Metal Complexes, an Untapped Source of Antibiotic Potential?
Abstract
:1. Introduction
2. Silver
3. Gold
4. Gallium
5. Bismuth
6. Ruthenium
7. Iridium
8. Rhenium
9. Metal Complexes vs. Organic Molecules
Funding
Conflicts of Interest
References
- Available online: https://www.pewtrusts.org/en/research-and-analysis/data-visualizations/2014/antibiotics-currently-in-clinical-development (accessed on 5 February 2020).
- Holm, R.H.; Kennepohl, P.; Solomon, E.I. Structural and Functional Aspects of Metal Sites in Biology. Chem. Rev. 1996, 96, 2239–2314. [Google Scholar] [CrossRef]
- Lovering, F.; Bikker, J.; Humblet, C. Escape from Flatland: Increasing Saturation as an Approach to Improving Clinical Success. J. Med. Chem. 2009, 52, 6752–6756. [Google Scholar] [CrossRef]
- Lovering, F. Escape from Flatland 2: Complexity and promiscuity. MedChemComm 2013, 4, 515–519. [Google Scholar] [CrossRef]
- Hung, A.W.; Ramek, A.; Wang, Y.; Kaya, T.; Wilson, J.A.; Clemons, P.A.; Young, D.W. Route to three-dimensional fragments using diversity-oriented synthesis. Proc. Natl. Acad. Sci. USA 2011, 108, 6799–6804. [Google Scholar] [CrossRef] [Green Version]
- Sauer, W.H.B.; Schwarz, M.K. Molecular Shape Diversity of Combinatorial Libraries: A Prerequisite for Broad Bioactivity. J. Chem. Inf. Comput. Sci. 2003, 43, 987–1003. [Google Scholar] [CrossRef]
- Galloway, W.R.J.D.; Isidro-Llobet, A.; Spring, D.R. Diversity-oriented synthesis as a tool for the discovery of novel biologically active small molecules. Nat. Comm. 2010, 1, 80. [Google Scholar] [CrossRef] [Green Version]
- Morrison, C.N.; Prosser, K.E.; Stokes, R.W.; Cordes, A.; Metzler-Nolte, N.; Cohen, S.M. Expanding medicinal chemistry into 3D space: Metallofragments as 3D scaffolds for fragment-based drug discovery. Chem. Sci. 2020. [Google Scholar] [CrossRef] [Green Version]
- Gasser, G. Metal Complexes and Medicine: A Successful Combination. Chimia 2015, 69, 442–446. [Google Scholar] [CrossRef] [Green Version]
- Johnstone, T.C.; Suntharalingam, K.; Lippard, S.J. The Next Generation of Platinum Drugs: Targeted Pt(II) Agents, Nanoparticle Delivery, and Pt(IV) Prodrugs. Chem. Rev. 2016, 116, 3436–3486. [Google Scholar] [CrossRef] [Green Version]
- Champion, G.D.; Graham, G.G.; Ziegler, J.B. The gold complexes. Clin. Rheumatol. 1990, 4, 491–534. [Google Scholar] [CrossRef]
- Kean, W.F.; Kean, I.R.L.J.I. Clinical pharmacology of gold. Inflammopharmacology 2008, 16, 112–125. [Google Scholar] [CrossRef]
- Barnard, P.J.; Berners-Price, S.J. Targeting the mitochondrial cell death pathway with gold compounds. Coord. Chem. Rev. 2007, 251, 1889–1902. [Google Scholar] [CrossRef]
- Mirzadeh, N.; Reddy, T.S.; Bhargava, S.K. Advances in diphosphine ligand-containing gold complexes as anticancer agents. Coord. Chem. Rev. 2019, 388, 343–359. [Google Scholar] [CrossRef]
- Harbut, M.B.; Vilchèze, C.; Luo, X.; Hensler, M.E.; Guo, H.; Yang, B.; Chatterjee, A.K.; Nizet, V.; Jacobs, W.R.; Schultz, P.G.; et al. Auranofin exerts broad-spectrum bactericidal activities by targeting thiol-redox homeostasis. Proc. Natl. Acad. Sci. USA 2015, 112, 4453–4458. [Google Scholar] [CrossRef] [Green Version]
- Wu, B.; Yang, X.; Yan, M. Synthesis and Structure–Activity Relationship Study of Antimicrobial Auranofin against ESKAPE Pathogens. J. Med. Chem. 2019, 62, 7751–7768. [Google Scholar] [CrossRef]
- ClinicalTrials.gov is a Database of Privately and Publicly Funded Clinical Studies Conducted around the World. Available online: www.clinicaltrials.gov (accessed on 5 December 2019).
