Use of Local Antibiogram Data and Antimicrobial Importance Ratings to Select Optimal Empirical Therapies for Urinary Tract Infections in Dogs and Cats
Abstract
:1. Introduction
2. Results
2.1. Antimicrobial Impact Factor
2.2. Whole-Population Antimicrobial Simulation and Antimicrobial Cost per Cure
2.3. Changes in Resistance
3. Discussion
4. Materials and Methods
4.1. Source and Handling of Data
4.2. Sample Processing
4.3. Antibiogram, Antimicrobial Impact Factors and Antimicrobial Cost per Cure
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Weese, J.S.; Blondeau, J.; Boothe, D.; Guardabassi, L.G.; Gumley, N.; Papich, M.; Jessen, L.R.; Lappin, M.; Rankin, S.; Westropp, J.L.; et al. International Society for Companion Animal Infectious Diseases (ISCAID) guidelines for the diagnosis and management of bacterial urinary tract infections in dogs and cats. Vet. J. 2019, 247, 8–25. [Google Scholar] [CrossRef] [PubMed]
- Hardefeldt, L.Y.; Selinger, J.; Stevenson, M.A.; Gilkerson, J.R.; Crabb, H.K.; Billman-Jacobe, H.; Thursky, K.; Bailey, K.E.; Awad, M.; Browning, G.F. Population wide assessment of antimicrobial use in dogs and cats using a novel data source—A cohort study using pet insurance data. Vet. Microbiol. 2018, 225, 34–39. [Google Scholar] [CrossRef] [PubMed]
- Hardefeldt, L.Y.; Holloway, S.; Trott, D.; Shipstone, M.; Barrs, V.; Malik, R.; Burrows, M.; Armstrong, S.; Browning, G.; Stevenson, M. Antimicrobial Prescribing in Dogs and Cats in Australia: Results of the Australasian Infectious Disease Advisory Panel Survey. J. Vet. Intern. Med. 2017, 31, 1100–1107. [Google Scholar] [CrossRef] [PubMed]
- De Briyne, N.; Atkinson, J.; Pokludová, L.; Borriello, S.P.; Price, S. Factors influencing antibiotic prescribing habits and use of sensitivity testing amongst veterinarians in Europe. Vet. Rec. 2013, 173, 475. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moyaert, H.; Morrissey, I.; De Jong, A.; El Garch, F.; Klein, U.; Ludwig, C.; Thiry, J.; Youala, M. Antimicrobial Susceptibility Monitoring of Bacterial Pathogens Isolated from Urinary Tract Infections in Dogs and Cats Across Europe: ComPath Results. Microb. Drug Resist. 2017, 23, 391–403. [Google Scholar] [CrossRef]
- Wong, C.; Epstein, S.E.; Westropp, J.L. Antimicrobial Susceptibility Patterns in Urinary Tract Infections in Dogs (2010–2013). J. Vet. Intern. Med. 2015, 29, 1045–1052. [Google Scholar] [CrossRef] [Green Version]
- Litster, A.; Moss, S.M.; Honnery, M.; Rees, B.; Trott, D. Prevalence of bacterial species in cats with clinical signs of lower urinary tract disease: Recognition of Staphylococcus felis as a possible feline urinary tract pathogen. Vet. Microbiol. 2007, 121, 182–188. [Google Scholar] [CrossRef]
- Jordan, D. Antimicrobial ratings: The importance of importance. Aust. Vet. J. 2019, 97, 283–284. [Google Scholar] [CrossRef]
- Australian Strategic and Technical Advisory Group on Antimicrobial Resistance. Importance Ratings and Summary of Antibacterial Uses in Human and Animal Health in Australia. Commonwealth of Australia: Canberra, Australia. 2018. Available online: https://www.amr.gov.au/resources/importance-ratings-and-summary-antibacterial-uses-human-and-animal-health-australia (accessed on 10 November 2020).
- Weese, J.S.; Blondeau, J.M.; Boothe, D.; Breitschwerdt, E.B.; Guardabassi, L.; Hillier, A.; Lloyd, D.H.; Papich, M.G.; Rankin, S.C.; Turnidge, J.D.; et al. Antimicrobial Use Guidelines for Treatment of Urinary Tract Disease in Dogs and Cats: Antimicrobial Guidelines Working Group of the International Society for Companion Animal Infectious Diseases. Vet. Med. Int. 2011, 2011, 263768. [Google Scholar] [CrossRef] [Green Version]
- British Small Animal Veterinary Association. PROTECT ME. BSAVA/SAMSoc Guide to Responsible Use of Antibacterials: PROTECT ME. 2018. Available online: https://www.bsavalibrary.com/content/chapter/10.22233/9781910443644.chap6_1 (accessed on 24 November 2020).
