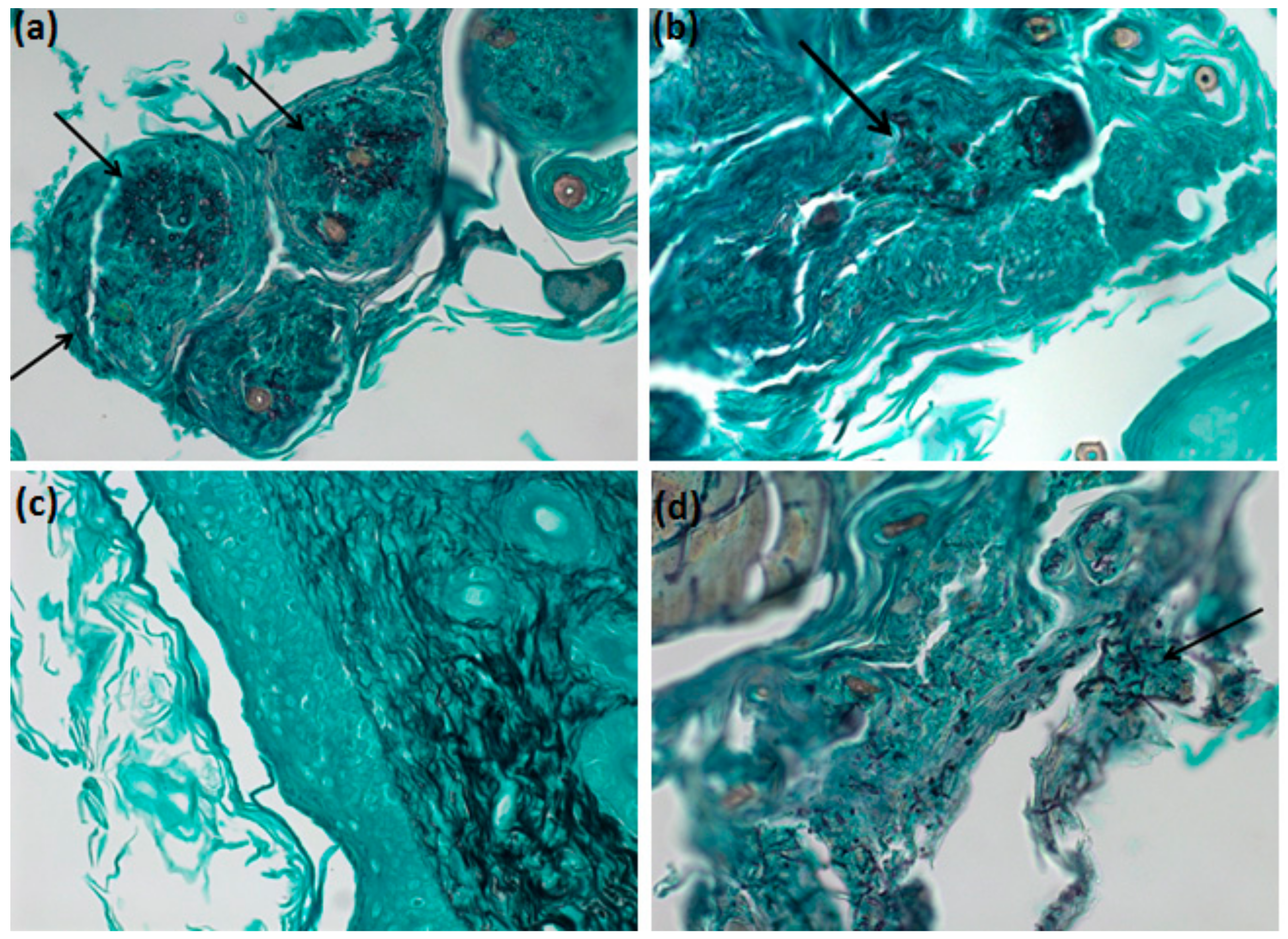
2.12. Grocott-Gomori Silver Methanamine Staining Microscopy
3. Results
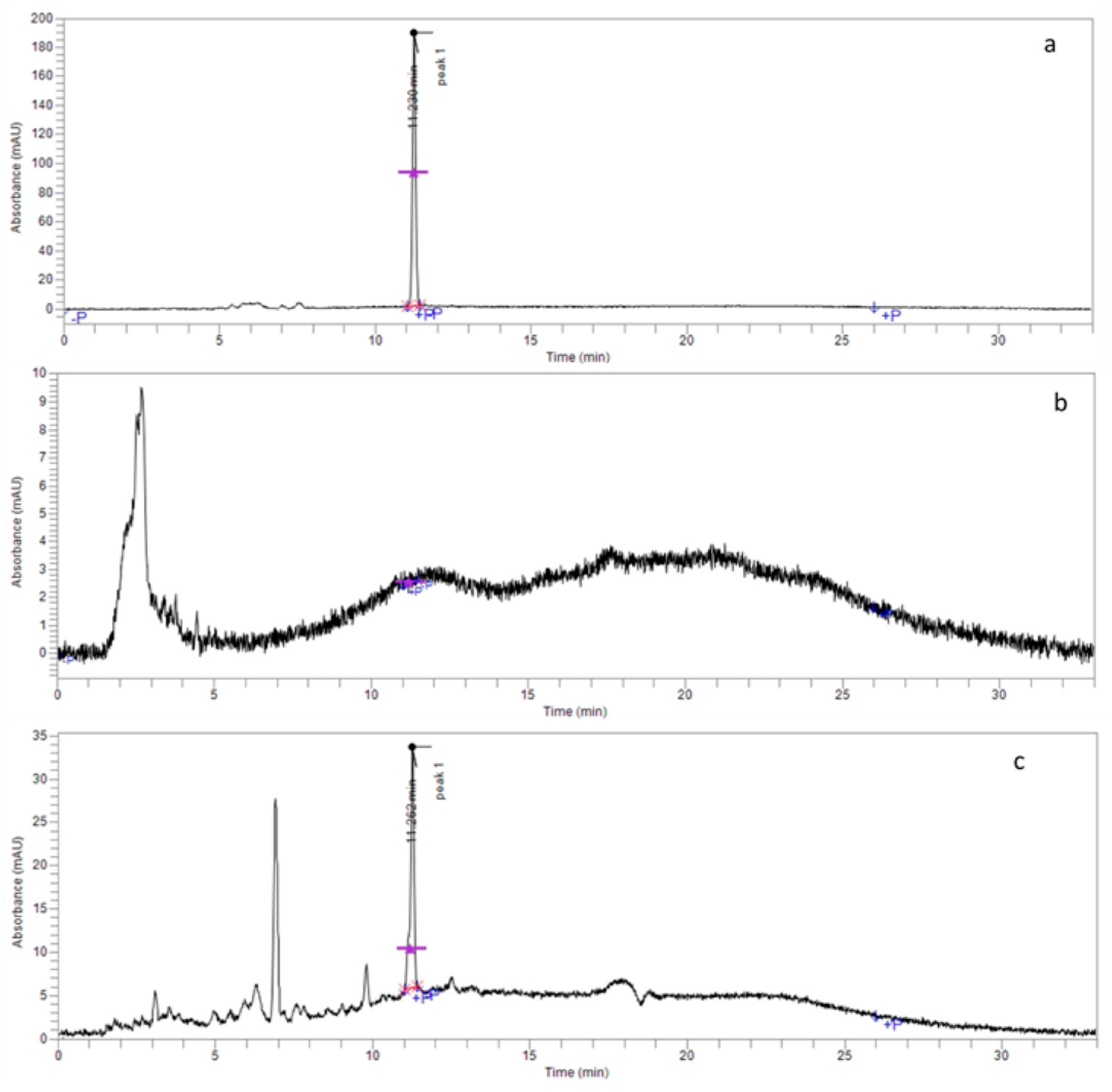
3.1. 4-NC Structural Determination
3.2. Encapsulation Efficiency
3.3. DLS, Zeta Potential and NTA Characterization
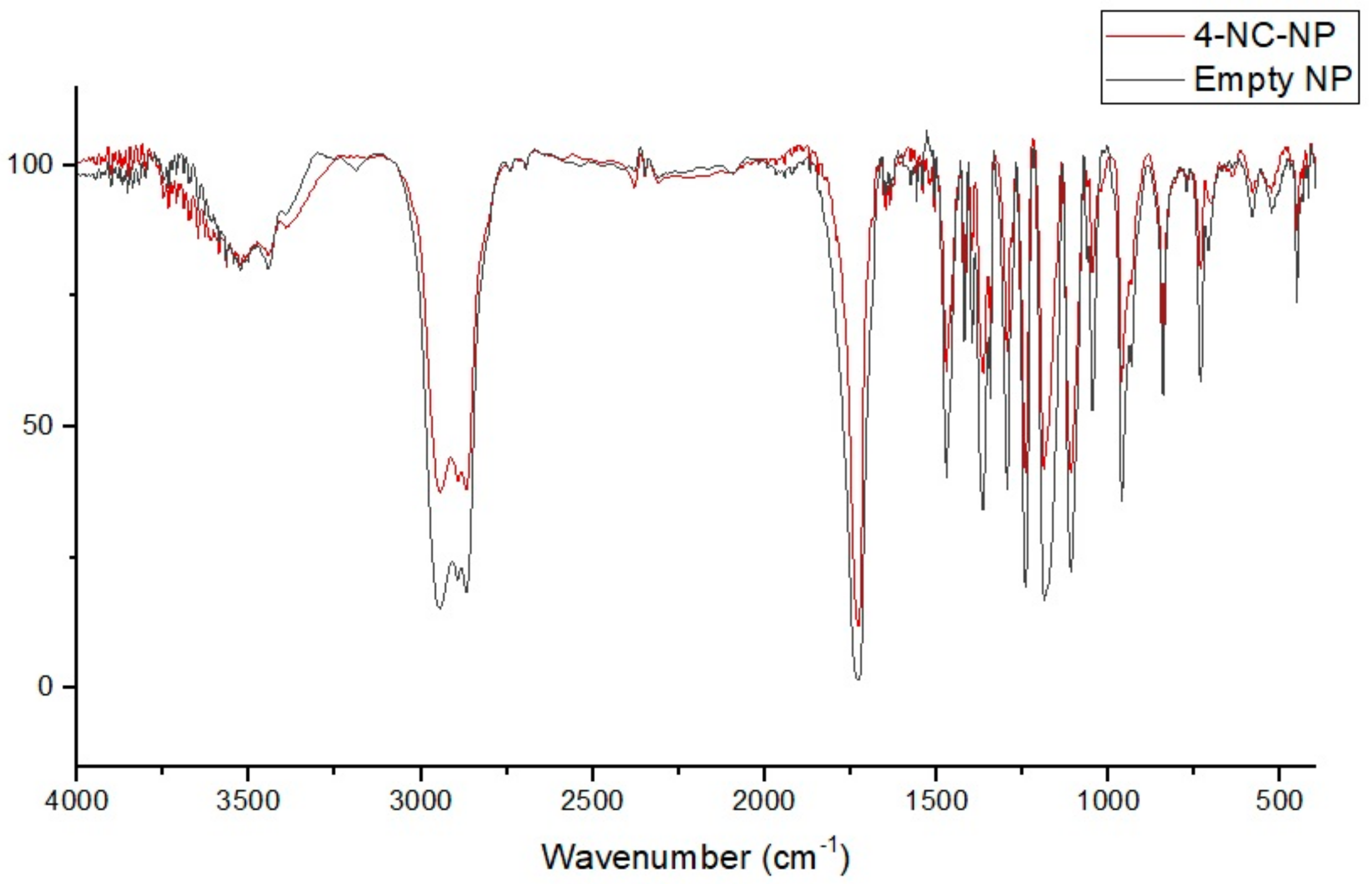
3.4. FT-IR Spectral of Nanoparticles and Compounds
3.5. Nanoparticles Antifungal Activity
3.6. In Vivo Analysis
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Maraki, S.; Mavromanolaki, V.E. Epidemiology of Dermatophytoses in Crete, Greece: A 5-year Survey. Med. Mycol. J. 2016, 57e, 69–75. [Google Scholar] [CrossRef]
- Vineetha, M.; Sheeja, S.; Celine, M.I.; Sadeep, M.S.; Palackal, S.; Shanimole, E.; Das, S.S. Profile of Dermatophytosis in a Tertiary Care Center. Indian J. Dermatol. 2018, 63, 490–495. [Google Scholar] [PubMed]
