Strategies to Combat Multidrug-Resistant and Persistent Infectious Diseases
Abstract
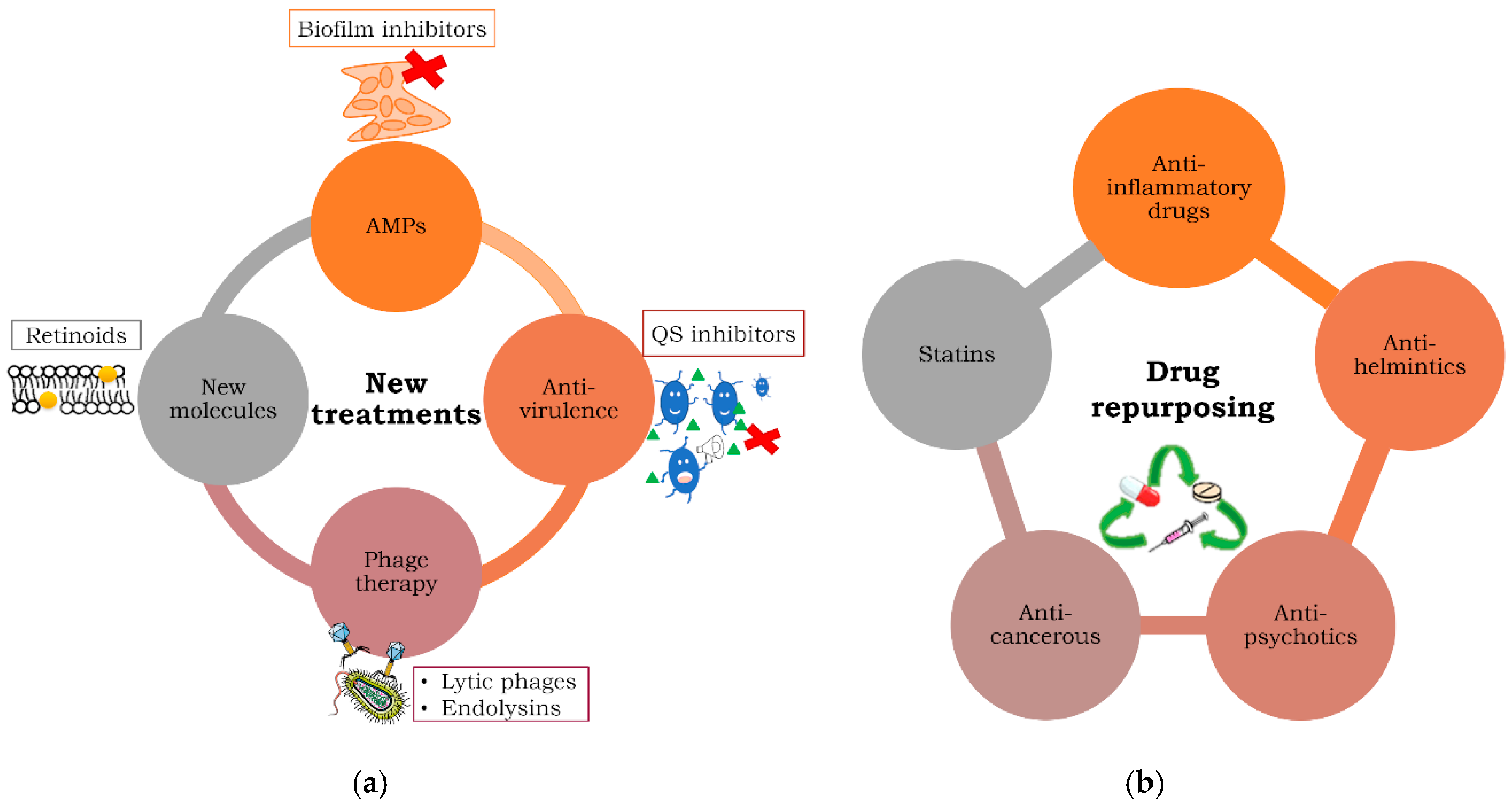
:1. Introduction
2. New Anti-Infectious Treatments against MDR and Persistent Bacteria
2.1. Antimicrobial Peptides (AMPs)
2.2. Anti-Virulence Compounds
2.3. Phage Therapy Alone or in Combination with Antibiotics to Treat MDR and Persistent Bacteria
2.4. New Molecules
3. Repurposing Treatments against MDR and Persistent Bacteria
3.1. Anti-Inflammatories as Antibacterial Agents
3.2. Anti-Psychotics
3.3. Anti-Helminthic Drugs
3.4. Anti-Cancerous Drugs as Antibacterials
3.5. Statins
4. Discussion
5. Conclusions
Funding
Conflicts of Interest
References
- WHO. Worldwide Country Situation Analysis: Response to Antimicrobial Resistance; WHO Library Cataloguing-in-Publication Data; World Health Organization: Geneva, Switzerland, 2015. [Google Scholar]
- Cerceo, E.; Deitelzweig, S.B.; Sherman, B.M.; Amin, A.N. Multidrug-Resistant Gram-Negative Bacterial Infections in the Hospital Setting: Overview, Implications for Clinical Practice, and Emerging Treatment Options. Microb. Drug Resist. 2016, 22, 412–431. [Google Scholar] [CrossRef]
- Medina, E.; Pieper, D.H. Tackling Threats and Future Problems of Multidrug-Resistant Bacteria. Curr. Top. Microbiol. Immunol. 2016, 398, 3–33. [Google Scholar] [CrossRef] [PubMed]
- Cassini, A.; Högberg, L.D.; Plachouras, D.; Quattrocchi, A.; Hoxha, A.; Simonsen, G.S.; Colomb-Cotinat, M.; Kretzschmar, M.E.; Devleesschauwer, B.; Cecchini, M.; et al. Attributable deaths and disability-adjusted life-years caused by infections with antibiotic-resistant bacteria in the EU and the European Economic Area in 2015: A population-level modelling analysis. Lancet Infect. Dis. 2018. [Google Scholar] [CrossRef] [Green Version]
- Abat, C.; Rolain, J.M.; Dubourg, G.; Fournier, P.E.; Chaudet, H.; Raoult, D. Evaluating the Clinical Burden and Mortality Attributable to Antibiotic Resistance: The Disparity of Empirical Data and Simple Model Estimations. Clin. Infect. Dis. 2017, 65, S58–S63. [Google Scholar] [CrossRef] [PubMed]
- Dye, C. After 2015: Infectious diseases in a new era of health and development. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2014, 369, 20130426. [Google Scholar] [CrossRef] [Green Version]
- Rice, L.B. Federal funding for the study of antimicrobial resistance in nosocomial pathogens: No ESKAPE. J. Infect. Dis. 2008, 197, 1079–1081. [Google Scholar] [CrossRef]
- Levin-Reisman, I.; Brauner, A.; Ronin, I.; Balaban, N.Q. Epistasis between antibiotic tolerance, persistence, and resistance mutations. Proc. Natl. Acad. Sci. USA 2019, 116, 14734–14739. [Google Scholar] [CrossRef] [Green Version]
- Brauner, A.; Fridman, O.; Gefen, O.; Balaban, N.Q. Distinguishing between resistance, tolerance and persistence to antibiotic treatment. Nat. Rev. Microbiol. 2016, 14, 320–330. [Google Scholar] [CrossRef]
- Tafoukt, R.; Leangapichart, T.; Hadjadj, L.; Bakour, S.; Diene, S.M.; Rolain, J.M.; Touati, A. Characterisation of blaOXA-538, a new variant of blaOXA-48, in Shewanella xiamenensis isolated from river water in Algeria. J. Glob. Antimicrob. Resist. 2018, 13, 70–73. [Google Scholar] [CrossRef]
- Tafoukt, R.; Touati, A.; Leangapichart, T.; Bakour, S.; Rolain, J.M. Characterization of OXA-48-like-producing Enterobacteriaceae isolated from river water in Algeria. Water Res. 2017, 120, 185–189. [Google Scholar] [CrossRef]