- Biot, C.; Nosten, F.; Fraisse, L.; Ter-Minassian, D.; Khalife, J.; Dive, D. The antimalarial ferroquine: From bench to clinic. Parasite 2011, 18, 207–214. [Google Scholar] [CrossRef] [Green Version]
- Monro, S.; Colón, K.L.; Yin, H.; Roque, J.; Konda, P.; Gujar, S.; Thummel, R.P.; Lilge, L.; Cameron, C.G.; McFarland, S.A. Transition Metal Complexes and Photodynamic Therapy from a Tumor-Centered Approach: Challenges, Opportunities, and Highlights from the Development of TLD1433. Chem. Rev. 2019, 119, 797–828. [Google Scholar] [CrossRef]
- Zeng, L.; Gupta, P.; Chen, Y.; Wang, E.; Ji, L.; Chao, H.; Chen, Z.-S. The development of anticancer ruthenium (ii) complexes: From single molecule compounds to nanomaterials. Chem. Soc. Rev. 2017, 46, 5771–5804. [Google Scholar] [CrossRef]
- Kenny, R.G.; Marmion, C.J. Toward Multi-Targeted Platinum and Ruthenium Drugs—A New Paradigm in Cancer Drug Treatment Regimens? Chem. Rev. 2019, 119, 1058–1137. [Google Scholar] [CrossRef]
- Korfel, A.; Scheulen, M.E.; Schmoll, H.J.; Gründel, O.; Harstrick, A.; Knoche, M.; Fels, L.M.; Skorzec, M.; Bach, F.; Baumgart, J.; et al. Phase I clinical and pharmacokinetic study of titanocene dichloride in adults with advanced solid tumors. Clin. Cancer Res. 1998, 4, 2701–2708. [Google Scholar]
- Hajipour, M.J.; Fromm, K.M.; Akbar Ashkarran, A.; Jimenez de Aberasturi, D.; Larramendi, I.R.D.; Rojo, T.; Serpooshan, V.; Parak, W.J.; Mahmoudi, M. Antibacterial properties of nanoparticles. Trends Biotechnol. 2012, 30, 499–511. [Google Scholar] [CrossRef] [Green Version]
- Zheng, K.; Setyawati, M.I.; Leong, D.T.; Xie, J. Antimicrobial silver nanomaterials. Coord. Chem. Rev. 2018, 357, 1–17. [Google Scholar] [CrossRef]
- Rajchakit, U.; Sarojini, V. Recent Developments in Antimicrobial-Peptide-Conjugated Gold Nanoparticles. Bioconjugate Chem. 2017, 28, 2673–2686. [Google Scholar] [CrossRef]
- Gupta, A.; Mumtaz, S.; Li, C.-H.; Hussain, I.; Rotello, V.M. Combatting antibiotic-resistant bacteria using nanomaterials. Chem. Soc. Rev. 2019, 48, 415–427. [Google Scholar] [CrossRef]
- Sierra, M.A.; Casarrubios, L.; de la torre, M.C. Bio-Organometallic Derivatives of Antibacterial Drugs. Chem. Eur. J. 2019, 25, 7232–7242. [Google Scholar] [CrossRef]
- Hill, W.R.; Pillsbury, D.M. Argyria: The Pharmacology of Silver; Williams & Wilkins: Baltimore, MD, USA, 1939. [Google Scholar]
- Alexander, J.W. History of the Medical Use of Silver. Surg. Infect. 2009, 10, 289–292. [Google Scholar] [CrossRef] [Green Version]
- Mjos, K.D.; Orvig, C. Metallodrugs in Medicinal Inorganic Chemistry. Chem. Rev. 2014, 114, 4540–4563. [Google Scholar] [CrossRef]
- Aziz, Z.; Abu, S.F.; Chong, N.J. A systematic review of silver-containing dressings and topical silver agents (used with dressings) for burn wounds. Burns 2012, 38, 307–318. [Google Scholar] [CrossRef]
- Wattanaploy, S.; Chinaroonchai, K.; Namviriyachote, N.; Muangman, P. Randomized Controlled Trial of Polyhexanide/Betaine Gel Versus Silver Sulfadiazine for Partial-Thickness Burn Treatment. Int. J. Lower Extrem. Wounds 2017, 16, 45–50. [Google Scholar] [CrossRef]
