In vitro Antibiotic and Modulatory Activity of Mesosphaerum suaveolens (L.) Kuntze against Candida strains
Abstract
1. Introduction
2. Results
2.1. Phytochemical Analysis
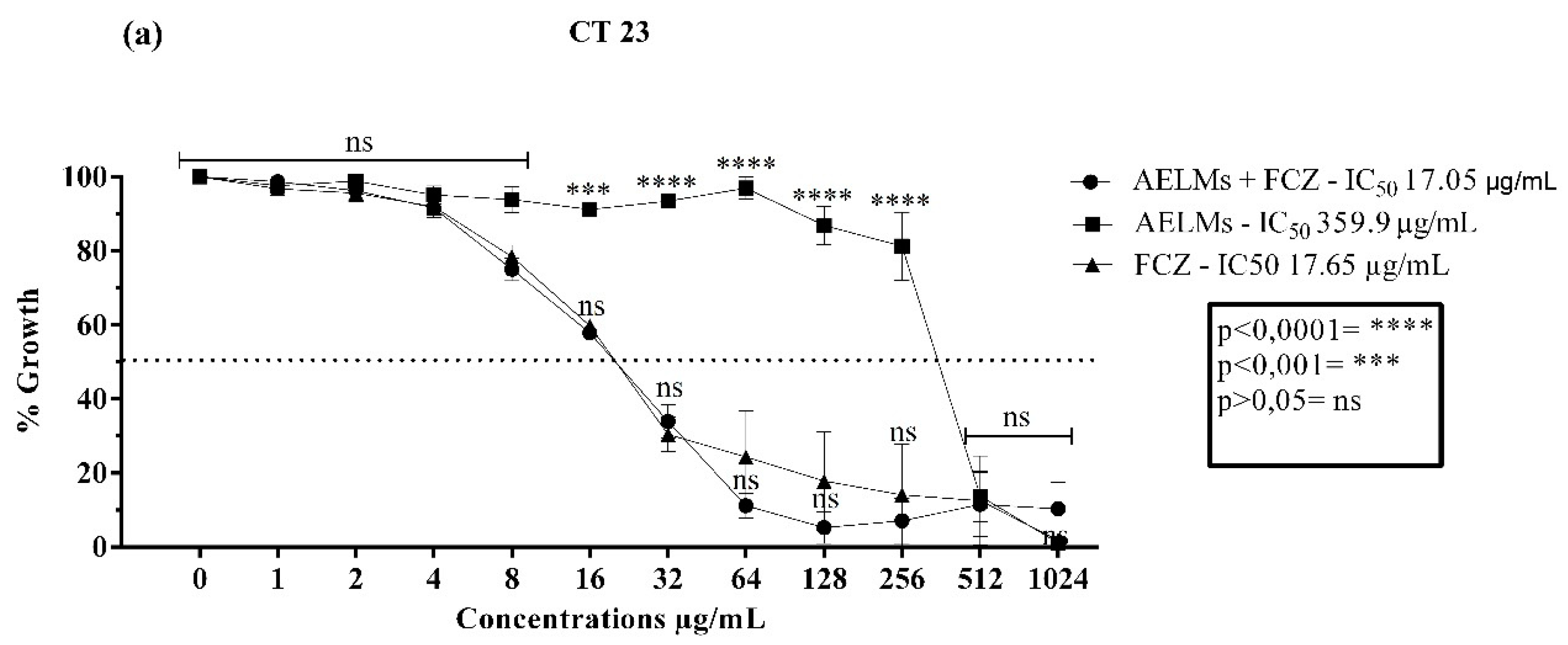
2.2. Microbiological Activity
3. Discussion
4. Materials and Methods
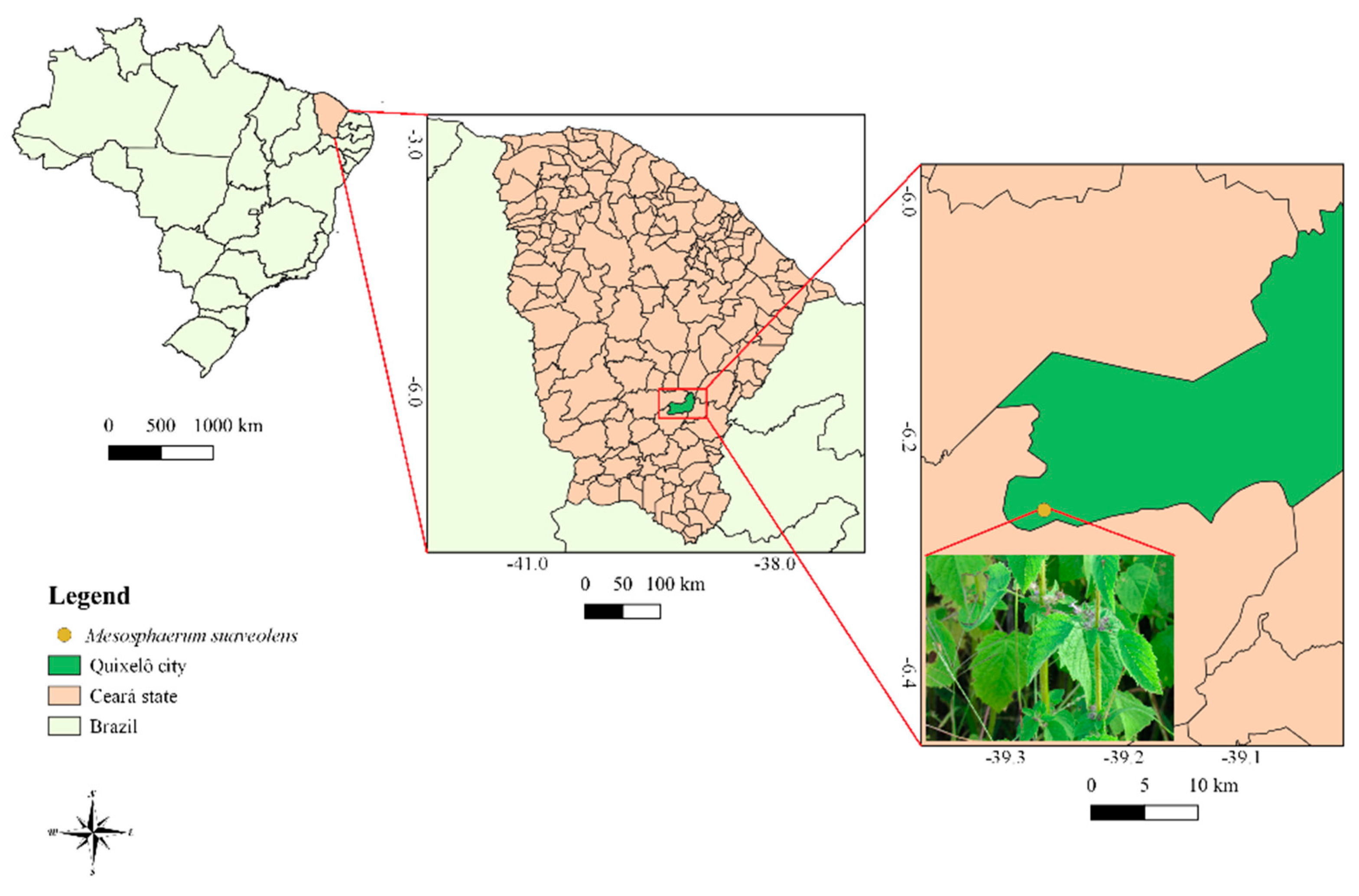
4.1. Plant Collection
4.2. Preparation of the Mesosphaerum Suaveolens Extracts
4.3. Quantification of Compounds by HPLC-DAD
4.4. Antifungal Activity
4.4.1. Fungal Strains and Culture Media Used
4.4.2. Drugs, Reagents, and Solution Preparation
4.4.3. IC50 Determination and Cellular Viability Curve
4.4.4. Modulatory Effect of Extracts from M. Suaveolens
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ziarrusta, G.B. Vulvogaginitis candidiásica. Rev. Iberoam. Micol. 2002, 19, 22–24. [Google Scholar]
- Morais-Braga, M.F.B.; Souza, T.M.; Santos, K.K.; Guedes, G.M.; Andrade, J.C.; Tintino, S.R.; Costa, J.G.M.; Menezes, I.R.A.; Saraiva, A.Á.F.; Coutinho, H.D.M. Atividade antibacteriana, antifúngica e moduladora da atividade antimicrobiana de frações obtidas de Lygodium venustum SW. B Latinoam. Caribe. Pl. 2013, 12, 38–43. [Google Scholar]
- Costa, A.R.; Silva, J.L.; Lima, K.R.R.; Rocha, M.I.; Barros, L.M.; Costa, J.G.M.; et al. Rhaphiodon echinus (Nees & Mart.) Schauer: Chemical, toxicological activity and increased antibiotic activity of antifungal drug activity and antibacterial. Microb. Pathogenesis. 2017, 107, 280–286. [Google Scholar]
- Bezerra, J.W.A.; Costa, A.R.; Freitas, M.A.; Rodrigues, F.C.; Souza, M.A.; Silva, A.R.P.; Santos, A.T.L.; Linhares, K.V.; Coutinho, H.D.M.; Silva, J.R.L.; et al. Chemical composition, antimicrobial, modulator and antioxidant activity of essential oil of Dysphania ambrosioides (L.) Mosyakin & Clemants. Comp. Immunol. Microb. 2019, 65, 58–64. [Google Scholar]
- Navarro-Arias, M.J.; Hernández-Chávez, M.J.; Garcia-Carnero, L.C.; Amezcua-Hernández, D.G.; Lozoya-Pérez, N.E.; Martínez-Duncker, I.; Franco, B.; Mora-Montes, H.M. Differential recognition of Candida tropicalis, Candida guilliermondii, Candida krusei, and Candida auris by human innate immune cells. Infect. Drug Resist. 2019, 12, 783–794. [Google Scholar] [CrossRef]
- Ferracin, I.; Oliveira, R.M.W. de. Corrimento vaginal: Causa, diagnóstico e tratamento farmacológico. Infarma 2005, 17, 82–86. [Google Scholar]
