Exposure of Mycobacterium avium subsp. homonissuis to Metal Concentrations of the Phagosome Environment Enhances the Selection of Persistent Subpopulation to Antibiotic Treatment
Abstract
:1. Introduction
2. Materials and Methods
2.1. Bacteria and Host Cells
2.2. Antibiotic Susceptibility and Killing Kinetics In Vitro
2.3. MAH104 Growth in Presence of Metals and in the Dropout Metal Mix
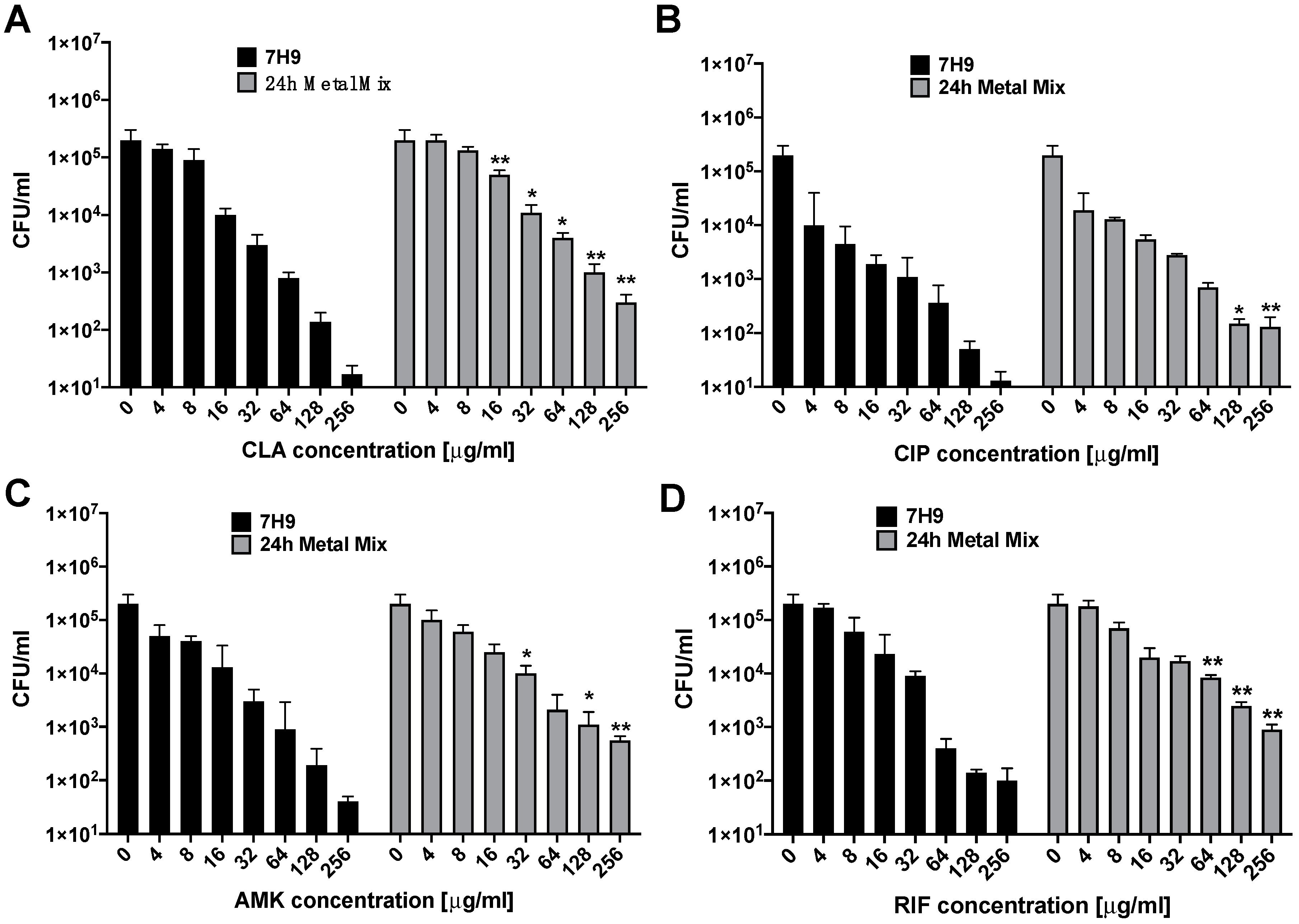
2.4. MAH104 Dose Response to Antibiotic Treatments before and after Exposure to Metal Mix
2.5. Macrophage Infection and Response to TNF-α and IFN-γ Activation
2.6. Statistical Analysis
3. Results
3.1. Susceptibility and Growth Dynamics of MAH104 to Antibiotics
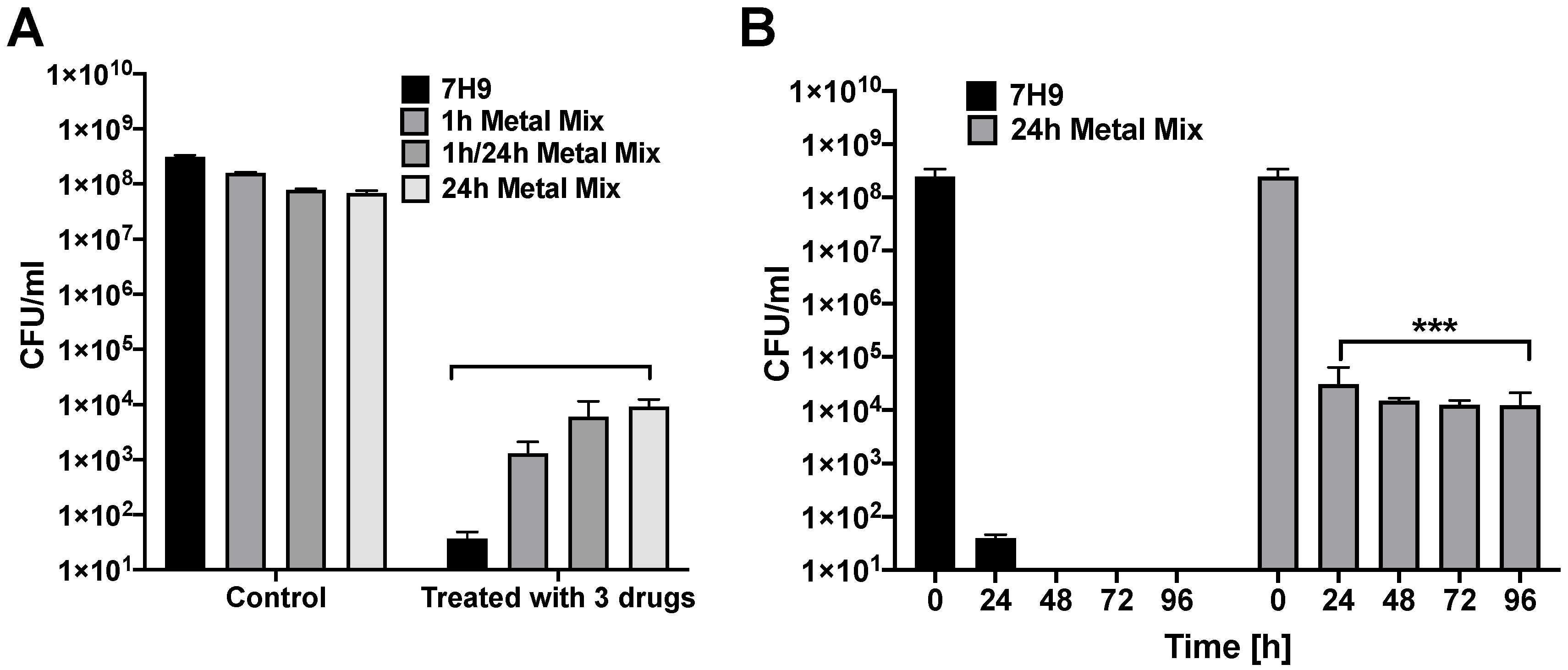
3.2. MAH104 Growth Curve in 7H9 Broth, 1 h and 24 h Metal Mix
3.3. Does MAH Exposure to an In Vitro Phagosome Model of Macrophage Lead to Selection of a Persistent Population?
3.4. Exposure to In Vitro Phagosome Models of Metal Environment Promote MAH104 Survival within Activated Macrophages
3.5. MAH104 Persistence in Metal Mix Lacking Specific Metals
4. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Griffith, D.E.; Aksamit, T.; Brown-Elliott, B.A.; Catanzaro, A.; Daley, C.; Gordin, F.; Holland, S.M.; Horsburgh, R.; Huitt, G.; Iademarco, M.F.; et al. An official ATS/IDSA statement: Diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am. J. Respir. Crit. Care Med. 2007, 175, 367–416. [Google Scholar] [CrossRef]
- Brode, S.K.; Daley, C.L.; Marras, T.K. The epidemiologic relationship between tuberculosis and non-tuberculous mycobacterial disease: A systematic review. Int. J. Tuberc. Lung Dis. 2014, 18, 1370–1377. [Google Scholar] [CrossRef]
- Appelberg, R. Pathogenesis of Mycobacterium avium infection: Typical responses to an atypical mycobacterium? Immunol. Res. 2006, 35, 179–190. [Google Scholar] [CrossRef]
- Awuh, J.A.; Flo, T.H. Molecular basis of mycobacterial survival in macrophages. Cell Mol. Life Sci. 2017, 74, 1625–1648. [Google Scholar] [CrossRef]
- Danelishvili, L.; Bermudez, L.E. Mycobacterium avium MAV_2941 mimics phosphoinositol-3-kinase to interfere with macrophage phagosome maturation. Microbes Infect. 2015, 17, 628–637. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prevots, D.R.; Marras, T.K. Epidemiology of human pulmonary infection with nontuberculous mycobacteria: A review. Clin. Chest Med. 2015, 36, 13–34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rojony, R.; Danelishvili, L.; Campeau, A.; Wozniak, J.M.; Gonzalez, D.J.; Bermudez, L.E. Exposure of Mycobacterium abscessus to Environmental Stress and Clinically Used Antibiotics Reveals Common Proteome Response among Pathogenic Mycobacteria. Microorganisms 2020, 8, 698. [Google Scholar] [CrossRef] [PubMed]