- University of Melbourne. Australian Veterinary Prescribing Guidelines, Dogs and Cats. 2019. Available online: https://vetantibiotics.fvas.unimelb.edu.au/about/resources/ (accessed on 24 May 2020).
- Jessen, L.R.; Sørensen, T.M.; Lilja, Z.L.; Kristensen, M.; Hald, T.; Damborg, P. Antibiotic Use Guidelines for Companion Animal Practice, 2nd ed.; The Danish Small Animal Veterinary Association, Ed.; Companion Animal Group, Danish Veterinary Association: Frederiksberg, Denmark, 2019. [Google Scholar]
- Lacy, M.K.; Klutman, N.E.; Horvat, R.T.; Zapantis, A. Antibiograms: New NCCLS Guidelines, Development, and Clinical Application. Hosp. Pharm. 2004, 39, 542–553. [Google Scholar] [CrossRef]
- Halstead, C.D.; Gomez, N.; McCarter, Y.S. Reality of Developing a Community-Wide Antibiogram. J. Clin. Microbiol. 2004, 42, 1–6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hur, B.A.; Hardefeldt, L.Y.; Verspoor, K.; Baldwin, T.; Gilkerson, J.R. Describing the antimicrobial usage patterns of companion animal veterinary practices; free text analysis of more than 4.4 million consultation records. PLoS ONE 2020, 15, e0230049. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Charani, E.; Cooke, J.; Holmes, A. Antibiotic stewardship programmes—What’s missing? J. Antimicrob. Chemother. 2010, 65, 2275–2277. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blondeau, M.J.; Tillotson, G.S. Formula to help select rational antimicrobial therapy (FRAT): Its application to community- and hospital-acquired urinary tract infections. Int. J. Antimicrob. Agents 1999, 12, 145–150. [Google Scholar] [CrossRef]
- Hernandez, J.; Bota, D.; Farbos, M.; Bernardin, F.; Ragetly, G.; Médaille, C. Risk factors for urinary tract infection with multiple drug-resistant Escherichia coli in cats. J. Feline Med. Surg. 2013, 16, 75–81. [Google Scholar] [CrossRef]
- Turnidge, J.; Paterson, D.L. Setting and Revising Antibacterial Susceptibility Breakpoints. Clin. Microbiol. Rev. 2007, 20, 391–408. [Google Scholar] [CrossRef] [Green Version]
- Doern, V.G.; Brecher, S.M. The Clinical Predictive Value (or Lack Thereof) of the Results of In Vitro Antimicrobial Susceptibility Tests. J. Clin. Microbiol. 2011, 49 (Suppl. 9), S11–S14. [Google Scholar] [CrossRef] [Green Version]