- Vasconcelos, C.N.E.D.; Silva, N.N.P.; Batista, P.N.; Souza, J.H.K.D. Estudo comparativo entre terapia oral e local no tratamento de corrimentos vaginais: Candidíase, tricomoníase e vaginose bacteriana. Braz. J. Surg. Clin. Res. 2016, 15, 123–128. [Google Scholar]
- Barbedo, L.S.; Sgarbi, D.B.G. Candidíase. J. Bras. Doenças Sex. Transm. 2010, 22, 22–38. [Google Scholar]
- Alnuaimi, A.D.; Wiesenfeld, D.; O’Brien-Simpson, N.M.; Reynolds, E.C.; McCullough, M.J. Oral Candida colonization in oral cancer patients and its relationship with traditional risk factors of oral cancer: A matched case-control study. Oral Oncol. 2015, 51, 139–145. [Google Scholar] [CrossRef] [PubMed]
- Mendling, W. Guideline: Vulvovaginal candidosis (AWMF 015/072), S2k (excluding chronic mucocutaneous candidosis). Mycoses 2015, 58, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Gama, M.R.D.; Silva, T.F.N.; Calixto, I.F.A.P.M.; Peixoto, F.B.; Ribeiro, C.M.B. CANDIDÍASE PSEUDOMEMBRANOSA ORAL EM NEONATO: Relato de caso. Revista da AcBO. 2017, 7, 116–120. [Google Scholar]
- Wiederhold, N.P. Antifungal resistance: Current trends and future strategies to combat. Infect. Drug Resist. 2017, 10, 249–259. [Google Scholar] [CrossRef] [PubMed]
- Vieira, A.J.H.; Santos, J.I. Mecanismos de resistência de Candida albicans aos antifúngicos anfotericina B, fluconazol e caspofungina. RBAC 2017, 49, 235–239. [Google Scholar] [CrossRef]
- Murugesh, J.; Annigeri, R.G.; Mangala, G.K.; Mythily, P.H.; Chandrakala, J. Evaluation of the antifungal efficacy of different concentrations of Curcuma longa on Candida albicans: An in vitro study. J. Oral Maxillofac. Pathol. 2019, 23, 305–311. [Google Scholar] [CrossRef] [PubMed]
- Goes, F.T.Z.; Gonçalves, A.P.P.; Cunha, P.N.A.; Deus Vieira, G.; Nicolete, R.; Hernandez, A.E.F.; Teles, C.B.G. Prospecção fitoquímica e antimicrobiana dos extratos de Lantana camara L. e Lantana trifolia L.(prospecção fitoquímica e antimicrobiana de L. camara e L. trifolia). Saber. Científico. 2016, 5, 1–11. [Google Scholar]
- Eddouzi, J.; Parker, J.E.; Vale-Silva, L.A.; Coste, A.; Ischer, F.; Kelly, S.; Manai, M.; Sanglard, D. Molecular mechanisms of drug resistance in clinical Candida species isolated from Tunisian hospitals. Antimicrob. Agents Chemother. 2013, 57, 3182–3193. [Google Scholar] [CrossRef]