- Rojony, R.; Martin, M.; Campeau, A.; Wozniak, J.M.; Gonzalez, D.J.; Jaiswal, P.; Danelishvili, L.; Bermudez, L.E. Quantitative analysis of Mycobacterium avium subsp. hominissuis proteome in response to antibiotics and during exposure to different environmental conditions. Clin. Proteome. 2019, 16, 39. [Google Scholar] [CrossRef] [Green Version]
- Stewart, G.R.; Robertson, B.D.; Young, D.B. Tuberculosis: A problem with persistence. Nat. Rev. Microbiol. 2003, 1, 97–105. [Google Scholar] [CrossRef]
- Esmail, H.; Barry, C.E., 3rd; Young, D.B.; Wilkinson, R.J. The ongoing challenge of latent tuberculosis. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2014, 369, 20130437. [Google Scholar] [CrossRef] [Green Version]
- McKinney, J.D.; Honer zu Bentrup, K.; Munoz-Elias, E.J.; Miczak, A.; Chen, B.; Chan, W.T.; Swenson, D.; Sacchettini, J.C.; Jacobs, W.R., Jr.; Russell, D.G. Persistence of Mycobacterium tuberculosis in macrophages and mice requires the glyoxylate shunt enzyme isocitrate lyase. Nature 2000, 406, 735–738. [Google Scholar] [CrossRef] [PubMed]
- Ramage, H.R.; Connolly, L.E.; Cox, J.S. Comprehensive functional analysis of Mycobacterium tuberculosis toxin-antitoxin systems: Implications for pathogenesis, stress responses, and evolution. PLoS Genet. 2009, 5, e1000767. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, P.R.; Vijjamarri, A.K.; Sarkar, D. Metabolic Switching of Mycobacterium tuberculosis during Hypoxia Is Controlled by the Virulence Regulator PhoP. J. Bacteriol. 2020, 202, e00705-19. [Google Scholar] [CrossRef] [PubMed]
- Park, H.D.; Guinn, K.M.; Harrell, M.I.; Liao, R.; Voskuil, M.I.; Tompa, M.; Schoolnik, G.K.; Sherman, D.R. Rv3133c/dosR is a transcription factor that mediates the hypoxic response of Mycobacterium tuberculosis. Mol. Microbiol. 2003, 48, 833–843. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gautam, U.S.; Sikri, K.; Vashist, A.; Singh, V.; Tyagi, J.S. Essentiality of DevR/DosR interaction with SigA for the dormancy survival program in Mycobacterium tuberculosis. J. Bacteriol. 2014, 196, 790–799. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parker, H.; Lorenc, R.; Ruelas Castillo, J.; Karakousis, P.C. Mechanisms of Antibiotic Tolerance in Mycobacterium avium Complex: Lessons from Related Mycobacteria. Front. Microbiol. 2020, 11, 573983. [Google Scholar] [CrossRef]