- Rashaan, Z.M.; Krijnen, P.; Kwa, K.A.A.; van der Vlies, C.H.; Schipper, I.B.; Breederveld, R.S. Flaminal® versus Flamazine® in the treatment of partial thickness burns: A randomized controlled trial on clinical effectiveness and scar quality (FLAM study). Wound Repair Regen. 2019, 27, 257–267. [Google Scholar] [CrossRef] [Green Version]
- Maciel, A.B.D.S.; Ortiz, J.F.; Siqueira, B.S.; Zanette, G.F. Tissue healing efficacy in burn patients treated with 1% silver sulfadiazine versus other treatments: A systematic review and meta-analysis of randomized controlled trials. An. Bras. Dermatol. 2019, 94, 204–210. [Google Scholar] [CrossRef] [Green Version]
- Kascatan-Nebioglu, A.; Panzner, M.J.; Tessier, C.A.; Cannon, C.L.; Youngs, W.J. N-Heterocyclic carbene–silver complexes: A new class of antibiotics. Coord. Chem. Rev. 2007, 251, 884–895. [Google Scholar] [CrossRef]
- Medici, S.; Peana, M.; Crisponi, G.; Nurchi, V.M.; Lachowicz, J.I.; Remelli, M.; Zoroddu, M.A. Silver coordination compounds: A new horizon in medicine. Coord. Chem. Rev. 2016, 327, 349–359. [Google Scholar] [CrossRef]
- Johnson, N.A.; Southerland, M.R.; Youngs, W.J. Recent Developments in the Medicinal Applications of Silver-NHC Complexes and Imidazolium Salts. Molecules 2017, 22, 1263. [Google Scholar] [CrossRef]
- Wang, H.; Yan, A.; Liu, Z.; Yang, X.; Xu, Z.; Wang, Y.; Wang, R.; Koohi-Moghadam, M.; Hu, L.; Xia, W.; et al. Deciphering molecular mechanism of silver by integrated omic approaches enables enhancing its antimicrobial efficacy in E. coli. PLoS Biol. 2019, 17, e3000292. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Wang, M.; Yang, X.; Xu, X.; Hao, Q.; Yan, A.; Hu, M.; Lobinski, R.; Li, H.; Sun, H. Antimicrobial silver targets glyceraldehyde-3-phosphate dehydrogenase in glycolysis of E. coli. Chem. Sci. 2019, 10, 7193–7199. [Google Scholar] [CrossRef] [Green Version]
- Koch, R. Über bakteriologische Forschung. Dtsch. Med. Wochenschr. 1890, 16, 756–757. [Google Scholar]
- Glišić, B.Đ.; Djuran, M.I. Gold complexes as antimicrobial agents: An overview of different biological activities in relation to the oxidation state of the gold ion and the ligand structure. Dalton Trans. 2014, 43, 5950–5969. [Google Scholar] [CrossRef]
- Mora, M.; Gimeno, M.C.; Visbal, R. Recent advances in gold–NHC complexes with biological properties. Chem. Soc. Rev. 2019, 48, 447–462. [Google Scholar] [CrossRef]
- Dominelli, B.; Correia, J.D.G.; Kühn, F.E. Medicinal Applications of Gold(I/III)-Based Complexes Bearing N-Heterocyclic Carbene and Phosphine Ligands. J. Organomet. Chem. 2018, 866, 153–164. [Google Scholar] [CrossRef]
- Marzo, T.; Cirri, D.; Pollini, S.; Prato, M.; Fallani, S.; Cassetta, M.I.; Novelli, A.; Rossolini, G.M.; Messori, L. Auranofin and its Analogues Show Potent Antimicrobial Activity against Multidrug-Resistant Pathogens: Structure–Activity Relationships. ChemMedChem 2018, 13, 2448–2454. [Google Scholar] [CrossRef]
- Thangamani, S.; Mohammad, H.; Abushahba, M.F.N.; Sobreira, T.J.P.; Hedrick, V.E.; Paul, L.N.; Seleem, M.N. Antibacterial activity and mechanism of action of auranofin against multi-drug resistant bacterial pathogens. Sci. Rep. 2016, 6, 22571. [Google Scholar] [CrossRef] [Green Version]