- Zenati, K.; Touati, A.; Bakour, S.; Sahli, F.; Rolain, J.M. Characterization of NDM-1- and OXA-23-producing Acinetobacter baumannii isolates from inanimate surfaces in a hospital environment in Algeria. J. Hosp. Infect. 2016, 92, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Rolain, J.M. Food and human gut as reservoirs of transferable antibiotic resistance encoding genes. Front. Microbiol. 2013, 4, 173. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, J.S.; Wood, T.K. Tolerant, Growing Cells from Nutrient Shifts Are Not Persister Cells. MBio 2017, 8, e00354-17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Windels, E.M.; Michiels, J.E.; Van den Bergh, B.; Fauvart, M.; Michiels, J. Antibiotics: Combatting Tolerance To Stop Resistance. MBio 2019, 10, e02095-19. [Google Scholar] [CrossRef] [Green Version]
- Fisher, R.A.; Gollan, B.; Helaine, S. Persistent bacterial infections and persister cells. Nat. Rev. Microbiol. 2017, 15, 453–464. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Yamasaki, R.; Song, S.; Wood, T.K. Interkingdom Signal Indole Inhibits Pseudomonas aeruginosa Persister Cell Waking. J. Appl. Microbiol. 2019, 127, 1768–1775. [Google Scholar] [CrossRef]
- Grant, S.S.; Hung, D.T. Persistent bacterial infections, antibiotic tolerance, and the oxidative stress response. Virulence 2013, 4, 273–283. [Google Scholar] [CrossRef] [Green Version]
- Lafleur, M.D.; Qi, Q.; Lewis, K. Patients with long-term oral carriage harbor high-persister mutants of Candida albicans. Antimicrob. Agents Chemother. 2010, 54, 39–44. [Google Scholar] [CrossRef] [Green Version]
- Mulcahy, L.R.; Burns, J.L.; Lory, S.; Lewis, K. Emergence of Pseudomonas aeruginosa strains producing high levels of persister cells in patients with cystic fibrosis. J. Bacteriol. 2010, 192, 6191–6199. [Google Scholar] [CrossRef] [Green Version]
- Goneau, L.W.; Yeoh, N.S.; MacDonald, K.W.; Cadieux, P.A.; Burton, J.P.; Razvi, H.; Reid, G. Selective target inactivation rather than global metabolic dormancy causes antibiotic tolerance in uropathogens. Antimicrob. Agents Chemother. 2014, 58, 2089–2097. [Google Scholar] [CrossRef] [Green Version]
- Schumacher, M.A.; Balani, P.; Min, J.; Chinnam, N.B.; Hansen, S.; Vulic, M.; Lewis, K.; Brennan, R.G. HipBA-promoter structures reveal the basis of heritable multidrug tolerance. Nature 2015, 524, 59–64. [Google Scholar] [CrossRef] [PubMed]
- Trastoy, R.; Manso, T.; Fernandez-Garcia, L.; Blasco, L.; Ambroa, A.; Perez Del Molino, M.L.; Bou, G.; Garcia-Contreras, R.; Wood, T.K.; Tomas, M. Mechanisms of Bacterial Tolerance and Persistence in the Gastrointestinal and Respiratory Environments. Clin. Microbiol. Rev. 2018, 31, e00023-18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moreillon, P.; Tomasz, A. Penicillin resistance and defective lysis in clinical isolates of pneumococci: Evidence for two kinds of antibiotic pressure operating in the clinical environment. J. Infect. Dis. 1988, 157, 1150–1157. [Google Scholar] [CrossRef]
- Novak, R.; Henriques, B.; Charpentier, E.; Normark, S.; Tuomanen, E. Emergence of vancomycin tolerance in Streptococcus pneumoniae. Nature 1999, 399, 590–593. [Google Scholar] [CrossRef] [PubMed]
- Sebastian, J.; Swaminath, S.; Nair, R.R.; Jakkala, K.; Pradhan, A.; Ajitkumar, P. De Novo Emergence of Genetically Resistant Mutants of Mycobacterium tuberculosis from the Persistence Phase Cells Formed against Antituberculosis Drugs In Vitro. Antimicrob. Agents Chemother. 2017, 61, e01343-16. [Google Scholar] [CrossRef] [Green Version]
- Levin-Reisman, I.; Ronin, I.; Gefen, O.; Braniss, I.; Shoresh, N.; Balaban, N.Q. Antibiotic tolerance facilitates the evolution of resistance. Science 2017, 355, 826–830. [Google Scholar] [CrossRef]
- Windels, E.M.; Michiels, J.E.; Fauvart, M.; Wenseleers, T.; Van den Bergh, B.; Michiels, J. Bacterial persistence promotes the evolution of antibiotic resistance by increasing survival and mutation rates. ISME J. 2019, 13, 1239–1251. [Google Scholar] [CrossRef]
- Garcia-Contreras, R.; Wood, T.K.; Tomas, M. Editorial: Quorum Network (Sensing/Quenching) in Multidrug-Resistant Pathogens. Front. Cell. Infect. Microbiol. 2019, 9, 80. [Google Scholar] [CrossRef] [Green Version]