- Abelson, B.; Sun, D.; Que, L.; A Nebel, R.; Baker, D.; Popiel, P.; Amundsen, C.L.; Chai, T.; Close, C.; Disanto, M.; et al. Sex differences in lower urinary tract biology and physiology. Biol. Sex. Differ. 2018, 9, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Sævik, B.K.; Trangerud, C.; Ottesen, N.; Sørum, H.; Eggertsdóttir, A.V. Causes of lower urinary tract disease in Norwegian cats. J. Feline Med. Surg 2011, 13, 410–417. [Google Scholar] [CrossRef]
- Piyarungsri, K.; Tangtrongsup, S.; Thitaram, N.; Lekklar, P.; Kittinuntasilp, A. Prevalence and risk factors of feline lower urinary tract disease in Chiang Mai, Thailand. Sci. Rep. 2020, 10, 196. [Google Scholar] [CrossRef] [Green Version]
- Dorsch, R.; Von Vopelius-Feldt, C.; Wolf, G.; Straubinger, R.K.; Hartmann, K. Feline urinary tract pathogens: Prevalence of bacterial species and antimicrobial resistance over a 10-year period. Vet. Rec. 2015, 176, 201. [Google Scholar] [CrossRef] [PubMed]
- Ball, K.R.; Rubin, J.E.; Chirino-Trejo, M.; Dowling, P.M. Antimicrobial resistance and prevalence of canine uropathogens at the Western College of Veterinary Medicine Veterinary Teaching Hospital, 2002–2007. Can. Vet. J. 2008, 49, 985–990. [Google Scholar]
- Marques, C.; Da Gama, L.T.; Belas, A.; Bergström, K.; Beurlet, S.; Briend-Marchal, A.; Broens, E.; Costa, M.; Criel, D.; Damborg, P.; et al. European multicenter study on antimicrobial resistance in bacteria isolated from companion animal urinary tract infections. BMC Vet. Res. 2016, 12, 213. [Google Scholar]
- McMeekin, C.H.; E Hill, K.; Gibson, I.R.; Bridges, J.P.; Benschop, J. Antimicrobial resistance patterns of bacteria isolated from canine urinary samples submitted to a New Zealand veterinary diagnostic laboratory between 2005–2012. N. Z. Vet. J. 2016, 65, 99–104. [Google Scholar] [CrossRef] [PubMed]
- Saputra, S.; Jordan, D.; Mitchell, T.; Wong, H.S.; Abraham, R.; Kidsley, A.; Turnidge, J.; Trott, D.J.; Abraham, S. Antimicrobial resistance in clinical Escherichia coli isolated from companion animals in Australia. Vet. Microbiol. 2017, 211, 43–50. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boothe, D.; Smaha, T.; Carpenter, D.M.; Shaheen, B.; Hatchcock, T. Antimicrobial resistance and pharmacodynamics of canine and feline pathogenic E. coli in the United States. J. Am. Anim. Hosp. Assoc. 2012, 48, 379–389. [Google Scholar] [CrossRef] [PubMed]
- Cummings, J.K.; Aprea, V.A.; Altier, C. Antimicrobial resistance trends among canine Escherichia coli isolates obtained from clinical samples in the northeastern USA, 2004–2011. Can. Vet. J. 2015, 56, 393–398. [Google Scholar] [PubMed]