- Lima, E.; Pinto, A.; Nogueira, K.; Silva, L.; Almeida, P.; Vasconcellos, M.; Chaves, F.; Tadei, W.; Pohlit, A. Stability and Antioxidant Activity of Semi-synthetic Derivatives of 4-Nerolidylcatechol. Molecules 2012, 18, 178–189. [Google Scholar] [CrossRef] [PubMed]
- Dorigoni, P.A.; Ghedini, P.C.; Fróes, L.F.; Baptista, K.C.; Ethur, A.B.M.; Baldisserotto, B.; Bürger, M.E.; Almeida, C.E.; Lopes, A.M.V.; Záchia, R.A. Survey of data on medicinal plants of popular use in the municipality of São João do Polêsine, RS, Brazil. i—Relationship between diseases and species used. Braz. J. Med. Plants 2001, 4, 69–79. [Google Scholar]
- Lopes, A.P.; Bagatela, B.S.; Rosa, P.C.P.; Nanayakkara, N.P.; Carvalho, J.C.T.; Mainstro, E.L.; Bastos, J.K.; Perazzo, F.F. Antioxidant and cytotoxic effects of crude extract, fractions and 4-nerolidylcathecol from aerial parts of Pothomorphe umbellata L. (Piperaceae). Biomed. Res. Int. 2013, 1, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Bagatela, B.S.; Lopes, A.P.; Fonseca, F.L.A.; Andreo, M.A.; Nanayakkara, D.N.P.; Bastos, J.K.; Perazzo, F.F. Evaluation of antimicrobial and antimalarial activities of crude extract, fractions and 4- nerolidylcathecol from the aerial parts of Piper umbellata L. (Piperaceae). Nat. Prod. Res. 2013, 27, 2202–2209. [Google Scholar] [CrossRef]
- Iwamoto, L.H.; Costa, D.B.V.; Monteiro, P.A.; Ruiz, A.L.T.G.; Sousa, I.M.O.; Foglio, M.A.; de Carvalho, J.E.; Rodrigues, R.A. Anticancer and Anti-Inflammatory Activities of a Standardized Dichloromethane Extract from Piper umbellatum L. Leaves. Evid. Based Complement. Alternat. Med. eCAM 2015, 1–8. [Google Scholar] [CrossRef]
- Roersch, C.M.F.B. Piper umbellatum L. A comparative cross-cultural analysis of its medicinal uses and an ethnopharmacological evaluation. J. Ethnopharmacol. 2010, 131, 522–537. [Google Scholar] [CrossRef]
- Rodrigues, E.R.; Nogueira, N.G.P.; Zocolo, J.G.; Leite, F.S.; Januario, A.H.; Fusco-Almeida, A.M.; Fachin, A.L.; de Marchi, M.R.; dos Santos, A.G.; Pietro, R.C. Pothomorphe umbellata: Antifungal activity against strains of Trichophyton rubrum. J. Mycol. Med. 2012, 22, 265–269. [Google Scholar] [CrossRef]
- PubChem. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/4-Nerolidylcatechol (accessed on 28 October 2020).