- Wagner, D.; Maser, J.; Lai, B.; Cai, Z.; Barry, C.E., 3rd; Honer Zu Bentrup, K.; Russell, D.G.; Bermudez, L.E. Elemental analysis of Mycobacterium avium-, Mycobacterium tuberculosis-, and Mycobacterium smegmatis-containing phagosomes indicates pathogen-induced microenvironments within the host cell’s endosomal system. J. Immunol. 2005, 174, 1491–1500. [Google Scholar] [CrossRef] [Green Version]
- Wagner, D.; Maser, J.; Moric, I.; Boechat, N.; Vogt, S.; Gicquel, B.; Lai, B.; Reyrat, J.M.; Bermudez, L. Changes of the phagosomal elemental concentrations by Mycobacterium tuberculosis Mramp. Microbiology 2005, 151, 323–332. [Google Scholar] [CrossRef] [Green Version]
- Wagner, D.; Maser, J.; Moric, I.; Vogt, S.; Kern, W.V.; Bermudez, L.E. Elemental analysis of the Mycobacterium avium phagosome in Balb/c mouse macrophages. Biochem. Biophys. Res. Commun. 2006, 344, 1346–1351. [Google Scholar] [CrossRef]
- Kurthkoti, K.; Amin, H.; Marakalala, M.J.; Ghanny, S.; Subbian, S.; Sakatos, A.; Livny, J.; Fortune, S.M.; Berney, M.; Rodriguez, G.M. The Capacity of Mycobacterium tuberculosis To Survive Iron Starvation Might Enable It to Persist in Iron-Deprived Microenvironments of Human Granulomas. mBio 2017, 8, e01092-17. [Google Scholar] [CrossRef] [Green Version]
- Marcela Rodriguez, G.; Neyrolles, O. Metallobiology of Tuberculosis. Microbiol. Spectr. 2014, 2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Neyrolles, O.; Wolschendorf, F.; Mitra, A.; Niederweis, M. Mycobacteria, metals, and the macrophage. Immunol. Rev. 2015, 264, 249–263. [Google Scholar] [CrossRef] [PubMed]
- Early, J.; Bermudez, L.E. Mimicry of the pathogenic mycobacterium vacuole in vitro elicits the bacterial intracellular phenotype, including early-onset macrophage death. Infect. Immun. 2011, 79, 2412–2422. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McGarvey, J.; Bermudez, L.E. Pathogenesis of nontuberculous mycobacteria infections. Clin. Chest Med. 2002, 23, 569–583. [Google Scholar] [CrossRef]
- Field, S.K.; Cowie, R.L. Treatment of Mycobacterium avium-intracellulare complex lung disease with a macrolide, ethambutol, and clofazimine. Chest 2003, 124, 1482–1486. [Google Scholar] [CrossRef] [Green Version]