- Blodgett, R.; Pietrusko, R. Long-term efficacy and safety of auranofin: A review of clinical experience. Scand. J. Rhenmatol. Suppl. 1986, 63, 67–78. [Google Scholar]
- Tharmalingam, N.; Ribeiro, N.Q.; Silva, D.L.D.; Naik, M.T.; Cruz, L.I.; Kim, W.; Shen, S.; Santos, J.D.d.; Ezikovich, K.; D’Agata, E.M.; et al. Auranofin is an effective agent against clinical isolates of Staphylococcus aureus. Future Med. Chem. 2019, 11, 1417–1425. [Google Scholar] [CrossRef]
- She, P.; Zhou, L.; Li, S.; Liu, Y.; Xu, L.; Chen, L.; Luo, Z.; Wu, Y. Synergistic Microbicidal Effect of Auranofin and Antibiotics Against Planktonic and Biofilm-Encased S. aureus and E. faecalis. Front. Microbiol. 2019, 10. [Google Scholar] [CrossRef]
- Epstein, T.D.; Wu, B.; Moulton, K.D.; Yan, M.; Dube, D.H. Sugar-Modified Analogs of Auranofin Are Potent Inhibitors of the Gastric Pathogen Helicobacter pylori. ACS Infect. Dis. 2019, 5, 1682–1687. [Google Scholar] [CrossRef]
- Bonchi, C.; Imperi, F.; Minandri, F.; Visca, P.; Frangipani, E. Repurposing of gallium-based drugs for antibacterial therapy. BioFactors 2014, 40, 303–312. [Google Scholar] [CrossRef]
- Choi, S.-R.; Britigan, B.E.; Narayanasamy, P. Dual Inhibition of Klebsiella pneumoniae and Pseudomonas aeruginosa Iron Metabolism Using Gallium Porphyrin and Gallium Nitrate. ACS Infect. Dis. 2019, 5, 1559–1569. [Google Scholar] [CrossRef]
- Ooi, M.L.; Richter, K.; Drilling, A.J.; Thomas, N.; Prestidge, C.A.; James, C.; Moratti, S.; Vreugde, S.; Psaltis, A.J.; Wormald, P.-J. Safety and Efficacy of Topical Chitogel- Deferiprone-Gallium Protoporphyrin in Sheep Model. Front. Cell. Infect. Microbiol. 2018, 9. [Google Scholar] [CrossRef]
- Banin, E.; Vasil, M.L.; Greenberg, E.P. Iron and Pseudomonas aeruginosa biofilm formation. Proc. Natl. Acad. Sci. USA 2005, 102, 11076–11081. [Google Scholar] [CrossRef] [Green Version]
- Banin, E.; Lozinski, A.; Brady, K.M.; Berenshtein, E.; Butterfield, P.W.; Moshe, M.; Chevion, M.; Greenberg, E.P.; Banin, E. The potential of desferrioxamine-gallium as an anti-Pseudomonas therapeutic agent. Proc. Natl. Acad. Sci. USA 2008, 105, 16761–16766. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Antunes, L.C.S.; Imperi, F.; Minandri, F.; Visca, P. In vitro and In vivo Antimicrobial Activities of Gallium Nitrate against Multidrug-Resistant Acinetobacter baumannii. Antimicrob. Agents Chemother. 2012, 56, 5961–5970. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hijazi, S.; Visaggio, D.; Pirolo, M.; Frangipani, E.; Bernstein, L.; Visca, P. Antimicrobial Activity of Gallium Compounds on ESKAPE Pathogens. Front. Cell. Infect. Microbiol. 2018, 8. [Google Scholar] [CrossRef] [Green Version]
- Goss, C.H.; Kaneko, Y.; Khuu, L.; Anderson, G.D.; Ravishankar, S.; Aitken, M.L.; Lechtzin, N.; Zhou, G.; Czyz, D.M.; McLean, K.; et al. Gallium disrupts bacterial iron metabolism and has therapeutic effects in mice and humans with lung infections. Sci. Transl. Med. 2018, 10, eaat7520. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Available online: https://www.cff.org/Trials/pipeline (accessed on 17 December 2019).