- Garcia-Contreras, R.; Wood, T.K.; Tomas, M. Editorial: Drug Re-purposing for the Treatment of Bacterial and Viral Infections. Front. Cell. Infect. Microbiol. 2019, 9, 387. [Google Scholar] [CrossRef]
- Yang, N.; Teng, D.; Mao, R.; Hao, Y.; Wang, X.; Wang, Z.; Wang, J. A recombinant fungal defensin-like peptide-P2 combats multidrug-resistant Staphylococcus aureus and biofilms. Appl. Microbiol. Biotechnol. 2019, 103, 5193–5213. [Google Scholar] [CrossRef]
- Li, C.; Zhu, C.; Ren, B.; Yin, X.; Shim, S.H.; Gao, Y.; Zhu, J.; Zhao, P.; Liu, C.; Yu, R.; et al. Two optimized antimicrobial peptides with therapeutic potential for clinical antibiotic-resistant Staphylococcus aureus. Eur. J. Med. Chem. 2019, 183, 111686. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Jia, Y.; Yang, K.; Li, R.; Xiao, X.; Wang, Z. Antagonizing Vancomycin Resistance in Enterococcus by Surface Localized Antimicrobial Display-Derived Peptides. ACS Infect. Dis. 2019. [Google Scholar] [CrossRef] [PubMed]
- Pan, J.; Xie, X.; Tian, W.; Bahar, A.A.; Lin, N.; Song, F.; An, J.; Ren, D. (Z)-4-bromo-5-(bromomethylene)-3-methylfuran-2(5H)-one sensitizes Escherichia coli persister cells to antibiotics. Appl. Microbiol. Biotechnol. 2013, 97, 9145–9154. [Google Scholar] [CrossRef] [PubMed]
- Conlon, B.P.; Nakayasu, E.S.; Fleck, L.E.; LaFleur, M.D.; Isabella, V.M.; Coleman, K.; Leonard, S.N.; Smith, R.D.; Adkins, J.N.; Lewis, K. Activated ClpP kills persisters and eradicates a chronic biofilm infection. Nature 2013, 503, 365–370. [Google Scholar] [CrossRef] [Green Version]
- Starkey, M.; Lepine, F.; Maura, D.; Bandyopadhaya, A.; Lesic, B.; He, J.; Kitao, T.; Righi, V.; Milot, S.; Tzika, A.; et al. Identification of anti-virulence compounds that disrupt quorum-sensing regulated acute and persistent pathogenicity. PLoS Pathog. 2014, 10, e1004321. [Google Scholar] [CrossRef] [PubMed]
- El Meouche, I.; Dunlop, M.J. Heterogeneity in efflux pump expression predisposes antibiotic-resistant cells to mutation. Science 2018, 362, 686–690. [Google Scholar] [CrossRef] [Green Version]
- Knoll, B.M.; Mylonakis, E. Antibacterial bioagents based on principles of bacteriophage biology: An overview. Clin. Infect. Dis. 2014, 58, 528–534. [Google Scholar] [CrossRef] [Green Version]
- Salmond, G.P.; Fineran, P.C. A century of the phage: Past, present and future. Nat. Rev. Microbiol. (Engl.) 2015, 13, 777–786. [Google Scholar] [CrossRef]
- Divya Ganeshan, S.; Hosseinidoust, Z. Phage Therapy with a Focus on the Human Microbiota. Antibiotics 2019, 8, 131. [Google Scholar] [CrossRef] [Green Version]
- Sarker, S.A.; Sultana, S.; Reuteler, G.; Moine, D.; Descombes, P.; Charton, F.; Bourdin, G.; McCallin, S.; Ngom-Bru, C.; Neville, T.; et al. Oral Phage Therapy of Acute Bacterial Diarrhea With Two Coliphage Preparations: A Randomized Trial in Children From Bangladesh. EBioMedicine 2016, 4, 124–137. [Google Scholar] [CrossRef] [Green Version]
- Roach, D.R.; Leung, C.Y.; Henry, M.; Morello, E.; Singh, D.; Di Santo, J.P.; Weitz, J.S.; Debarbieux, L. Synergy between the Host Immune System and Bacteriophage Is Essential for Successful Phage Therapy against an Acute Respiratory Pathogen. Cell Host Microbe 2017, 22, 38–47.e34. [Google Scholar] [CrossRef] [PubMed]
- Schooley, R.T.; Biswas, B.; Gill, J.J.; Hernandez-Morales, A.; Lancaster, J.; Lessor, L.; Barr, J.J.; Reed, S.L.; Rohwer, F.; Benler, S.; et al. Development and Use of Personalized Bacteriophage-Based Therapeutic Cocktails To Treat a Patient with a Disseminated Resistant Acinetobacter baumannii Infection. Antimicrob. Agents Chemother. 2017, 61, e00954-17. [Google Scholar] [CrossRef] [Green Version]