- Goring, S.M.; Wilson, J.B.; Risebrough, N.R.; Gallagher, J.; Carroll, S.; Heap, K.J.; Obradovic, M.; Loebinger, M.R.; Diel, R. The cost of Mycobacterium avium complex lung disease in Canada, France, Germany, and the United Kingdom: A nationally representative observational study. BMC Health Serv. Res. 2018, 18, 700. [Google Scholar] [CrossRef] [Green Version]
- Kwak, N.; Park, J.; Kim, E.; Lee, C.H.; Han, S.K.; Yim, J.J. Treatment Outcomes of Mycobacterium avium Complex Lung Disease: A Systematic Review and Meta-analysis. Clin. Infect. Dis. 2017, 65, 1077–1084. [Google Scholar] [CrossRef]
- Park, Y.; Lee, E.H.; Jung, I.; Park, G.; Kang, Y.A. Clinical characteristics and treatment outcomes of patients with macrolide-resistant Mycobacterium avium complex pulmonary disease: A systematic review and meta-analysis. Respir. Res. 2019, 20, 286. [Google Scholar] [CrossRef] [Green Version]
- Lee, B.Y.; Kim, S.; Hong, Y.; Lee, S.D.; Kim, W.S.; Kim, D.S.; Shim, T.S.; Jo, K.W. Risk factors for recurrence after successful treatment of Mycobacterium avium complex lung disease. Antimicrob. Agents Chemother. 2015, 59, 2972–2977. [Google Scholar] [CrossRef] [Green Version]
- Kwon, Y.S.; Koh, W.J.; Daley, C.L. Treatment of Mycobacterium avium Complex Pulmonary Disease. Tuberc. Respir. Dis. 2019, 82, 15–26. [Google Scholar] [CrossRef]
- Silva, C.; Rojony, R.; Bermudez, L.E.; Danelishvili, L. Short-Chain Fatty Acids Promote Mycobacterium avium subsp. hominissuis Growth in Nutrient-Limited Environments and Influence Susceptibility to Antibiotics. Pathogens 2020, 9, 700. [Google Scholar] [CrossRef] [PubMed]
- Kussell, E.; Kishony, R.; Balaban, N.Q.; Leibler, S. Bacterial persistence: A model of survival in changing environments. Genetics 2005, 169, 1807–1814. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harms, A.; Maisonneuve, E.; Gerdes, K. Mechanisms of bacterial persistence during stress and antibiotic exposure. Science 2016, 354. [Google Scholar] [CrossRef] [PubMed]
- Windels, E.M.; Michiels, J.E.; Fauvart, M.; Wenseleers, T.; Van den Bergh, B.; Michiels, J. Bacterial persistence promotes the evolution of antibiotic resistance by increasing survival and mutation rates. ISME J. 2019, 13, 1239–1251. [Google Scholar] [CrossRef] [PubMed]