- Calixto Júnior, J.T.; Morais, S.M.; Martins, C.G.; Vieira, L.G.; Morais-Braga, M.F.B.; Carneiro, J.N.; Machado, A.J.; Menezes, I.R.A.; Tintino, S.R.; Coutinho, H.D.M. Phytochemical analysis and modulation os antibiotic activity by Luehea paniculata Mart. & Zucc. (Malvaceae) in multiresistant clinical isolates of Candida Spp. Biomed. Res. Int. 2015, 2015, 1–10. [Google Scholar]
- Coutinho, H.D.; Costa, J.G.; Lima, E.O.; Falcão-Silva, V.S.; Siqueira-Júnior, J.P. Enhancement of the antibiotic activity against a multiresistant Escherichia coli by Mentha arvensis L. and chlorpromazine. Chemotherapy 2008, 54, 328–330. [Google Scholar] [CrossRef]
- Duarte, A.E.; Waczuk, E.P.; Roversi, K.; Silva, M.A.P.; Barros, L.M.; Cunha, F.A.B.; Menezes, I.R.A.; Costa, J.G.M.; Boligon, A.A.; Ademiluyi, A.O.; et al. Polyphenolic composition and evaluation of antioxidant activity, osmotic fragility and cytotoxic effects of Raphiodon echinus (Nees & Mart.) Schauer. Molecules 2016, 21, 1–15. [Google Scholar]
- Souza, L.K.H.; Oliveira, C.M.O.; Ferri, P.H.; Júnior, J.G.O.; Júnior, A.H.S.; Fátima, O.; Silva, M.R.R. Antimicrobial Activity of Hyptis ovalifolia Towards Dermatophytes. Mem. Inst. Oswaldo. Cruz. 2003, 98, 963–965. [Google Scholar] [CrossRef]
- Coutinho, H.D.; Brito, S.M.; Leite, N.F.; Vandesmet, V.C.; Oliveira, M.T.; Martins, G.M.; Silva, A.R.P.; Costa, M.S. Avaliação comparativa da modulação de antibióticos, frente às cepas bacterianas de Escherichia coli, Staphylococcus aureus. Rev. Cien. Salud. 2015, 13, 345–354. [Google Scholar] [CrossRef][Green Version]
- Bezerra, J.W.A.; Costa, A.R.; Silva, M.A.P.; Rocha, M.I.; Boligon, A.A.; Rocha, J.B.T.; Barros, L.M.; Kamdem, J.P. Chemical composition and toxicological evaluation of Hyptis suaveolens (L.) Poiteau (LAMIACEAE) in Drosophila melanogaster and Artemia salina. S. Afr. J. Bot. 2017, 113, 437–442. [Google Scholar] [CrossRef]
- Bezerra, J.W.A.; Costa, A.R.; Rodrigues, F.C.; Cunha, F.A.B.; Silva, V.B.; Boligon, A.A.; Anraku, M.M. Mesosphaerum suaveolens (L.) Kuntze (bamburral): Planta medicinal com potencial antioxidante e rica em polifenóis. Rev. Cub. Plant. Medic. 2018, 24, 1–15. [Google Scholar]
- Sharma, A.; Singh, H.P.; Batish, D.R.; Kohli, R.K. Chemical profiling, cytotoxicity and phytotoxicity of foliar volatiles of Hyptis suaveolens. Ecotox. Environ. Safe. 2019, 171, 863–870. [Google Scholar] [CrossRef] [PubMed]