- Khawaldeh, A.; Morales, S.; Dillon, B.; Alavidze, Z.; Ginn, A.N.; Thomas, L.; Chapman, S.J.; Dublanchet, A.; Smithyman, A.; Iredell, J.R. Bacteriophage therapy for refractory Pseudomonas aeruginosa urinary tract infection. J. Med. Microbiol. 2011, 60, 1697–1700. [Google Scholar] [CrossRef] [PubMed]
- Torres-Barcelo, C.; Arias-Sanchez, F.I.; Vasse, M.; Ramsayer, J.; Kaltz, O.; Hochberg, M.E. A window of opportunity to control the bacterial pathogen Pseudomonas aeruginosa combining antibiotics and phages. PLoS ONE 2014, 9, e106628. [Google Scholar] [CrossRef] [Green Version]
- Chaudhry, W.N.; Concepcion-Acevedo, J.; Park, T.; Andleeb, S.; Bull, J.J.; Levin, B.R. Synergy and Order Effects of Antibiotics and Phages in Killing Pseudomonas aeruginosa Biofilms. PLoS ONE 2017, 12, e0168615. [Google Scholar] [CrossRef]
- Torres-Barcelo, C. The disparate effects of bacteriophages on antibiotic-resistant bacteria. Emerg. Microbes Infect. 2018, 7, 168. [Google Scholar] [CrossRef]
- Chan, B.K.; Sistrom, M.; Wertz, J.E.; Kortright, K.E.; Narayan, D.; Turner, P.E. Phage selection restores antibiotic sensitivity in MDR Pseudomonas aeruginosa. Sci. Rep. 2016, 6, 26717. [Google Scholar] [CrossRef]
- Blasco, L.; Ambroa, A.; Lopez, M.; Fernandez-Garcia, L.; Bleriot, I.; Trastoy, R.; Ramos-Vivas, J.; Coenye, T.; Fernandez-Cuenca, F.; Vila, J.; et al. Combined Use of the Ab105-2φΔCI Lytic Mutant Phage and Different Antibiotics in Clinical Isolates of Multi-Resistant. Microorganisms 2019, 7, 556. [Google Scholar] [CrossRef] [Green Version]
- Donovan, D.M.; Lardeo, M.; Foster-Frey, J. Lysis of staphylococcal mastitis pathogens by bacteriophage phi11 endolysin. FEMS Microbiol. Lett. 2006, 265, 133–139. [Google Scholar] [CrossRef] [Green Version]
- Fenton, M.; Keary, R.; McAuliffe, O.; Ross, R.P.; O’Mahony, J.; Coffey, A. Bacteriophage-Derived Peptidase CHAP(K) Eliminates and Prevents Staphylococcal Biofilms. Int. J. Microbiol. 2013, 2013, 625341. [Google Scholar] [CrossRef] [Green Version]
- Shen, Y.; Koller, T.; Kreikemeyer, B.; Nelson, D.C. Rapid degradation of Streptococcus pyogenes biofilms by PlyC, a bacteriophage-encoded endolysin. J. Antimicrob. Chemother. 2013, 68, 1818–1824. [Google Scholar] [CrossRef] [PubMed]
- Larpin, Y.; Oechslin, F.; Moreillon, P.; Resch, G.; Entenza, J.M.; Mancini, S. In vitro characterization of PlyE146, a novel phage lysin that targets Gram-negative bacteria. PLoS ONE 2018, 13, e0192507. [Google Scholar] [CrossRef] [PubMed]
- Peng, S.Y.; You, R.I.; Lai, M.J.; Lin, N.T.; Chen, L.K.; Chang, K.C. Highly potent antimicrobial modified peptides derived from the Acinetobacter baumannii phage endolysin LysAB2. Sci. Rep. 2017, 7, 11477. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, W.; Zhu, W.; Hendricks, G.L.; Van Tyne, D.; Steele, A.D.; Keohane, C.E.; Fricke, N.; Conery, A.L.; Shen, S.; Pan, W.; et al. A new class of synthetic retinoid antibiotics effective against bacterial persisters. Nature 2018, 556, 103–107. [Google Scholar] [CrossRef]
- Alvarez, R.; Vaz, B.; Gronemeyer, H.; de Lera, A.R. Functions, therapeutic applications, and synthesis of retinoids and carotenoids. Chem. Rev. 2014, 114, 1–125. [Google Scholar] [CrossRef]
- Vijayashree Priyadharsini, J. In silico validation of non-antibiotic drugs, acetaminophen, and ibuprofen as antibacterial agents against red complex pathogens. J. Periodontol. 2019, 90, 1441–1448. [Google Scholar] [CrossRef]
- Giannoni, E.; Guignard, L.; Knaup Reymond, M.; Perreau, M.; Roth-Kleiner, M.; Calandra, T.; Roger, T. Estradiol and progesterone strongly inhibit the innate immune response of mononuclear cells in newborns. Infect. Immun. 2011, 79, 2690–2698. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sautebin, L.; Carnuccio, R.; Ialenti, A.; Di Rosa, M. Lipocortin and vasocortin: Two species of anti-inflammatory proteins mimicking the effects of glucocorticoids. Pharmacol. Res. 1992, 25, 1–12. [Google Scholar] [CrossRef]