- Yamanaka, A.R.; Hayakawa, A.T.; Rocha Ícaro, S.M.; Dutra, V.; Sousa, V.R.F.; Cruz, J.N.; Camargo, L.M.; Nakazato, L. The Occurrence of Multidrug Resistant Bacteria in the Urine of Healthy Dogs and Dogs with Cystitis. Animals 2019, 9, 1087. [Google Scholar] [CrossRef] [Green Version]
- Australian Commission on Safety and Quality in Healthcare. AURA 2019: Third Australian Report on Antimicrobial Use and Resistance in Human Health; Australian Commission on Safety and Quality in Healthcare: Sydney, Australia, 2019. [Google Scholar]
- Arsène, P.S.; Leclercq, R. Role of a qnr-Like Gene in the Intrinsic Resistance of Enterococcus faecalis to Fluoroquinolones. Antimicrob. Agents Chemother. 2007, 51, 3254–3258. [Google Scholar] [CrossRef] [Green Version]
- KuKanich, S.K.; Lubbers, B.V. Review of enterococci isolated from canine and feline urine specimens from 2006 to 2011. J. Am. Anim Hosp. Assoc. 2015, 51, 148–154. [Google Scholar] [CrossRef]
- Horsley, H.; Malone-Lee, J.; Holland, D.; Tuz, M.; Hibbert, A.; Kelsey, M.; Kupelian, A.; Rohn, J. Enterococcus faecalis Subverts and Invades the Host Urothelium in Patients with Chronic Urinary Tract Infection. PLoS ONE 2013, 8, e83637. [Google Scholar] [CrossRef] [PubMed]
- Hall, L.J.; Holmes, M.A.; Baines, S.J. Prevalence and antimicrobial resistance of canine urinary tract pathogens. Vet. Rec. 2013, 173, 549. [Google Scholar] [CrossRef] [PubMed]
- Papich, M.G. Antibiotic Treatment of Resistant Infections in Small Animals. Vet. Clin. N. Am. Small Anim. Pract. 2013, 43, 1091–1107. [Google Scholar] [CrossRef] [PubMed]
- Westropp, J.L.; Sykes, J.; Irom, S.; Daniels, J.; Smith, A.; Keil, D.; Settje, T.; Wang, Y.; Chew, D. Evaluation of the Efficacy and Safety of High Dose Short Duration Enrofloxacin Treatment Regimen for Uncomplicated Urinary Tract Infections in Dogs. J. Vet. Intern. Med. 2012, 26, 506–512. [Google Scholar] [CrossRef] [PubMed]
- Clare, S.; Hartmann, F.; Jooss, M.; Bachar, E.; Wong, Y.; Trepanier, L.; Viviano, K. Short- and long-term cure rates of short-duration trimethoprim-sulfamethoxazole treatment in female dogs with uncomplicated bacterial cystitis. J. Vet. Intern. Med. 2014, 28, 818–826. [Google Scholar] [CrossRef] [Green Version]
- Hall, I.A.; Campbell, K.L.; Chambers, M.D.; Davis, C.N. Effect of trimethoprim/sulfamethoxazole on thyroid function in dogs with pyoderma. J. Am. Vet. Med. Assoc. 1993, 202, 1959–1962. [Google Scholar]