- Pandey, A.; Savino, C.; Ahn, S.H.; Yang, Z.; Van Lanen, S.G.; Boros, E. Theranostic Gallium Siderophore Ciprofloxacin Conjugate with Broad Spectrum Antibiotic Potency. J. Med. Chem. 2019, 62, 9947–9960. [Google Scholar] [CrossRef]
- Chitambar, C.R. Gallium and its competing roles with iron in biological systems. Biochim. Biophys. Acta Mol. Cell Res. 2016, 1863, 2044–2053. [Google Scholar] [CrossRef]
- Wang, Y.; Han, B.; Xie, Y.; Wang, H.; Wang, R.; Xia, W.; Li, H.; Sun, H. Combination of gallium(iii) with acetate for combating antibiotic resistant Pseudomonas aeruginosa. Chem. Sci. 2019, 10, 6099–6106. [Google Scholar] [CrossRef] [Green Version]
- Sun, H. Biological Chemistry of Arsenic, Antimony and Bismuth; Wiley: Chichester, UK, 2011. [Google Scholar]
- Fock, K.M.; Graham, D.Y.; Malfertheiner, P. Helicobacter pylori research: Historical insights and future directions. Nat. Rev. Gastroenterol. Hepatol. 2013, 10, 495–500. [Google Scholar] [CrossRef]
- Li, H.; Wang, R.; Sun, H. Systems Approaches for Unveiling the Mechanism of Action of Bismuth Drugs: New Medicinal Applications beyond Helicobacter Pylori Infection. Acc. Chem. Res. 2019, 52, 216–227. [Google Scholar] [CrossRef]
- Li, H.; Sun, H. Recent advances in bioinorganic chemistry of bismuth. Curr. Opin. Chem. Biol. 2012, 16, 74–83. [Google Scholar] [CrossRef] [Green Version]
- Hong, Y.; Lai, Y.-T.; Chan, G.C.-F.; Sun, H. Glutathione and multidrug resistance protein transporter mediate a self-propelled disposal of bismuth in human cells. Proc. Natl. Acad. Sci. USA 2015, 112, 3211–3216. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Hu, L.; Xu, F.; Quan, Q.; Lai, Y.-T.; Xia, W.; Yang, Y.; Chang, Y.-Y.; Yang, X.; Chai, Z.; et al. Integrative approach for the analysis of the proteome-wide response to bismuth drugs in Helicobacter pylori. Chem. Sci. 2017, 8, 4626–4633. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, R.; Lai, T.-P.; Gao, P.; Zhang, H.; Ho, P.-L.; Woo, P.C.-Y.; Ma, G.; Kao, R.Y.-T.; Li, H.; Sun, H. Bismuth antimicrobial drugs serve as broad-spectrum metallo-β-lactamase inhibitors. Nat. Comm. 2018, 9, 439. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dwyer, F.P.; Gyarfas, E.C.; Rogers, W.P.; Koch, J.H. Biological Activity of Complex Ions. Nature 1952, 170, 190–191. [Google Scholar] [CrossRef]
- Dwyer, F.; Reid, I.; Shulman, A.; Laycock, G.M.; Dixson, S. The biological actions of 1,10-phenanthroline and 2,2′-bipyridine hydrochlorides, quaternary salts and metal chelates and related compounds. Aust. J. Exp. Biol. Med. 1969, 47, 203–218. [Google Scholar] [CrossRef]
- Brandt, W.W.; Dwyer, F.P.; Gyarfas, E.D. Chelate Complexes of 1,10-Phenanthroline and Related Compounds. Chem. Rev. 1954, 54, 959–1017. [Google Scholar] [CrossRef]
- Li, F.; Collins, J.G.; Keene, F.R. Ruthenium complexes as antimicrobial agents. Chem. Soc. Rev. 2015, 44, 2529–2542. [Google Scholar] [CrossRef] [Green Version]
- Bolhuis, A.; Hand, L.; Marshall, J.E.; Richards, A.D.; Rodger, A.; Aldrich-Wright, J. Antimicrobial activity of ruthenium-based intercalators. Eur. J. Pharm. Sci. 2011, 42, 313–317. [Google Scholar] [CrossRef] [Green Version]
- Heinemann, F.; Karges, J.; Gasser, G. Critical Overview of the Use of Ru (II) Polypyridyl Complexes as Photosensitizers in One-Photon and Two-Photon Photodynamic Therapy. Acc. Chem. Res. 2017, 50, 2727–2736. [Google Scholar] [CrossRef]
- Cieplik, F.; Deng, D.; Crielaard, W.; Buchalla, W.; Hellwig, E.; Al-Ahmad, A.; Maisch, T. Antimicrobial photodynamic therapy – what we know and what we don’t. Crit. Rev. Microbiol. 2018, 44, 571–589. [Google Scholar] [CrossRef] [Green Version]
- Wainwright, M.; Maisch, T.; Nonell, S.; Plaetzer, K.; Almeida, A.; Tegos, G.P.; Hamblin, M.R. Photoantimicrobials—Are we afraid of the light? Lancet Infect. Dis. 2017, 17, e49–e55. [Google Scholar] [CrossRef]