- Emgard, P.; Hellstrom, S.; Holm, S. External otitis caused by infection with Pseudomonas aeruginosa or Candida albicans cured by use of a topical group III steroid, without any antibiotics. Acta Otolaryngol. 2005, 125, 346–352. [Google Scholar] [CrossRef]
- Jiang, M.; Ma, L.; Huang, Y.; Wu, H.; Dou, J.; Zhou, C. Antimicrobial activities of peptide Cbf-K16 against drug-resistant Helicobacter pylori infection in vitro and in vivo. Microb. Pathog. 2019, 138, 103847. [Google Scholar] [CrossRef]
- Lieberman, L.A.; Higgins, D.E. Inhibition of Listeria monocytogenes infection by neurological drugs. Int. J. Antimicrob. Agents 2010, 35, 292–296. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ordway, D.; Viveiros, M.; Leandro, C.; Arroz, M.J.; Amaral, L. Intracellular activity of clinical concentrations of phenothiazines including thioridiazine against phagocytosed Staphylococcus aureus. Int. J. Antimicrob. Agents 2002, 20, 34–43. [Google Scholar] [CrossRef]
- van Ingen, J.; van der Laan, T.; Amaral, L.; Dekhuijzen, R.; Boeree, M.J.; van Soolingen, D. In vitro activity of thioridazine against mycobacteria. Int. J. Antimicrob. Agents 2009, 34, 190–191. [Google Scholar] [CrossRef] [PubMed]
- Andersson, J.A.; Fitts, E.C.; Kirtley, M.L.; Ponnusamy, D.; Peniche, A.G.; Dann, S.M.; Motin, V.L.; Chauhan, S.; Rosenzweig, J.A.; Sha, J.; et al. New Role for FDA-Approved Drugs in Combating Antibiotic-Resistant Bacteria. Antimicrob. Agents Chemother. 2016, 60, 3717–3729. [Google Scholar] [CrossRef] [Green Version]
- Rajamuthiah, R.; Fuchs, B.B.; Conery, A.L.; Kim, W.; Jayamani, E.; Kwon, B.; Ausubel, F.M.; Mylonakis, E. Repurposing salicylanilide anthelmintic drugs to combat drug resistant Staphylococcus aureus. PLoS ONE 2015, 10, e0124595. [Google Scholar] [CrossRef] [Green Version]
- Imperi, F.; Massai, F.; Ramachandran Pillai, C.; Longo, F.; Zennaro, E.; Rampioni, G.; Visca, P.; Leoni, L. New life for an old drug: The anthelmintic drug niclosamide inhibits Pseudomonas aeruginosa quorum sensing. Antimicrob. Agents Chemother. 2013, 57, 996–1005. [Google Scholar] [CrossRef] [Green Version]
- Ayerbe-Algaba, R.; Gil-Marques, M.L.; Jimenez-Mejias, M.E.; Sanchez-Encinales, V.; Parra-Millan, R.; Pachon-Ibanez, M.E.; Pachon, J.; Smani, Y. Synergistic Activity of Niclosamide in Combination With Colistin Against Colistin-Susceptible and Colistin-Resistant Acinetobacter baumannii and Klebsiella pneumoniae. Front. Cell. Infect. Microbiol. 2018, 8, 348. [Google Scholar] [CrossRef] [Green Version]
- Gooyit, M.; Janda, K.D. Reprofiled anthelmintics abate hypervirulent stationary-phase Clostridium difficile. Sci. Rep. 2016, 6, 33642. [Google Scholar] [CrossRef] [Green Version]
- Omansen, T.F.; Porter, J.L.; Johnson, P.D.; van der Werf, T.S.; Stienstra, Y.; Stinear, T.P. In-vitro activity of avermectins against Mycobacterium ulcerans. PLoS Negl. Trop. Dis. 2015, 9, e0003549. [Google Scholar] [CrossRef] [Green Version]
- Ashraf, S.; Chaudhry, U.; Raza, A.; Ghosh, D.; Zhao, X. In vitro activity of ivermectin against Staphylococcus aureus clinical isolates. Antimicrob. Resist. Infect. Control 2018, 7, 27. [Google Scholar] [CrossRef]
- Zhang, X.; Song, Y.; Ci, X.; An, N.; Ju, Y.; Li, H.; Wang, X.; Han, C.; Cui, J.; Deng, X. Ivermectin inhibits LPS-induced production of inflammatory cytokines and improves LPS-induced survival in mice. Inflamm. Res. 2008, 57, 524–529. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.S.; Sun, W.; Xu, M.; Shen, M.; Khraiwesh, M.; Sciotti, R.J.; Zheng, W. Repurposing Screen Identifies Unconventional Drugs With Activity Against Multidrug Resistant Acinetobacter baumannii. Front. Cell. Infect. Microbiol. 2018, 8, 438. [Google Scholar] [CrossRef] [PubMed]