- Levin-Reisman, I.; Ronin, I.; Gefen, O.; Braniss, I.; Shoresh, N.; Balaban, N.Q. Antibiotic tolerance facilitates the evolution of resistance. Science 2017, 355, 826–830. [Google Scholar] [CrossRef] [PubMed]
- Brown-Elliott, B.A.; Nash, K.A.; Wallace, R.J., Jr. Antimicrobial susceptibility testing, drug resistance mechanisms, and therapy of infections with nontuberculous mycobacteria. Clin. Microbiol. Rev. 2012, 25, 545–582. [Google Scholar] [CrossRef] [Green Version]
- Goldstein, B.P. Resistance to rifampicin: A review. J. Antibiot. 2014, 67, 625–630. [Google Scholar] [CrossRef] [Green Version]
- Hu, Y.; Coates, A.R. Enhancement by novel anti-methicillin-resistant Staphylococcus aureus compound HT61 of the activity of neomycin, gentamicin, mupirocin and chlorhexidine: In vitro and in vivo studies. J. Antimicrob. Chemother. 2013, 68, 374–384. [Google Scholar] [CrossRef] [Green Version]
- Walters, M.C., 3rd; Roe, F.; Bugnicourt, A.; Franklin, M.J.; Stewart, P.S. Contributions of antibiotic penetration, oxygen limitation, and low metabolic activity to tolerance of Pseudomonas aeruginosa biofilms to ciprofloxacin and tobramycin. Antimicrob. Agents Chemother. 2003, 47, 317–323. [Google Scholar] [CrossRef] [Green Version]
- Lisher, J.P.; Giedroc, D.P. Manganese acquisition and homeostasis at the host-pathogen interface. Front. Cell Infect. Microbiol. 2013, 3, 91. [Google Scholar] [CrossRef] [Green Version]
- De Voss, J.J.; Rutter, K.; Schroeder, B.G.; Su, H.; Zhu, Y.; Barry, C.E., 3rd. The salicylate-derived mycobactin siderophores of Mycobacterium tuberculosis are essential for growth in macrophages. Proc. Natl. Acad. Sci. USA 2000, 97, 1252–1257. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Juttukonda, L.J.; Skaar, E.P. Manganese homeostasis and utilization in pathogenic bacteria. Mol. Microbiol. 2015, 97, 216–228. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mashabela, G.T.; de Wet, T.J.; Warner, D.F. Mycobacterium tuberculosis Metabolism. Microbiol. Spectr. 2019, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| Supplements | Metal Mix (mL/L of 7H9 Broth) | Dropout 24 h Metal Mix (mL/L of 7H9 Broth) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 h | 24 h | -Ni | -Fe | -Zn | -Cu | -Mn | -Ca | -K | |
| 1 M potassium chloride (KCl) | 14.7 | 0.925 | 0.925 | 0.925 | 0.925 | 0.925 | 0.925 | 0.925 | - |
| 1 M calcium chloride (CaCl2) | 2 | 1.25 | 1.25 | 1.25 | 1.25 | 1.25 | 1.25 | - | 1.25 |
| 1 M manganese chloride (MnCl2) | 5.9 | 11.9 | 11.9 | 11.9 | 11.9 | 11.9 | - | 11.9 | 11.9 |