- Kashef, N.; Hamblin, M.R. Can microbial cells develop resistance to oxidative stress in antimicrobial photodynamic inactivation? Drug Resist. Updat. 2017, 31, 31–42. [Google Scholar] [CrossRef]
- Donnelly, R.F.; Fletcher, N.C.; McCague, P.J.; Donnelly, J.; McCarron, P.A.; Tunney, M.M. Design, Synthesis and Photodynamic Antimicrobial Activity of Ruthenium Trischelate Diimine Complexes. Lett. Drug Des. Discov. 2007, 4, 175–179. [Google Scholar] [CrossRef]
- Frei, A.; Rubbiani, R.; Tubafard, S.; Blacque, O.; Anstaett, P.; Felgenträger, A.; Maisch, T.; Spiccia, L.; Gasser, G. Synthesis, Characterization, and Biological Evaluation of New Ru(II) Polypyridyl Photosensitizers for Photodynamic Therapy. J. Med. Chem. 2014, 57, 7280–7292. [Google Scholar] [CrossRef] [PubMed]
- Le Gall, T.; Lemercier, G.; Chevreux, S.; Tücking, K.-S.; Ravel, J.; Thétiot, F.; Jonas, U.; Schönherr, H.; Montier, T. Ruthenium (II) Polypyridyl Complexes as Photosensitizers for Antibacterial Photodynamic Therapy: A Structure—Activity Study on Clinical Bacterial Strains. ChemMedChem 2018, 13, 2229–2239. [Google Scholar] [CrossRef]
- Feng, Y.; Sun, W.-Z.; Wang, X.-S.; Zhou, Q.-X. Selective Photoinactivation of Methicillin-Resistant Staphylococcus aureus by Highly Positively Charged RuII Complexes. Chem. Eur. J. 2019, 25, 13879–13884. [Google Scholar] [CrossRef] [PubMed]
- Smith, N.A.; Zhang, P.; Greenough, S.E.; Horbury, M.D.; Clarkson, G.J.; McFeely, D.; Habtemariam, A.; Salassa, L.; Stavros, V.G.; Dowson, C.G.; et al. Combatting AMR: Photoactivatable ruthenium(ii)-isoniazid complex exhibits rapid selective antimycobacterial activity. Chem. Sci. 2017, 8, 395–404. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lewandowski, E.M.; Skiba, J.; Torelli, N.J.; Rajnisz, A.; Solecka, J.; Kowalski, K.; Chen, Y. Antibacterial properties and atomic resolution X-ray complex crystal structure of a ruthenocene conjugated β-lactam antibiotic. Chem. Commun. 2015, 51, 6186–6189. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Skiba, J.; Rajnisz, A.; de Oliveira, K.N.; Ott, I.; Solecka, J.; Kowalski, K. Ferrocenyl bioconjugates of ampicillin and 6-aminopenicillinic acid – Synthesis, electrochemistry and biological activity. Eur. J. Med. Chem. 2012, 57, 234–239. [Google Scholar] [CrossRef]
- Li, F.; Mulyana, Y.; Feterl, M.; Warner, J.M.; Collins, J.G.; Keene, F.R. The antimicrobial activity of inert oligonuclear polypyridylruthenium(ii) complexes against pathogenic bacteria, including MRSA. Dalton Trans. 2011, 40, 5032–5038. [Google Scholar] [CrossRef]
- Li, F.; Feterl, M.; Mulyana, Y.; Warner, J.M.; Collins, J.G.; Keene, F.R. In vitro susceptibility and cellular uptake for a new class of antimicrobial agents: Dinuclear ruthenium (II) complexes. J. Antimicrob. Chemother. 2012, 67, 2686–2695. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, F.; Harry, E.J.; Bottomley, A.L.; Edstein, M.D.; Birrell, G.W.; Woodward, C.E.; Keene, F.R.; Collins, J.G. Dinuclear ruthenium (ii) antimicrobial agents that selectively target polysomes in vivo. Chem. Sci. 2014, 5, 685–693. [Google Scholar] [CrossRef] [Green Version]
- Weber, D.K.; Sani, M.-A.; Downton, M.T.; Separovic, F.; Keene, F.R.; Collins, J.G. Membrane Insertion of a Dinuclear Polypyridylruthenium(II) Complex Revealed by Solid-State NMR and Molecular Dynamics Simulation: Implications for Selective Antibacterial Activity. J. Am. Chem. Soc. 2016, 138, 15267–15277. [Google Scholar] [CrossRef]
- Li, X.; Gorle, A.K.; Ainsworth, T.D.; Heimann, K.; Woodward, C.E.; Grant Collins, J.; Richard Keene, F. RNA and DNA binding of inert oligonuclear ruthenium (ii) complexes in live eukaryotic cells. Dalton Trans. 2015, 44, 3594–3603. [Google Scholar] [CrossRef] [Green Version]