- Rocha e Silva, L.F.; Nogueira, K.L.; Pinto, A.C.S.; Katzin, A.M.; Sussmann, R.A.C.; Muniz, M.P.; Andrade Neto, V.F.; Chaves, F.C.M.; Coutinho, J.P.; Lima, E.S.; et al. In Vivo Antimalarial Activity and Mechanisms of Action of 4-Nerolidylcatechol Derivatives. Antimicrob. Agents Chemother. 2015, 59, 3271–3280. [Google Scholar] [CrossRef]
- Alves-Fernandes, D.K.; Oliveira, E.A.; Hastreiter, A.A.; Faião-Flores, F.; Felipe-Silva, A.S.; Turato, W.; Fock, R.A.; Maria-Engler, S.S.; Barros, S.B. In vivo antitumoral effect of 4-nerolidylcatechol (4-NC) in NRAS-mutant human melanoma. Food Chem. Toxicol. 2020, 141, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Severino, P.; Andreani, T.; Macedo, A.S.; Fangueiro, J.F.; Santana, M.H.A.; Silva, A.M.; Souto, E.B. Current state of art and new trends on lipid nanoparticles (SLN and NLC) for oral drug delivery. J. Drug Deliv. 2012, 2012, 750891. [Google Scholar] [CrossRef] [PubMed]
- Joseph, S.; Bunjes, H. Solid Lipid Nanoparticles for Drug Delivery. In Drug Delivery Strategies for Poorly Water-Soluble Drugs; Douroumis, D., Fahr, A., Eds.; John Wiley & Sons Ltd.: Chichester, UK, 2013; pp. 103–149. ISBN 9780470711972. [Google Scholar]
- Zhang, W.; Saliba, M.; Stranks, S.D.; Sun, Y.; Shi, X.; Wiesner, U.; Snaith, H.J. Enhancement of Perovskite-Based Solar Cells Employing Core–Shell Metal Nanoparticles. Nano Lett. 2013, 13, 4505–4510. [Google Scholar] [CrossRef] [PubMed]
- Dash, T.K.; Konkimalla, V.B. Poly-є-caprolactone based formulations for drug delivery and tissue engineering: A review. J. Control. Release 2012, 158, 15–33. [Google Scholar] [CrossRef] [PubMed]
- Silva, L.G. Chemical Physical Characterization and Evaluation of the In Vitro Release of Poly (ε-Caprolactone)/Tetracycline Conjugates and Poly (ε-Caprolactone)/Ethylsalicylic Acid Conjugates. Master’s Thesis, Federal University of Itajubá, Itajubá, Brazil, 2012. [Google Scholar]
- Valeriano, V.S.; Leal, A.F.V.B.; Soares, L.A.; Rezende, K.R.; Resck, I.S.; Kato, M.J. Inclusion complex of 4-Nerolidylcatechol in hydroxypropyl-β-cyclodextrin. Rev. Eletrônica Farmácia 2005, 2, 224–227. [Google Scholar]
- Elsabahy, M.; Wooley, K.L. Design of polymeric nanoparticles for biomedical delivery applications. Chem. Soc. Rev. 2012, 41, 2545–2561. [Google Scholar] [CrossRef] [PubMed]
- Souza, A.C.O.; Amaral, A.C. Antifungal Therapy for Systemic Mycosis and the Nanobiotechnology Era: Improving Efficacy, Biodistribution and Toxicity. Front. Microbiol. 2017, 8, 1–13. [Google Scholar] [CrossRef]