- Rangel-Vega, A.; Bernstein, L.R.; Mandujano-Tinoco, E.A.; Garcia-Contreras, S.J.; Garcia-Contreras, R. Drug repurposing as an alternative for the treatment of recalcitrant bacterial infections. Front. Microbiol. 2015, 6, 282. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hijazi, S.; Visaggio, D.; Pirolo, M.; Frangipani, E.; Bernstein, L.; Visca, P. Antimicrobial Activity of Gallium Compounds on ESKAPE Pathogens. Front. Cell. Infect. Microbiol. 2018, 8, 316. [Google Scholar] [CrossRef] [Green Version]
- Goss, C.H.; Kaneko, Y.; Khuu, L.; Anderson, G.D.; Ravishankar, S.; Aitken, M.L.; Lechtzin, N.; Zhou, G.; Czyz, D.M.; McLean, K.; et al. Gallium disrupts bacterial iron metabolism and has therapeutic effects in mice and humans with lung infections. Sci. Transl. Med. 2018, 10. [Google Scholar] [CrossRef] [Green Version]
- Kwan, B.W.; Chowdhury, N.; Wood, T.K. Combatting bacterial infections by killing persister cells with mitomycin C. Environ. Microbiol. 2015, 17, 4406–4414. [Google Scholar] [CrossRef]
- Chowdhury, N.; Wood, T.L.; Martínez-Vázquez, M.; García-Contreras, R.; Wood, T.K. DNA-crosslinker cisplatin eradicates bacterial persister cells. Biotechnol. Bioeng. 2016, 113, 1984–1992. [Google Scholar] [CrossRef]
- Farha, M.A.; Czarny, T.L.; Myers, C.L.; Worrall, L.J.; French, S.; Conrady, D.G.; Wang, Y.; Oldfield, E.; Strynadka, N.C.; Brown, E.D. Antagonism screen for inhibitors of bacterial cell wall biogenesis uncovers an inhibitor of undecaprenyl diphosphate synthase. Proc. Natl. Acad. Sci. USA 2015, 112, 11048–11053. [Google Scholar] [CrossRef] [Green Version]
- Jerwood, S.; Cohen, J. Unexpected antimicrobial effect of statins. J. Antimicrob. Chemother. 2008, 61, 362–364. [Google Scholar] [CrossRef]
- McTaggart, F.; Buckett, L.; Davidson, R.; Holdgate, G.; McCormick, A.; Schneck, D.; Smith, G.; Warwick, M. Preclinical and clinical pharmacology of Rosuvastatin, a new 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitor. Am. J. Cardiol. 2001, 87, 28b–32b. [Google Scholar] [CrossRef]
- Graziano, T.S.; Cuzzullin, M.C.; Franco, G.C.; Schwartz-Filho, H.O.; de Andrade, E.D.; Groppo, F.C.; Cogo-Muller, K. Statins and Antimicrobial Effects: Simvastatin as a Potential Drug against Staphylococcus aureus Biofilm. PLoS ONE 2015, 10, e0128098. [Google Scholar] [CrossRef] [PubMed]
- Miro-Canturri, A.; Ayerbe-Algaba, R.; Smani, Y. Drug Repurposing for the Treatment of Bacterial and Fungal Infections. Front. Microbiol. 2019, 10, 41. [Google Scholar] [CrossRef] [PubMed]
- Thangamani, S.; Mohammad, H.; Abushahba, M.F.; Hamed, M.I.; Sobreira, T.J.; Hedrick, V.E.; Paul, L.N.; Seleem, M.N. Exploring simvastatin, an antihyperlipidemic drug, as a potential topical antibacterial agent. Sci. Rep. 2015, 5, 16407. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ko, H.H.T.; Lareu, R.R.; Dix, B.R.; Hughes, J.D. In vitro antibacterial effects of statins against bacterial pathogens causing skin infections. Eur. J. Clin. Microbiol. Infect. Dis. 2018, 37, 1125–1135. [Google Scholar] [CrossRef]
- Fauvart, M.; De Groote, V.N.; Michiels, J. Role of persister cells in chronic infections: Clinical relevance and perspectives on anti-persister therapies. J. Med. Microbiol. 2011, 60, 699–709. [Google Scholar] [CrossRef] [PubMed]
- Theuretzbacher, U.; Outterson, K.; Engel, A.; Karlen, A. The global preclinical antibacterial pipeline. Nat. Rev. Microbiol. 2019. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kang, H.K.; Kim, C.; Seo, C.H.; Park, Y. The therapeutic applications of antimicrobial peptides (AMPs): A patent review. J. Microbiol. 2017, 55, 1–12. [Google Scholar] [CrossRef]
- Sierra, J.M.; Fuste, E.; Rabanal, F.; Vinuesa, T.; Vinas, M. An overview of antimicrobial peptides and the latest advances in their development. Expert Opin. Biol. Ther. 2017, 17, 663–676. [Google Scholar] [CrossRef]