- Adjou, E.S.; Chougourou, D.; Soumanou, M.M. Insecticidal and repellent effects of essential oils from leaves of Hyptis suaveolens and Ocimum canum against Tenebroides mauritanicus (L.) isolated from peanut in post-harvest. J. Cons. Protect. F. Saf. 2019, 14, 25–30. [Google Scholar] [CrossRef]
- Batista, J.M.; Birman, E.G.; Cury, A.E. Suscetibilidade a antifúngicos de cepas de Candida albicans isoladas de pacientes com estomatite protética. Rev. odontol. 1999, 13, 343–348. [Google Scholar] [CrossRef]
- Castro, T.L.; Coutinho, H.D.M.; Gedeon, C.C.; Santos, J.D.; Santana, W.J.; Souza, L.D. Mecanismos de resistência da Candida Sp. Wwa antifúngicos. Infarma 2006, 18, 30–35. [Google Scholar]
- Jesus, N.Z.T.; Falcão, H.S.; Lima, G.R.M.; Caldas-Filho, M.R.D.; Sales, I.R.P.; Gomes, I.F.; Santos, S.G.; Tavares, J.F.; Barbosa-Filho, J.M.; Batista, L.M. Hyptis suaveolens (L.) Poit (Lamiaceae), a medicinal plant protects the stomach against several gastric ulcer models. J. Ethnopharmacol. 2013, 150, 982–988. [Google Scholar] [CrossRef]
- Ghaffari, H.; Ghassam, B.J.G.; Prakash, H.S. Hepatoprotective and cytoprotective properties of Hyptis suaveolens against oxidative stress-induced damage by CCl4 and H2O2. Asian. Pac. J. Trop. Med. 2013, 5, 868–874. [Google Scholar] [CrossRef]
- Malele, R.S.; Mutayabarwa, C.K.; Mwangi, J.W.; Thoithi, G.N.; Lopez, A.G.; Lucini, E.I.; Zygadlo, J.A. Essential oil of Hyptis suaveolens (L.) Poit. from Tanzania: Composition and antifungal activity. J. Essent. Oil Res. 2003, 15, 438–440. [Google Scholar] [CrossRef]
- Bachheti, R.; Rai, I.; Joshi, A.; Satyan, R. Chemical composition and antimicrobial activity of Hyptis suaveolens Poit. seed oil from Uttarakhand State, India, Oriental. Ph. Exp. Med. 2015, 15, 141–146. [Google Scholar]
- Jaya, P.S.; Bhanu-Prakash, N.K.D. Insecticidal activity of Ageratum conyzoides L., Coleus aromaticus Benth. and Hyptis suaveolens (L.) Poit essencial oils as fumigant against storage grain insect Triobolium castaneum Herbs. J. Sci. Tecnol. 2014, 51, 2210–2215. [Google Scholar]
- Sakthivadivel, M.; Ganasekaran, P.; Sivakumar, M.; Arivoli, S.; Raveen, R.; Tennyson, S. Mosquito larvicidal activity of Hyptis suaveolens (L.) Poit (Lamiaceae) aerial extracts against the filarial vector Culex quinquefasciatus Say (Diptera: Culicidae). J. Med. Plant Studies 2015, 3, 1–5. [Google Scholar]