- Gorle, A.K.; Feterl, M.; Warner, J.M.; Wallace, L.; Keene, F.R.; Collins, J.G. Tri- and tetra-nuclear polypyridyl ruthenium (ii) complexes as antimicrobial agents. Dalton Trans. 2014, 43, 16713–16725. [Google Scholar] [CrossRef] [Green Version]
- Pandrala, M.; Li, F.; Feterl, M.; Mulyana, Y.; Warner, J.M.; Wallace, L.; Keene, F.R.; Collins, J.G. Chlorido-containing ruthenium (ii) and iridium (iii) complexes as antimicrobial agents. Dalton Trans. 2013, 42, 4686–4694. [Google Scholar] [CrossRef] [PubMed]
- Smitten, K.L.; Southam, H.M.; de la Serna, J.B.; Gill, M.R.; Jarman, P.J.; Smythe, C.G.W.; Poole, R.K.; Thomas, J.A. Using Nanoscopy To Probe the Biological Activity of Antimicrobial Leads That Display Potent Activity against Pathogenic, Multidrug Resistant, Gram-Negative Bacteria. ACS Nano 2019, 13, 5133–5146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gross, M.; Cramton, S.E.; Götz, F.; Peschel, A. Key Role of Teichoic Acid Net Charge in Staphylococcus aureus Colonization of Artificial Surfaces. Infect. Immun. 2001, 69, 3423–3426. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smitten, K.L.; Fairbanks, S.D.; Robertson, C.C.; Bernardino de la Serna, J.; Foster, S.J.; Thomas, J.A. Ruthenium based antimicrobial theranostics—Using nanoscopy to identify therapeutic targets and resistance mechanisms in Staphylococcus aureus. Chem. Sci. 2019. [Google Scholar] [CrossRef] [Green Version]
- Pandrala, M.; Li, F.; Wallace, L.; Steel, P.J.; Moore II, B.; Autschbach, J.; Collins, J.G.; Keene, F.R. Iridium (iii) Complexes Containing 1,10-Phenanthroline and Derivatives: Synthetic, Stereochemical, and Structural Studies, and their Antimicrobial Activity. Aust. J. Chem. 2013, 66, 1065–1073. [Google Scholar] [CrossRef]
- Lu, L.; Liu, L.-J.; Chao, W.-C.; Zhong, H.-J.; Wang, M.; Chen, X.-P.; Lu, J.-J.; Li, R.-N.; Ma, D.-L.; Leung, C.-H. Identification of an iridium (III) complex with anti-bacterial and anti-cancer activity. Sci. Rep. 2015, 5, 14544. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jain, N.; Alam, P.; Laskar, I.R.; Panwar, J. Aggregation induced phosphorescence active iridium (iii) complexes for integrated sensing and inhibition of bacterial growth in aqueous solution. RSC Adv. 2015, 5, 61983–61988. [Google Scholar] [CrossRef]
- Sauvageot, E.; Elie, M.; Gaillard, S.; Daniellou, R.; Fechter, P.; Schalk, I.J.; Gasser, V.; Renaud, J.L.; Mislin, G.L.A. Antipseudomonal activity enhancement of luminescent iridium(iii) dipyridylamine complexes under visible blue light. Metallomics 2017, 9, 1820–1827. [Google Scholar] [CrossRef]
- Huang, H.; Banerjee, S.; Sadler, P.J. Recent Advances in the Design of Targeted Iridium(III) Photosensitizers for Photodynamic Therapy. ChemBioChem 2018, 19, 1574–1589. [Google Scholar] [CrossRef] [PubMed]
- Karpin, G.W.; Merola, J.S.; Falkinham, J.O. Transition Metal–α-Amino Acid Complexes with Antibiotic Activity against Mycobacterium spp. Antimicrob. Agents Chemother. 2013, 57, 3434–3436. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karpin, G.W.; Morris, D.M.; Ngo, M.T.; Merola, J.S.; Falkinham, J.O. Transition metal diamine complexes with antimicrobial activity against Staphylococcus aureus and methicillin-resistant S. aureus (MRSA). MedChemComm 2015, 6, 1471–1478. [Google Scholar] [CrossRef] [Green Version]
- DuChane, C.M.; Karpin, G.W.; Ehrich, M.; Falkinham, J.O.; Merola, J.S. Iridium piano stool complexes with activity against S. aureus and MRSA: It is past time to truly think outside of the box. MedChemComm 2019, 10, 1391–1398. [Google Scholar] [CrossRef]
- Chen, F.; Moat, J.; McFeely, D.; Clarkson, G.; Hands-Portman, I.J.; Furner-Pardoe, J.P.; Harrison, F.; Dowson, C.G.; Sadler, P.J. Biguanide Iridium (III) Complexes with Potent Antimicrobial Activity. J. Med. Chem. 2018, 61, 7330–7344. [Google Scholar] [CrossRef]
- Metalsdaily. Available online: https://www.metalsdaily.com (accessed on 5 December 2019).