- Dickey, S.W.; Cheung, G.Y.C.; Otto, M. Different drugs for bad bugs: Antivirulence strategies in the age of antibiotic resistance. Nat. Rev. Drug Discov. 2017, 16, 457–471. [Google Scholar] [CrossRef]
- López, M.; Barbosa, B.; Gato, E.; Bou, G.; Tomás, M. Patents on antivirulence therapies. World J. Pharmacol. 2014, 3, 97–109. [Google Scholar] [CrossRef]
- Furfaro, L.L.; Payne, M.S.; Chang, B.J. Bacteriophage Therapy: Clinical Trials and Regulatory Hurdles. Front. Cell. Infect. Microbiol. 2018, 8, 376. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harper, D.R. Criteria for Selecting Suitable Infectious Diseases for Phage Therapy. Viruses 2018, 10, 177. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chhibber, S.; Kaur, S.; Kumari, S. Therapeutic potential of bacteriophage in treating Klebsiella pneumoniae B5055-mediated lobar pneumonia in mice. J. Med. Microbiol. 2008, 57, 1508–1513. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Torres-Barcelo, C.; Hochberg, M.E. Evolutionary Rationale for Phages as Complements of Antibiotics. Trends Microbiol. 2016, 24, 249–256. [Google Scholar] [CrossRef]
- Tagliaferri, T.L.; Jansen, M.; Horz, H.P. Fighting Pathogenic Bacteria on Two Fronts: Phages and Antibiotics as Combined Strategy. Front. Cell. Infect. Microbiol. 2019, 9, 22. [Google Scholar] [CrossRef]
- Love, M.J.; Bhandari, D.; Dobson, R.C.J.; Billington, C. Potential for Bacteriophage Endolysins to Supplement or Replace Antibiotics in Food Production and Clinical Care. Antibiotics 2018, 7, 17. [Google Scholar] [CrossRef] [Green Version]
- Gondil, V.S.; Harjai, K.; Chhibber, S. Endolysins as emerging alternative therapeutic agent to counter drug resistant infections. Int. J. Antimicrob. Agents 2019. [Google Scholar] [CrossRef]
- Peyclit, L.; Baron, S.A.; Rolain, J.M. Drug Repurposing to Fight Colistin and Carbapenem-Resistant Bacteria. Front. Cell. Infect. Microbiol. 2019, 9, 193. [Google Scholar] [CrossRef]
- Mercorelli, B.; Palu, G.; Loregian, A. Drug Repurposing for Viral Infectious Diseases: How Far Are We? Trends Microbiol. 2018, 26, 865–876. [Google Scholar] [CrossRef]
- Gupta, P.; Sharma, M.; Arora, N.; Pruthi, V.; Poluri, K.M. Chemistry and Biology of Farnesol and its Derivatives: Quorum Sensing Molecules with Immense Therapeutic Potential. Curr. Top. Med. Chem. 2018, 18, 1937–1954. [Google Scholar] [CrossRef]
- Liu, H.; Long, S.; Rakesh, K.P.; Zha, G.F. Structure-activity relationships (SAR) of triazine derivatives: Promising antimicrobial agents. Eur. J. Med. Chem. 2020, 185, 111804. [Google Scholar] [CrossRef] [PubMed]
- Tharmalingam, N.; Port, J.; Castillo, D.; Mylonakis, E. Repurposing the anthelmintic drug niclosamide to combat Helicobacter pylori. Sci. Rep. 2018, 8, 3701. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Costabile, G.; d’Angelo, I.; Rampioni, G.; Bondi, R.; Pompili, B.; Ascenzioni, F.; Mitidieri, E.; d’Emmanuele di Villa Bianca, R.; Sorrentino, R.; Miro, A.; et al. Toward Repositioning Niclosamide for Antivirulence Therapy of Pseudomonas aeruginosa Lung Infections: Development of Inhalable Formulations through Nanosuspension Technology. Mol. Pharm. 2015, 12, 2604–2617. [Google Scholar] [CrossRef] [PubMed]
- Ko, H.H.T.; Lareu, R.R.; Dix, B.R.; Hughes, J.D. Statins: Antimicrobial resistance breakers or makers? PeerJ 2017, 5, e3952. [Google Scholar] [CrossRef] [Green Version]

| Name of Drug | Type of Drug | Active against | Mechanism of Action | Reference |
|---|---|---|---|---|
| P5 and P9 | AMPs | MRSA | Inhibition of biofilm, disruption of membrane integrity, downregulation of virulence genes | [32] |
| Cationic peptides | Inhibition of TCS vanRS | VRE | Restoration of vancomycin activity | [33] |