- Magalhães, K.N.; Guarniz, W.A.S.; Sá, K.M.; Freire, A.B.; Monteiro, M.P.; Nojosa, R.T.; Bieski, I.G.C.; Custódio, J.B.; Balogun, S.O.; et al. Medicinal plants of the Caatinga, northeastern Brazil: Ethnopharmacopeia (1980–1990) of the late professor Francisco José de Abreu Matos. J. Ethnopharmacol. 2019, 237, 314–353. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, R.V.; Bieski, I.G.C.; Balogun, S.O.; Martins, D.T.O. Ethnobotanical study of medicinal plants used by Ribeirinhos in the North Araguaia microregion, Mato Grosso, Brazil. J. Ethnopharmacol. 2017, 205, 69–102. [Google Scholar] [CrossRef]
- Anand, J.; Rai, N. Anticandidal synergistic activity of green tea catechins, antimycotics and copper sulphate as a mean of combinational drug therapy against candidiasis. J. Mycol. Med. 2017, 27, 33–45. [Google Scholar] [CrossRef]
- Gadkari, P.V.; Balaraman, M. Catechins: Sources, extraction and encapsulation: A review. Food Bioprod. Process. 2015, 93, 122–138. [Google Scholar] [CrossRef]
- Zhou, X.; Wang, F.; Zhou, R.; Song, X.; Xie, M. Apigenin: A current review on its beneficial biological activities. J. Food Biochem. 2017, 41, 1–11. [Google Scholar] [CrossRef]
- Mbatchou, V.C.; Abdullatif, S.; Glover, R. Phytochemical screening of solvent extracts from Hyptis suaveolens LAM for fungal growth inhibition. Pak. J. Nutr. 2010, 9, 358–361. [Google Scholar] [CrossRef][Green Version]
- Shi, C.; Liu, J.; Li, W.; Zhao, Y.; Meng, L.; Xiang, M. Expression of fluconazole resistance-associated genes in biofilm from 23 clinical isolates of Candida albicans. Brazilian J. Microbio. 2019, 50, 157–163. [Google Scholar] [CrossRef]
- Córdoba, S.; Taverna, C.; Vivot, W.; Szusz, W.; Vivot, M.; Isla, G.; Davel, G. Emergence of Resistance to Fluconazole in Candida albicans Isolated from Vaginal Discharge. Curr. Fungal Infect. Rep. 2018, 12, 155–160. [Google Scholar] [CrossRef]
- Cruz, R.P.; Freitas, F.T.; Costa, S.; Santos, L.; Thassya, A.; Campina, F.F.; Pereira, R.L.S.; Bezerra, J.W.A.; Quintans-Júnior, L.J.; Araújo, A.A.S.; et al. Effect of α-Bisabolol and Its β-Cyclodextrin Complex as TetK and NorA Efflux Pump Inhibitors in Staphylococcus aureus Strains. Antibiotics 2020, 9, 1–8. [Google Scholar]
- Rodrigues, F.C.; Santos, A.T.L.; Machado, A.J.T.; Bezerra, C.F.; Freitas, T.S.; Coutinho, H.D.M.; Morais-Braga, M.F.B.; Bezerra, J.W.A.; Duarte, A.E.; Kamdem, J.P.; et al. Chemical composition and anti-Candida potencial of the extracts of Tarenaya spinosa (Jacq.) Raf. (Cleomaceae). Comp. Immunol. Microbiol. Infect. Dis. 2019, 64, 14–19. [Google Scholar] [CrossRef] [PubMed]