- Noli, C.; Koeman, J.P.; Willemse, T. A retrospective evaluation of adverse reactions to trimethoprim-sulphonamide combinations in dogs and cats. Vet. Q. 1995, 17, 123–128. [Google Scholar] [CrossRef] [Green Version]
- Williamson, L.N.; Frank, L.A.; Hnilica, K.A. Effects of short-term trimethoprim-sulfamethoxazole administration on thyroid function in dogs. J. Am. Vet. Med. Assoc. 2002, 221, 802–806. [Google Scholar]
- Sykes, J.E. Antimicrobial Drug Use in Dogs and Cats. In Antimicrobial Therapy in Veterinary Medicine, 5th ed.; Giguère, S., Prescott, J.F., Dowling, P.M., Eds.; Wiley-Blackwell: Hoboken, NJ, USA, 2013; pp. 473–494. [Google Scholar]
- CDS Reference Laboratory. Antibiotic Susceptibility Testing by the CDS Method, 10th ed.; The CDS Reference Laboratory: Sydney, Australia, 2019; Available online: http://cdstest.net/wordpress/wp-content/uploads/10th-Edition-Modified-September-2019.pdf (accessed on 26 May 2020).
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2013; Available online: http://www.R-project.org/ (accessed on 30 March 2020).
- Wickham, H.; François, R.; Henry, L.; Müller, K.; RStudio. dplyr: A Grammar of Data Manipulation. Available online: https://CRAN.R-project.org/package=dplyr (accessed on 20 May 2020).
- Wickham, H.; Averick, M.; Bryan, J.; Chang, W.; McGowan, L.D.; François, R.; Grolemund, G.; Hayes, A.; Henry, L.; Hester, J.; et al. Welcome to the tidyverse. J. Open Source Softw. 2019, 4, 1686. [Google Scholar] [CrossRef]
- Chongsuvivatwong, V. epiDisplay: Epidemiological Data Display Package. Available online: https://CRAN.R-project.org/package=epiDisplay (accessed on 20 May 2020).
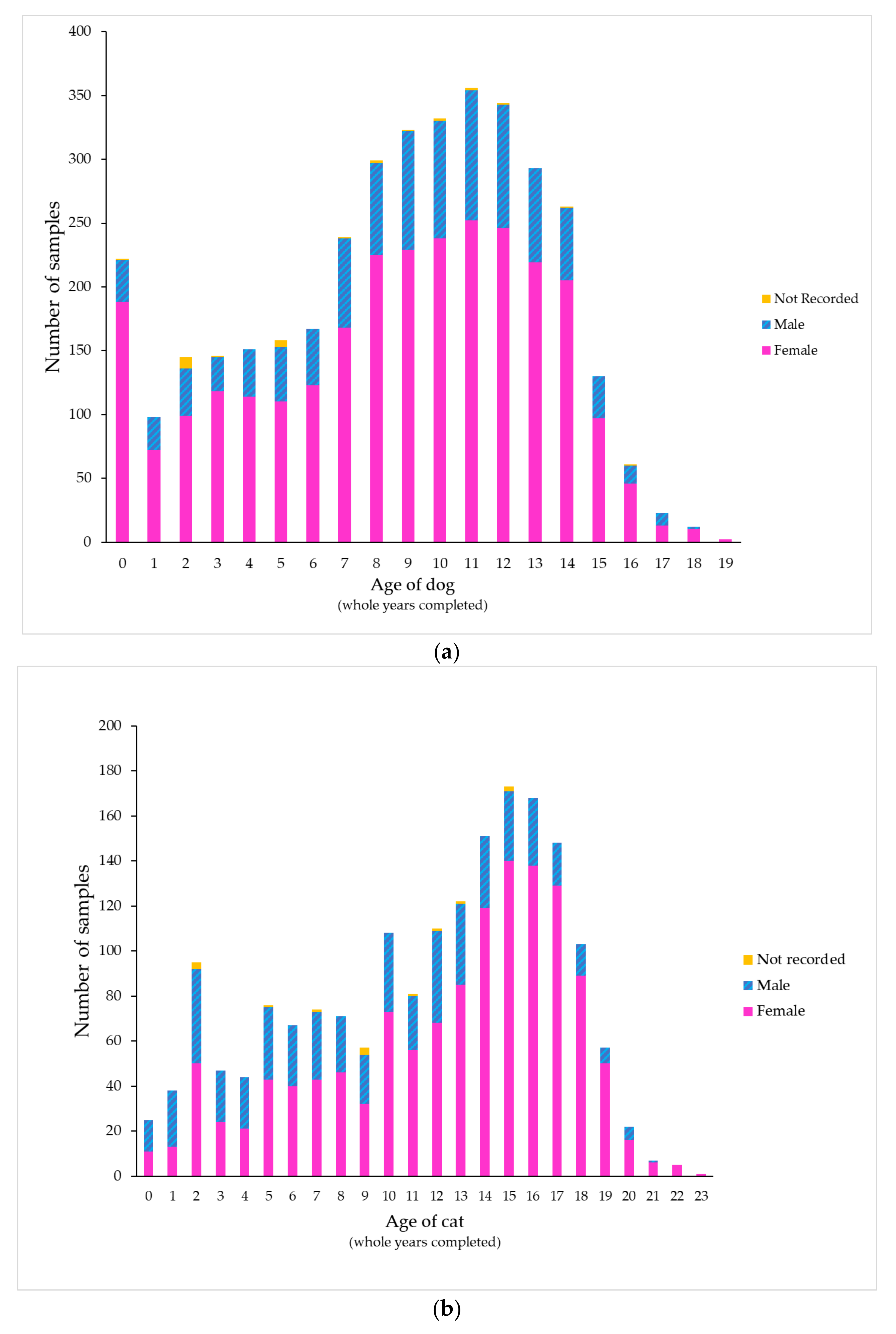



| Organism Species or Group | % of All Samples | Urine Collection Method, n (% as Pure Growth) | ||||
|---|---|---|---|---|---|---|
| Cystocentesis | Catheter | Free Catch | Not Stated | Total | ||
| Escherichia coli | 59% | 1004 (94) | 78 (89) | 357 (86) | 1855 (90) | 3294 (90) |
| Enterococcus faecalis | 11% | 192 (72) | 8 (63) | 63 (54) | 373 (55) | 636 (61) |
| Staphylococcus pseudintermedius | 9.1% | 155 (92) | 22 (96) | 55 (87) | 281 (75) | 513 (87) |
| Proteus mirabilis | 7.8% | 110 (86) | 8 (100) | 72 (81) | 250 (85) | 440 (88) |
| Proteus spp. | 5.5% | 80 (89) | 4 (50) | 43 (88) | 183 (78) | 310 (76) |
| Enterobacter spp. | 3.4% | 36 (91) | 8 (100) | 35 (88) | 111 (80) | 190 (88) |
| Coagulase-negative Staphylococcus spp. | 3.2% | 55 (91) | 8 (100) | 16 (88) | 98 (88) | 177 (92) |
| Streptococcus canis | 2.2% | 13 (69) | 8 (75) | 31 (55) | 73 (50) | 125 (62) |