| Inhibitors of AcrAB–TolC | Inhibition of AcrAB–TolC | MDR Escherichia coli | Restoration of antibiotic activity, induction of spontaneous mutations, inhibition of DNA mismatch repair system | [37] |
| Cocktail of 9 lytic phages | Lytic phages | MDR Acinetobacter baumannii | Lysis of MDR A. baumannii cells | [43] |
| Ab105-2phiΔCI | Engineered phage | MDR A. baumannii | Lysis of MDR A. baumannii cells, synergy with carbapenems | [49] |
| LysAB2 | Endolysin from a phage | MRSA | Bactericidal activity against MRSA, A. baumannii, and E. coli | [54] |
| Cbf-K16 | AMP anti-inflammatory | Clarithromycin and amoxicillin-resistant Helicobacter pylori SS1 | Inhibition of IL-8, downregulation of virulence and adhesion genes | [61] |
| 5-Fluorouracil and 6-thioguanine | Anti-cancerous | A. baumannii | Pirimidin and purin analogues | [73] |
| Gallium | Anti-cancerous | MDR ESKAPE | Competition with Fe+3 | [76] |
| Clomiphene | Anti-cancerous (SERM) | MRSA | Inhibition of UPPS, synergy with β-lactams | [79] |
| Simvastatin | Statin | MRSA skin infections | Anti-biofilm activity against staphylococcal biofilms in vivo | [84] |
| Name of Drug | Type of Drug | Active against | Mechanism of Action | Reference (PMID) |
|---|---|---|---|---|
| P2* (Defensin-like peptide) | Permeabilizer | Staphylococcus aureus | Binding to DNA, biofilm inhibition | [31] |
| BF8* | QS inhibitor | E. coli | Disruption of E. coli biofilm, restoration of ofloxacin activity | [34] |
| ADEP4 | ClpP activator | S. aureus | Degradation of hundreds of proteins | [35] |
| M64 | QS inhibitor | Pseudomonas aeruginosa | Inhibition of PqsR, down-regulation of virulence genes | [36] |
| Cocktail of 6 lytic phages | Lytic phages | Recurrent P. aeruginosa | Lysis of P. aeruginosa bacterial cells | [44] |
| phi11 endolysin | Endolysin from a phage | S. aureus | Disruption of S. aureus biofilms and bactericidal activity | [50] |
| CHAP(K) | Endolysin from a phage | S. aureus | Removal of staphylococcal biofilms | [51] |
| PlyC | Endolysin from a phage | Streptococcus spp. | Removal of streptococcal biofilms | [52] |
| PlyE146 | Endolysin from a phage | E. coli, P. aeruginosa, and A. baumannii | Disruption of E. coli, P. aeruginosa and A. baumannii biofilms | [53] |
| CD437* and CD1530* | Retinoids (analogues of vitamin A) | S. aureus | Membrane disruption | [55] |
| Cisplatin | Anti-cancerous | E. coli K-12, S. aureus, P. aeruginosa | Forms intra-strand DNA crosslinks | [78] |
| Mitomycin C | Anti-cancerous | Broad range of persisters | Forms inter-strand DNA crosslinks | [78] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pacios, O.; Blasco, L.; Bleriot, I.; Fernandez-Garcia, L.; González Bardanca, M.; Ambroa, A.; López, M.; Bou, G.; Tomás, M. Strategies to Combat Multidrug-Resistant and Persistent Infectious Diseases. Antibiotics 2020, 9, 65. https://doi.org/10.3390/antibiotics9020065
Pacios O, Blasco L, Bleriot I, Fernandez-Garcia L, González Bardanca M, Ambroa A, López M, Bou G, Tomás M. Strategies to Combat Multidrug-Resistant and Persistent Infectious Diseases. Antibiotics. 2020; 9(2):65. https://doi.org/10.3390/antibiotics9020065
Chicago/Turabian StylePacios, Olga, Lucia Blasco, Inès Bleriot, Laura Fernandez-Garcia, Mónica González Bardanca, Antón Ambroa, María López, German Bou, and Maria Tomás. 2020. "Strategies to Combat Multidrug-Resistant and Persistent Infectious Diseases" Antibiotics 9, no. 2: 65. https://doi.org/10.3390/antibiotics9020065
APA StylePacios, O., Blasco, L., Bleriot, I., Fernandez-Garcia, L., González Bardanca, M., Ambroa, A., López, M., Bou, G., & Tomás, M. (2020). Strategies to Combat Multidrug-Resistant and Persistent Infectious Diseases. Antibiotics, 9(2), 65. https://doi.org/10.3390/antibiotics9020065