| 1 M copper sulfate (CuSO4) | 1.85 | 5.5 | 5.5 | 5.5 | 5.5 | - | 5.5 | 5.5 | 5.5 |
| 1 M zinc chloride (ZnCl2) | 33 | 58.7 | 58.7 | 58.7 | - | 58.7 | 58.7 | 58.7 | 58.7 |
| 0.25 M ferric pyrophosphate (FePO4) | 288 | 2 | 2 | - | 2 | 2 | 2 | 2 | 2 |
| 1 M nickel chloride (NiCl2) | 5 | 5 | - | 5 | 5 | 5 | 5 | 5 | 5 |
| Antibiotic | M. avium subsp. hominissuis Strain 104 | |
|---|---|---|
| MIC (μg/mL) | BC (μg/mL) | |
| Amikacin (AMK) | 1 | 4 |
| Clarithromycin (CLA) | 1 | 16 |
| Ciprofloxacin (CIP) | 0.5 | 8 |
| Rifampicin (RIF) | 8 | 32 |
| Condition | Invasion (1 h) | Intracellular MAH104 (CFU/Well) at 48 h | ||
|---|---|---|---|---|
| No Treatment | TNF-α | IFN-γ | ||
| 7H9 | 1.8 ± 0.3 × 105 | 3.6 ± 0.4 × 105 | 6.1 ± 0.3 × 103 (1) | 2.7 ± 0.3 × 104 (1) |
| 1 h Metal Mix | 5.0 ± 0.6 × 105 | 6.4 ± 06 × 105 | 5.6 ± 0.4 × 104 (1,2) | 8.8 ± 0.3 × 104 (1,2) |
| 24 h Metal Mix | 7.1 ± 0.4 × 104 | 1.8 ± 0.4 × 105 | 1.9 ± 0.3 × 105 | 1.8 ± 0.5 × 105 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Danelishvili, L.; Armstrong, E.; Miyasako, E.; Jeffrey, B.; Bermudez, L.E. Exposure of Mycobacterium avium subsp. homonissuis to Metal Concentrations of the Phagosome Environment Enhances the Selection of Persistent Subpopulation to Antibiotic Treatment. Antibiotics 2020, 9, 927. https://doi.org/10.3390/antibiotics9120927
Danelishvili L, Armstrong E, Miyasako E, Jeffrey B, Bermudez LE. Exposure of Mycobacterium avium subsp. homonissuis to Metal Concentrations of the Phagosome Environment Enhances the Selection of Persistent Subpopulation to Antibiotic Treatment. Antibiotics. 2020; 9(12):927. https://doi.org/10.3390/antibiotics9120927
Chicago/Turabian StyleDanelishvili, Lia, Elyssa Armstrong, Emily Miyasako, Brendan Jeffrey, and Luiz E. Bermudez. 2020. "Exposure of Mycobacterium avium subsp. homonissuis to Metal Concentrations of the Phagosome Environment Enhances the Selection of Persistent Subpopulation to Antibiotic Treatment" Antibiotics 9, no. 12: 927. https://doi.org/10.3390/antibiotics9120927
APA StyleDanelishvili, L., Armstrong, E., Miyasako, E., Jeffrey, B., & Bermudez, L. E. (2020). Exposure of Mycobacterium avium subsp. homonissuis to Metal Concentrations of the Phagosome Environment Enhances the Selection of Persistent Subpopulation to Antibiotic Treatment. Antibiotics, 9(12), 927. https://doi.org/10.3390/antibiotics9120927