| All other organisms | 8.2% | 82 | 13 | 55 | 309 | 459 |
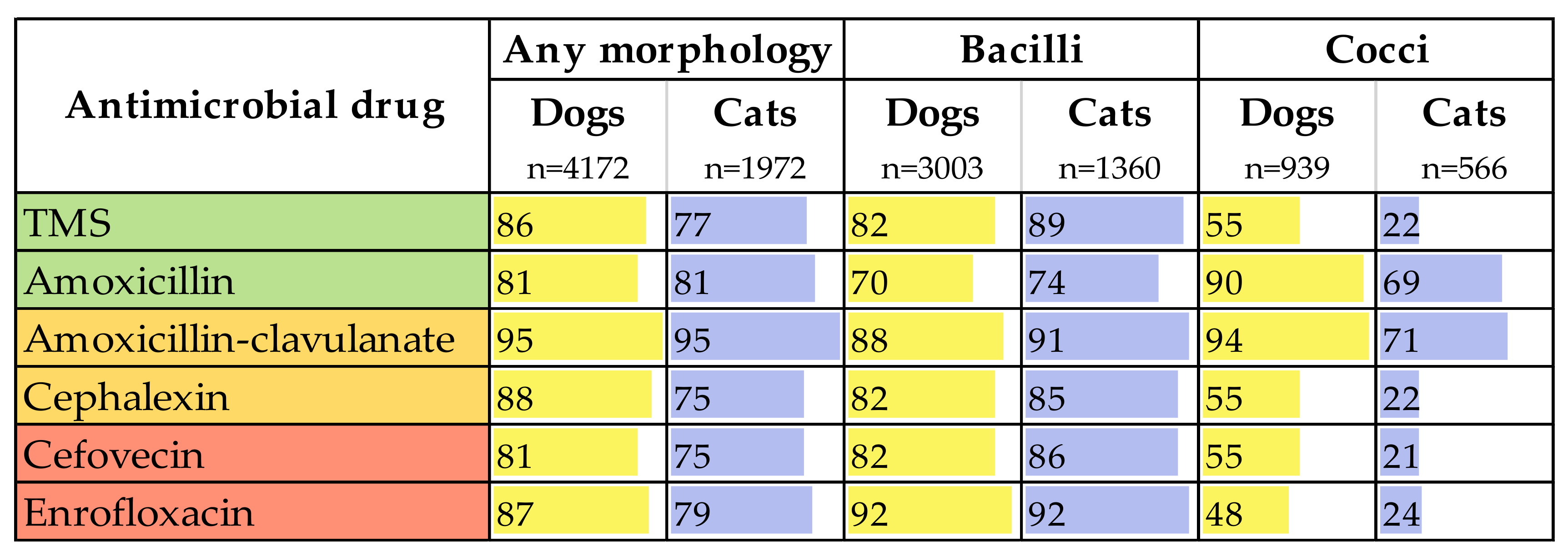
| Low-Importance Antimicrobials | Medium-Importance Antimicrobials | High-Importance Antimicrobials | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Organism | n | % of Dog Isolates | Amox | TMS | Tetra | Doxy | Erythro | Gentam | Amox-Clav | Ceph | Cefovecin | Enroflox |
| Escherichia coli | 2058 | 55% | 75% | 93% | 82% | 98% | IR | 93% | 97% | 90% | 90% | 95% |
| Proteus mirabilis | 401 | 11% | 91% | 94% | IR | IR | IR | 100% | 99% | 95% | 97% | 99% |
| Staphylococcus pseudintermedius | 380 | 10% | 91% | 93% | 76% | 85% | 75% | 89% | 98% | 95% | 95% | 98% |
| Enterococcus faecalis | 351 | 9.3% | 96% | IR | 54% | 91% | 76% | 95% * | 95% | IR | IR | 0.9% |
| Proteus spp. | 288 | 7.7% | 87% | 92% | 1.6% ** | 0% ** | IR | 100% | 98% | 96% | 94% | 98% |
| Enterobacter spp. | 157 | 4.2% | IR | 86% | 86% | 100% | IR | 100% | IR | IR | IR | 96% |
| Streptococcus canis | 111 | 2.9% | 96% | 96% | 84% | 97% | 89% | IR | 97% | 90% | 89% | 4.8% |
| Coagulase-negative Staphylococcus spp. | 89 | 2.4% | 87% | 93% | 86% | 86% | 93% | 95% | 97% | 94% | 92% | 98% |
| Low-Importance Antimicrobials | Medium-Importance Antimicrobials | High-Importance Antimicrobials | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Organism | n | % of Cat Isolates | Amox | TMS | Tetra | Doxy | Erythro | Gentam | Amox-Clav | Ceph | Cefovecin | Enroflox |
| Escherichia coli | 1236 | 67% | 80% | 94% | 82% | 82% | IR | 100% | 99% | 92% | 93% | 97% |
| Enterococcus faecalis | 285 | 15% | 98% | IR | 53% | 87% | 77% | 95% * | 99% | IR | IR | 1.4% |
| Staphylococcus pseudintermedius | 133 | 7.2% | 91% | 97% | 89% | 100% | 100% | 100% | 100% | 93% | 94% | 100% |
| Coagulase-negative Staphylococcus spp. | 88 | 4.8% | 98% | 100% | 100% | 100% | 100% | 100% | 99% | 98% | 98% | 100% |
| Proteus mirabilis | 39 | 2.1% | 87% | 92% | IR | IR | IR | 0.0% | 100% | 95% | 95% | 97% |
| Enterobacter spp. | 33 | 1.8% | IR | 76% | 100% | 100% | IR | 100% | IR | IR | IR | 97% |
| Proteus spp. | 22 | 1.2% | 82% | 73% | 0% ** | 0% ** | IR | 100% | 96% | 87% | 82% | 100% |
| Streptococcus canis | 14 | 0.8% | 100% | 86% | 86% | 100% | 100% | IR | 100% | 100% | 100% | 0% |
| Organism | Total Number of Isolates | Intrinsic Resistances | Isolates with no Acquired Resistance Detected n (%) | MDR = Resistance to Three or More of: | MDR n (%) | Most Common MDR Combination (n) | Prevalence of Most Common MDR Combination ## n (%) |