- Gaeti, M.P.N.; Benfica, P.L.; Mendes, L.P.; Vieira, M.S.; Anjos, J.L.V.; Alonso, A.; Rezende, K.R.; Valadares, M.C.; Lima, E.M. Liposomal entrapment of 4-nerolidylcatechol: Impact on phospholipid dynamics, drug stability and bioactivity. J. Nanosci. Nanotech. 2015, 15, 838–847. [Google Scholar] [CrossRef]
- Mondal, D.; Griffth, M.; Venkatraman, S.S. Polycaprolactone-based biomaterials for tissue engineering and drug delivery: Current scenario and challenges. Int. J. Polym. Mater. Polym. Biomater. 2016, 65, 255–265. [Google Scholar] [CrossRef]
- Tammaro, R.; Saturnino, C.; D’Aniello, S.; Vigliotta, G.; Vittoria, V. Polymorphic solidification of Linezolid confined in electrospun PCL fibers for controlled release in topical applications. Int. J. Pharm. 2015, 490, 32–38. [Google Scholar] [CrossRef]
- Wang, H.; Xia, Y.; Liu, J.; Ma, Z.; Shi, Q.; Yin, J. Programmable release of 2-O-D-glucopyranosyl-L-ascorbic acid and heparin from PCL-based nanofiber scaffold for reduction of inflammation and thrombosis. Mater. Today Chem. 2020, 17, 100303. [Google Scholar] [CrossRef]
- Abriata, J.P.; Eloy, J.O.; Riul, T.B.; Campos, P.M.; Baruffi, M.D.; Marchetti, J.M. Poly-epsilon-caprolactone nanoparticles enhance ursolic acid in vivo efficacy against Trypanosoma cruzi infection. Mat. Sci. Eng. C-Mater. 2017, 77, 1196–1203. [Google Scholar] [CrossRef] [PubMed]
- Filipe, V.; Hawe, A.; Jiskoot, W. Critical Evaluation of Nanoparticle Tracking Analysis (NTA) by NanoSight for the Measurement of Nanoparticles and Protein Aggregates. Pharm. Res. 2010, 27, 796–810. [Google Scholar] [CrossRef] [PubMed]
- Adura, C.; Grerrero, S.; Salas, E.; Medel, L.; Riveros, A.; Mena, J.; Arbiol, J.; Albericio, F.; Giralt, E.; Kogan, M.J. Stable conjugates of peptides with gold nanorods for biomedical applications with reduced effects on cell viability. ACS Appl. Mater. Inter. 2013, 5, 4076–4085. [Google Scholar] [CrossRef] [PubMed]
- Clinical and Laboratory Standards Institute (CLSI). Reference Method for Broth Dilution Antifungal Susceptibility Testing of Filamentous Fungi; Approved Standard, 2nd ed.; CLSI document M38-A2; CLSI: Wayne, PA, USA, 2008. [Google Scholar]
- Sharma, B.; Kumar, P.; Joshi, S. Topical Treatment of Dermatophytic Lesion on Mice (Mus musculus) Model. Indian J. Microbiol. 2011, 51, 217–222. [Google Scholar] [CrossRef]
- Golde, W.T.; Gollobin, P.; Rodriguez, L.L. A rapid, simple, and humane method for submandibular bleeding of mice using a lancet. Lab. Anim. 2005, 34, 39–43. [Google Scholar] [CrossRef]
- Ozer, J.; Ratner, M.; Shaw, M.; Bailey, W.; Schomaker, S. The current state of serum biomarkers of hepatotoxicity. Toxicology 2008, 245, 194–205. [Google Scholar] [CrossRef]