- Santos, A.T.L.; Carneiro, P.; Nályda, J.; Cruz, R.P.; Sales, D.L.; Andrade, J.C.; Almeida, W.O.; Costa, J.G.M.; Ribeiro, P.R.V.; Brito, E.S.; et al. UPLC-MS-ESI-QTOF Analysis and Antifungal Activity of the Spondias tuberosa Arruda Leaf and Root Hydroalcoholic Extracts. Antibiotics 2019, 8, 240–253. [Google Scholar] [CrossRef] [PubMed]
- Spitzer, M.; Griffiths, E.; Blakely, K.M.; Wildenhain, J.; Ejim, L.; Rossi, L.; Pascale, G.; Curak, J.; Brown, E.; Tyers, M.; et al. Cross-species discovery of syncretic drug combinations that potentiate the antifungal fluconazole. Mol. Syst. Biol. 2009, 7, 1–14. [Google Scholar] [CrossRef]
- Pyne, M.E.; Narcross, L.; Martin, V.J. Metabolismo secundário de plantas de engenharia em sistemas microbianos. Plant Phys. 2019, 179, 3–844. [Google Scholar]
- Obata, T. Metabolons in plant primary and secondary metabolism. Phytochem. Rev. 2019, 18, 6–1483. [Google Scholar] [CrossRef]
- Arif, T.; Mandal, T.K.; Dabur, R. Natural products: Anti—fungal agents derived from plants. In Opportunity, Challenge and Scope of Natural Products in Medicinal Chemistry; Research Signpost: Thiruvananthapuram, India, 2011; Volume 81, pp. 283–311. [Google Scholar]
- Salas, P.M.; Céliz, G.; Geronazzo, H.; Daz, M.; Resnik, S.L. Antifungal activity and enzymatically—modified flavonoids isolated from citrus species. Food Chem. 2011, 124, 1411–1415. [Google Scholar] [CrossRef]
- Freitas, M.A.; Alves, A.I.S.; Andrade, J.C.; Leite-Andrade, M.C.; Santos, A.T.L.; Oliveira, T.F.; Santos, F.A.G.; Buonafina, M.D.S.; Coutinho, H.D.M.; Menezes, I.R.A.; et al. Evaluation of the Antifungal Activity of the Licania Rigida Leaf Ethanolic Extract against Biofilms Formed by Candida Sp. Isolates in Acrylic Resin Discs. Antibiotics 2019, 8, 1–11. [Google Scholar] [CrossRef]
- Khonkarn, R.; Okonogi, S.; Ampasavate, C.; Anuchapreeda, S. Investigation of fruit peel extracts as sources for compounds with antioxidant and antiproliferative activities against human cell lines. Food Chem. Toxicol. 2010, 48, 2122–2129. [Google Scholar] [CrossRef]