|---|---|---|---|---|---|---|---|
| Escherichia coli | 3294 | - | 2450 (74) | AMX, AMC, LEX, CVN, TET, GEN, FQN, SXT | 320 (9.7) | AMX + LEX + CVN | 276 (8.4) |
| Enterococcus faecalis | 636 | LEX, CVN, SXT | 22 (3.5) | AMX, TET, GEN*, ERY, FQN | 19 (3.0) | TET + ERY + FQN | 8 (1.3) |
| Staphylococcus pseudintermedius | 513 | - | 433 (84) | AMX, AMC, LEX, CVN, TET, GEN, FQN, SXT | 34 (6.6) | AMX + LEX + CVN | 28 (5.5) |
| Proteus mirabilis | 440 | TET | 378 (86) | AMX, AMC, LEX, CVN, FQN, SXT | 16 (3.6) | AMX + LEX + CVN | 13 (2.9) |
| Proteus spp. | 310 | # | 249 (80) | AMX, AMC, LEX, CVN, FQN, SXT | 16 (5.2) | AMX + LEX + CVN | 8 (2.6) |
| Enterobacter spp. | 190 | AMX, AMC, LEX, CVN | 157 (83) | Not assessable ** | |||
| Coagulase-negative Staphylococcus spp. | 177 | - | 155 (88) | AMX, AMC, LEX, CVN, TET, GEN, FQN, SXT | 8 (4.5) | AMX + LEX + CVN | 6 (3.4) |
| Strep. canis | 125 | GEN | 8 (6.4) | AMX, AMC, LEX, CVN, TET, GEN, FQN, SXT | 2 (1.6) | AMX + FQN + SXT | 1 (0.8) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Scarborough, R.; Bailey, K.; Galgut, B.; Williamson, A.; Hardefeldt, L.; Gilkerson, J.; Browning, G. Use of Local Antibiogram Data and Antimicrobial Importance Ratings to Select Optimal Empirical Therapies for Urinary Tract Infections in Dogs and Cats. Antibiotics 2020, 9, 924. https://doi.org/10.3390/antibiotics9120924
Scarborough R, Bailey K, Galgut B, Williamson A, Hardefeldt L, Gilkerson J, Browning G. Use of Local Antibiogram Data and Antimicrobial Importance Ratings to Select Optimal Empirical Therapies for Urinary Tract Infections in Dogs and Cats. Antibiotics. 2020; 9(12):924. https://doi.org/10.3390/antibiotics9120924
Chicago/Turabian StyleScarborough, Ri, Kirsten Bailey, Bradley Galgut, Adam Williamson, Laura Hardefeldt, James Gilkerson, and Glenn Browning. 2020. "Use of Local Antibiogram Data and Antimicrobial Importance Ratings to Select Optimal Empirical Therapies for Urinary Tract Infections in Dogs and Cats" Antibiotics 9, no. 12: 924. https://doi.org/10.3390/antibiotics9120924
APA StyleScarborough, R., Bailey, K., Galgut, B., Williamson, A., Hardefeldt, L., Gilkerson, J., & Browning, G. (2020). Use of Local Antibiogram Data and Antimicrobial Importance Ratings to Select Optimal Empirical Therapies for Urinary Tract Infections in Dogs and Cats. Antibiotics, 9(12), 924. https://doi.org/10.3390/antibiotics9120924