- Bergamo, D.C.B. Chemical Evaluation of Nonvolatile and Volatile Components and Biosynthetic Study of 4-Nerolidylcatecol in Potomorphe umbellata. Ph.D. Thesis, Institute of Chemistry, UNESP, Araraquara, Brazil, 2003. [Google Scholar]
- Almeida, R.L. Participation of Fractions of 4-Nerolidylcatecol-Free Hydroalcoholic Root Extract of Pothomorphe umbellata Root on the Antioxidant and Inhibitory Activity of Metalloproteinases 2 and 9 on Skin. Ph.D. Thesis, School of Pharmaceutical Sciences, UNESP, São Paulo, Brazil, 2011. [Google Scholar]
- Freitas, J.A. Investigation of Antifungal Activity, Mechanisms of Action and Protein Profile Analysis against Pothomorphe umbellata Extracts. Master´s Thesis, School of Pharmaceutical Sciences, UNESP, Araraquara, Brazil, 2015. [Google Scholar]
- Das, S.; Suresh, P.K.; Desmukh, R. Design of Eudragit RL 100 nanoparticles by nanoprecipitation method for ocular drug delivery. Nanomedicine 2010, 6, 318–323. [Google Scholar] [CrossRef]
- Shao, X.R.; Wei, X.Q.; Song, X.; Hao, L.Y.; Cai, X.X.; Zhang, Z.R. Independent effect of polymeric nanoparticle zeta potential/surface charge, on their cytotoxicity and affinity to cells. Cell Proliferat. 2015, 48, 465–474. [Google Scholar] [CrossRef]
- Souza, P.M.S.; Lobo, F.A.; Rosa, A.H.; Fraceto, L.F. Development of poly-caprolactone nanocapsules containing the atrazine herbicide. Quim. Nova 2012, 35, 132–137. [Google Scholar] [CrossRef]
- James, A.E.; Driskell, J.D. Monitoring gold nanoparticle conjugation and analysis of biomolecular binding with nanoparticle tracking analysis (NTA) and dynamic light scattering (DLS). Analyst 2013, 138, 1212–1218. [Google Scholar] [CrossRef] [PubMed]
- Perecin, C.; Cerize, N.; Chitta, V.; Gratens, X.; Léo, P.; de Oliveira, A.; Yoshioka, S. Magnetite Nanoparticles Encapsulated with PCL and Poloxamer by Nano Spray Drying Technology. Nanosci. Nanotechnol. 2016, 6, 68–73. [Google Scholar] [CrossRef]
- Soares, L.A.; Leal, A.F.V.B.; Fraceto, L.F.; Maia, E.R.; Resck, I.S.; Kato, M.J.; Gil, E.S.; de Sousa, A.R.; Cunha, L.C.; Rezende, K.R. Host-guest system of 4-nerolidylcatechol in 2-hydroxypropyl-β-cliclodextrin: Preparation, characterization and molecular modeling. J. Incl. Phenom. Macrocycl. Chem. 2009, 64, 23–35. [Google Scholar] [CrossRef]
- Nogueira-Neto, J.D.; Almeida, A.A.C.; Silva, A.O.; Carvalho, R.B.F.; de Sousa, D.P.; Freitas, R.M. Evaluation of acute toxicity and anxiolytic properties of nerolidol in mice. BioFar 2012, 8, 42–56. [Google Scholar]