- Lima, V.N.; Oliveira-Tintino, C.D.M.; Santos, E.S.; Morais, L.P.; Tintino, S.R.; Freitas, T.S.; Gerado, Y.S.; Pereira, R.L.S.; Cruz, R.P.; Menezes, I.R.A.; et al. Antimicrobial and enhancement of the antibiotic activity by phenolic compounds: Gallic acid, caffeic acid and pyrogallol. Microbial. Pathogenesis 2016, 99, 56–61. [Google Scholar] [CrossRef] [PubMed]
- Bufalo, M.C. Efeito da própolis e de compostos isolados sobre a expressão de receptores, produção de citocinas e atividades fungicida de monócitos humanos. Ph.D. Thesis, Paulista State University Julio de Mesquita Filho, Botucatu, SP, Brazil, 2013. [Google Scholar]
- Cheah, H.L.; Lim, V.; Sandai, D. Inhibitors of the glyoxylate cycle enzyme ICL1 in Candida albicans for potential use as antifungal agents. PLoS ONE 2014, 9, 91–95. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.N.; Upreti, D.K.; Singh, B.R.; Pandey, G.; Verma, S.; Roy, S.; Naqvi, A.H.; Rawat, A.K.S. Quercetin sensitizes fluconazole-resistant Candida albicans to induce apoptotic cell death by modulating quorum sensing. Antimicrob. Agents Chemother. 2015, 59, 2153–2168. [Google Scholar] [CrossRef]
- Ferry, D.R.; Smith, A.; Malkhandi, J.; Fyfe, D.W.; Takats, P.G.; Anderson, D.; Baker, J.; Kerr, D.J. Phase I clinical trial of the flavonoid quercetin: Pharmacokinetics and evidence for in vivo tyrosine kinase inhibition. Clin. Cancer Res. 1996, 2, 659–668. [Google Scholar]
- Silva, C.R.; Neto, J.B.A.; Campos, R.S.; Figueiredo, N.S.; Sampaio, L.S.; Magalhães, H.I.F.; Cavalcanti, B.C.; Gaspar, D.M.; Andrade, G.M.; Lima, I.S.P.; et al. Synergistic effect of the flavonoid catechin, quercetin, or epigallocatechin gallate with fluconazole induces apoptosis in Candida tropicalis resistant to fluconazole. Antimicrob. Agents Chemother. 2014, 58, 1468–1478. [Google Scholar] [CrossRef]
- Waczuk, E.P.; Kamdem, J.P.; Abolaji, A.O.; Meinerz, D.F.; Bueno, D.C.; Nascimento Gonzaga, T.K.S.; Dorow, T.S.C.; Boligon, A.A.; Athayde, M.L.; Rocha, J.B.T.; et al. Euphorbia tirucalli aqueous extract induces cytotoxicity, genotoxicity and changes in antioxidant gene expression in human leukocytes. Toxicol. Res. 2015, 4, 739–748. [Google Scholar] [CrossRef]
- Morais-Braga, M.F.B.; Carneiro, J.N.P.; Machado, A.J.T.; Sales, D.L.; Santos, A.T.L.; Boligon, A.A.; Athayde, M.L.; Souza, D.S.L.; Costa, J.G.M.; Coutinho, H.D.M. Phenolic composition and medicinal usage of Psidium guajava Linn.: Antifungal activity or inhibition of virulence? Saudi. J. Biol. Sci. 2017, 24, 302–313. [Google Scholar] [CrossRef]