- Araujo, F.T.M. Establishment of Reference Values for Hematological and Biochemical Parameters and Evaluation of the Immunological Profile of Strains of Mice Produced in the Research Center René Rachou/FIOCRUZ—Minas and of Laboratory Breeding Center/FIOCRUZ. Ph.D. Thesis, Fundação Oswaldo Cruz Research Center René Rachou, Belo Horizonte, Brazil, 2012. [Google Scholar]
- Branco, A.C.S.C.; Diniz, M.F.F.M.; Almeida, R.N.; Santos, H.B.; Oliveira, K.M.; Ramalho, J.A.; Dantas, J.G.; Diniz, M.F.F.M.; Almeida, R.N.; Santos, H.B.; et al. Biochemical and Hematological Parameters of Wistar Rats and Swiss Mice in the Professor Thomas George Animal Laboratory. Rev. Bras. Ciênc. Saúde 2011, 15, 209–214. [Google Scholar] [CrossRef]



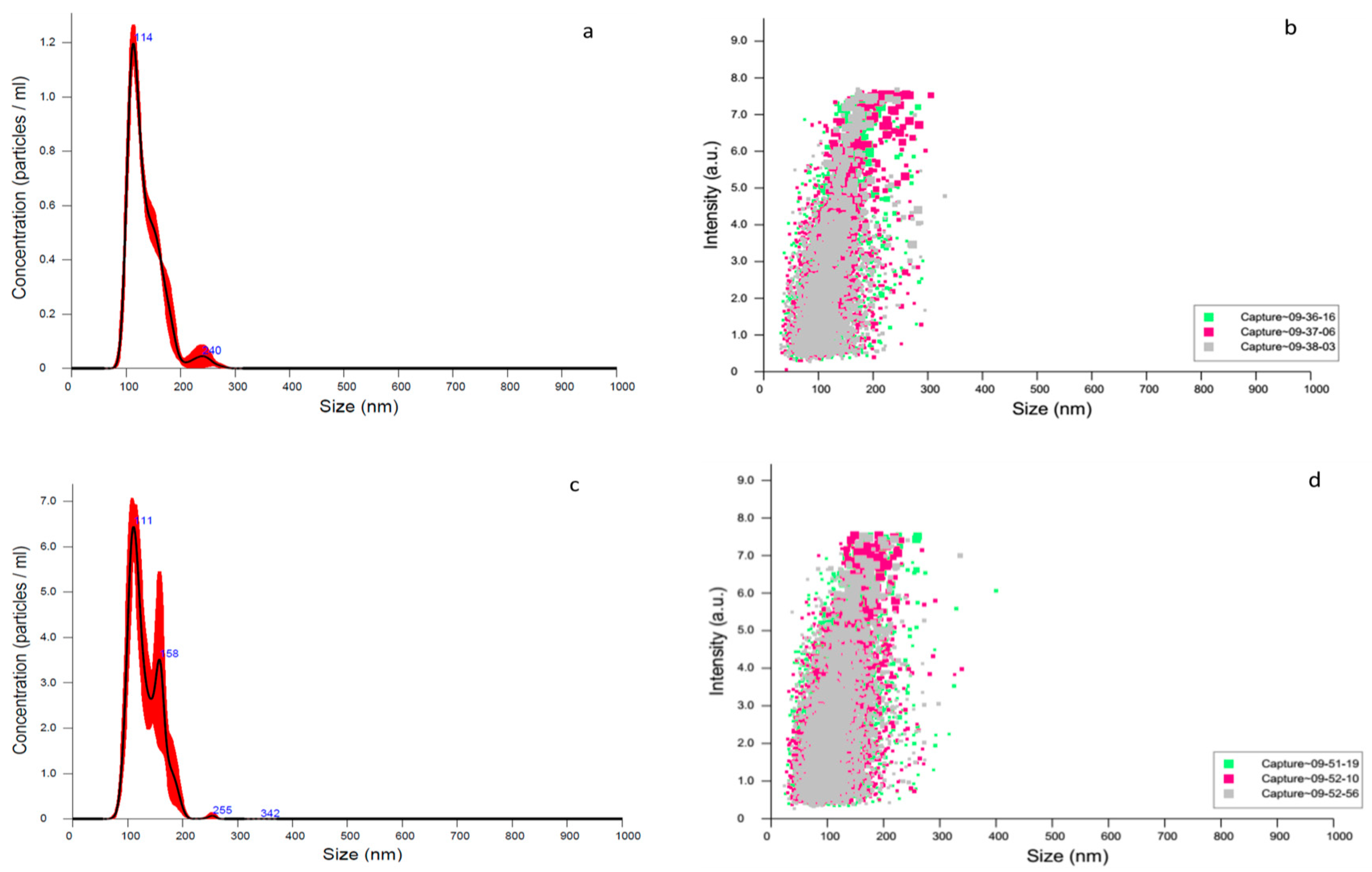



| Zeta Potential (mV) | pdi | Size (nm) | |
|---|---|---|---|
| Empty NP | −7.15 ± 0.16 | 0.149 ± 0.01 | 148.1 ± 1.12 |
| 4-NC NP | −9.30 ± 0.17 | 0.232 ± 0.00 | 143.5 ± 1.36 |
| Samples * | MIC | MFC |
|---|---|---|
| 4-NC | 7.8 | 7.8 |
| NP | - | - |
| 4-NCNP | 75 | 150 |
| Amphotericin B | 0.5 | 0.5 |
| Terbinafine | 0.625 | 0.625 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Greatti, V.R.; Oda, F.; Sorrechia, R.; Kapp, B.R.; Seraphim, C.M.; Weckwerth, A.C.V.B.; Chorilli, M.; Silva, P.B.D.; Eloy, J.O.; Kogan, M.J.; et al. Poly-ε-caprolactone Nanoparticles Loaded with 4-Nerolidylcatechol (4-NC) for Growth Inhibition of Microsporum canis. Antibiotics 2020, 9, 894. https://doi.org/10.3390/antibiotics9120894
Greatti VR, Oda F, Sorrechia R, Kapp BR, Seraphim CM, Weckwerth ACVB, Chorilli M, Silva PBD, Eloy JO, Kogan MJ, et al. Poly-ε-caprolactone Nanoparticles Loaded with 4-Nerolidylcatechol (4-NC) for Growth Inhibition of Microsporum canis. Antibiotics. 2020; 9(12):894. https://doi.org/10.3390/antibiotics9120894
Chicago/Turabian StyleGreatti, Vanessa Raquel, Fernando Oda, Rodrigo Sorrechia, Bárbara Regina Kapp, Carolina Manzato Seraphim, Ana Carolina Villas Bôas Weckwerth, Marlus Chorilli, Patrícia Bento Da Silva, Josimar O. Eloy, Marcelo J. Kogan, and et al. 2020. "Poly-ε-caprolactone Nanoparticles Loaded with 4-Nerolidylcatechol (4-NC) for Growth Inhibition of Microsporum canis" Antibiotics 9, no. 12: 894. https://doi.org/10.3390/antibiotics9120894
APA StyleGreatti, V. R., Oda, F., Sorrechia, R., Kapp, B. R., Seraphim, C. M., Weckwerth, A. C. V. B., Chorilli, M., Silva, P. B. D., Eloy, J. O., Kogan, M. J., Morales, J. O., & Pietro, R. C. L. R. (2020). Poly-ε-caprolactone Nanoparticles Loaded with 4-Nerolidylcatechol (4-NC) for Growth Inhibition of Microsporum canis. Antibiotics, 9(12), 894. https://doi.org/10.3390/antibiotics9120894