- CLSI—Clinical and Laboratory Standards Institute. Reference Method for Broth Dilution Antifungal Susceptibility Testing of Filamentous Fungi; Aproved Standard M27-A2; CLSI—Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2002. [Google Scholar]
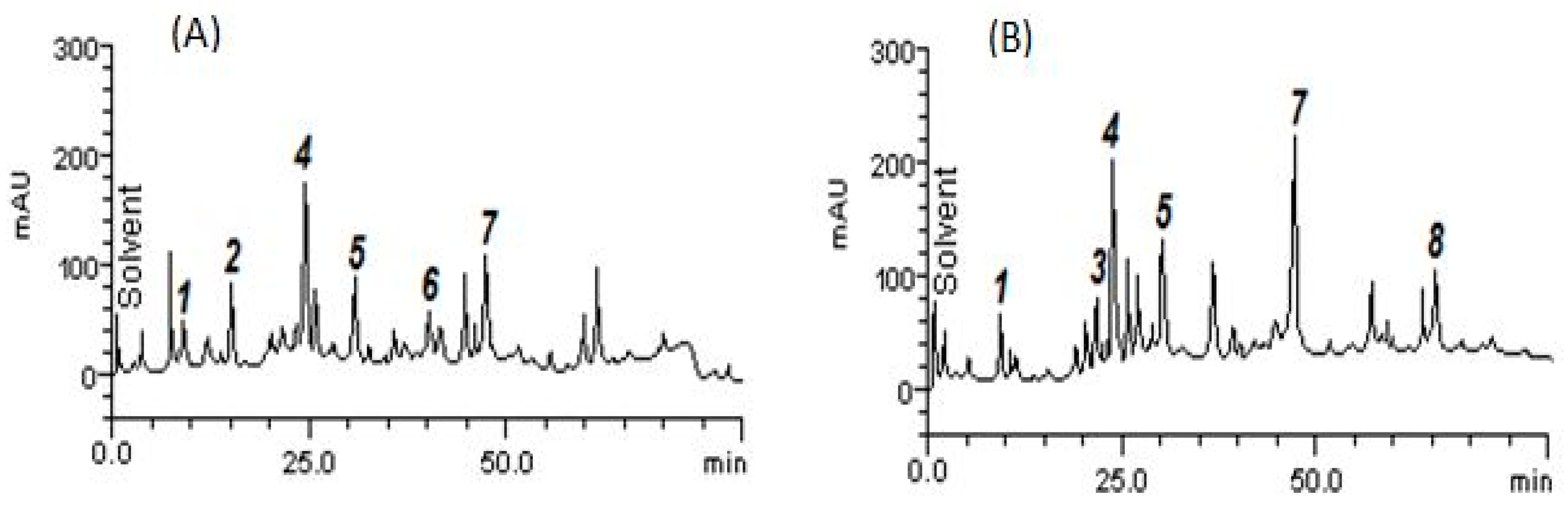




| Compounds | AELMs | AEAPMs |
|---|---|---|
| mg/g | mg/g | |
| Catechin | 6.05 ± 0.01 b | - |
| Chlorogenic acid | - | 3.89 ± 0.01 a |
| Caffeic acid | 13.27 ± 0.02 c | 14.25 ± 0.04 b |
| Ellagic acid | 5.98 ± 0.03 b | 8.01 ± 0.02 c |
| Rutin | 2.81 ± 0.01 a | - |
| Quercetin | 7.03 ± 0.01 d | 14.70 ± 0.01 b |
| Apigenin | - | 4.11 ± 0.01 a |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodrigues Costa, A.; Bezerra, J.W.A.; Pereira da Cruz, R.; de Freitas, M.A.; da Silva, V.B.; Neto, J.C.; dos Santos, A.T.L.; Bezerra Morais Braga, M.F.; da Silva, L.A.; Ivaneide Rocha, M.; et al. In vitro Antibiotic and Modulatory Activity of Mesosphaerum suaveolens (L.) Kuntze against Candida strains. Antibiotics 2020, 9, 46. https://doi.org/10.3390/antibiotics9020046
Rodrigues Costa A, Bezerra JWA, Pereira da Cruz R, de Freitas MA, da Silva VB, Neto JC, dos Santos ATL, Bezerra Morais Braga MF, da Silva LA, Ivaneide Rocha M, et al. In vitro Antibiotic and Modulatory Activity of Mesosphaerum suaveolens (L.) Kuntze against Candida strains. Antibiotics. 2020; 9(2):46. https://doi.org/10.3390/antibiotics9020046
Chicago/Turabian StyleRodrigues Costa, Adrielle, José Weverton Almeida Bezerra, Rafael Pereira da Cruz, Maria Audilene de Freitas, Viviane Bezerra da Silva, João Cruz Neto, Antonia Thassya Lucas dos Santos, Maria Flaviana Bezerra Morais Braga, Leomara Andrade da Silva, Maria Ivaneide Rocha, and et al. 2020. "In vitro Antibiotic and Modulatory Activity of Mesosphaerum suaveolens (L.) Kuntze against Candida strains" Antibiotics 9, no. 2: 46. https://doi.org/10.3390/antibiotics9020046
APA StyleRodrigues Costa, A., Bezerra, J. W. A., Pereira da Cruz, R., de Freitas, M. A., da Silva, V. B., Neto, J. C., dos Santos, A. T. L., Bezerra Morais Braga, M. F., da Silva, L. A., Ivaneide Rocha, M., Kamdem, J. P., Iriti, M., Vitalini, S., Duarte, A. E., & Barros, L. M. (2020). In vitro Antibiotic and Modulatory Activity of Mesosphaerum suaveolens (L.) Kuntze against Candida strains. Antibiotics, 9(2), 46. https://doi.org/10.3390/antibiotics9020046