- Konkankit, C.C.; Marker, S.C.; Knopf, K.M.; Wilson, J.J. Anticancer activity of complexes of the third row transition metals, rhenium, osmium, and iridium. Dalton Trans. 2018, 47, 9934–9974. [Google Scholar] [CrossRef]
- Bauer, E.B.; Haase, A.A.; Reich, R.M.; Crans, D.C.; Kühn, F.E. Organometallic and coordination rhenium compounds and their potential in cancer therapy. Coord. Chem. Rev. 2019, 393, 79–117. [Google Scholar] [CrossRef]
- Patra, M.; Gasser, G.; Bobukhov, D.; Merz, K.; Shtemenko, A.V.; Metzler-Nolte, N. Sequential insertion of three different organometallics into a versatile building block containing a PNA backbone. Dalton Trans. 2010, 39, 5617–5619. [Google Scholar] [CrossRef] [PubMed]
- Patra, M.; Wenzel, M.; Prochnow, P.; Pierroz, V.; Gasser, G.; Bandow, J.E.; Metzler-Nolte, N. An organometallic structure-activity relationship study reveals the essential role of a Re(CO)3 moiety in the activity against gram-positive pathogens including MRSA. Chem. Sci. 2015, 6, 214–224. [Google Scholar] [CrossRef] [PubMed]
- Wenzel, M.; Patra, M.; Senges, C.H.R.; Ott, I.; Stepanek, J.J.; Pinto, A.; Prochnow, P.; Vuong, C.; Langklotz, S.; Metzler-Nolte, N.; et al. Analysis of the Mechanism of Action of Potent Antibacterial Hetero-tri-organometallic Compounds: A Structurally New Class of Antibiotics. ACS Chem. Biol. 2013, 8, 1442–1450. [Google Scholar] [CrossRef] [PubMed]
- Frei, A.; Amado, M.; Cooper, M.A.; Blaskovich, M.A.T. Light-activated Rhenium Complexes with Dual Mode of Action against Bacteria. Chem. Eur. J. 2019. [Google Scholar] [CrossRef]
- Siegmund, D.; Lorenz, N.; Gothe, Y.; Spies, C.; Geissler, B.; Prochnow, P.; Nuernberger, P.; Bandow, J.E.; Metzler-Nolte, N. Benzannulated Re (i)–NHC complexes: Synthesis, photophysical properties and antimicrobial activity. Dalton Trans. 2017, 46, 15269–15279. [Google Scholar] [CrossRef]
- Metzler-Nolte, N.; Siegmund, D.; Bandow, J.E.; Schäkermann, S. EF-Tu-binding antibiotics containing benzimidazolylidene NHC-carbene rhenium complexes with chelating diimine ligands. Patent WO2019007664, 2019. [Google Scholar]
- Feng, Q.L.; Wu, J.; Chen, G.Q.; Cui, F.Z.; Kim, T.N.; Kim, J.O. A mechanistic study of the antibacterial effect of silver ions on Escherichia coli and Staphylococcus aureus. J. Biomed. Mater. Res. 2000, 52, 662–668. [Google Scholar] [CrossRef]
- Hijazi, S.; Visca, P.; Frangipani, E. Gallium-Protoporphyrin IX Inhibits Pseudomonas aeruginosa Growth by Targeting Cytochromes. Front. Cell. Infect. Microbiol. 2017, 7. [Google Scholar] [CrossRef] [Green Version]
- Patra, M.; Gasser, G.; Pinto, A.; Merz, K.; Ott, I.; Bandow, J.E.; Metzler-Nolte, N. Synthesis and Biological Evaluation of Chromium Bioorganometallics Based on the Antibiotic Platensimycin Lead Structure. ChemMedChem 2009, 4, 1930–1938. [Google Scholar] [CrossRef]
- Patra, M.; Gasser, G.; Wenzel, M.; Merz, K.; Bandow, J.E.; Metzler-Nolte, N. Synthesis and Biological Evaluation of Ferrocene-Containing Bioorganometallics Inspired by the Antibiotic Platensimycin Lead Structure. Organometallics 2010, 29, 4312–4319. [Google Scholar] [CrossRef]
- Zobi, F. CO and CO-releasing molecules in medicinal chemistry. Future Med. Chem. 2013, 5, 175–188. [Google Scholar] [CrossRef]
- Ward, J.S.; Lynam, J.M.; Moir, J.; Fairlamb, I.J.S. Visible-Light-Induced CO Release from a Therapeutically Viable Tryptophan-Derived Manganese (I) Carbonyl (TryptoCORM) Exhibiting Potent Inhibition against E. coli. Chem. Eur. J. 2014, 20, 15061–15068. [Google Scholar] [CrossRef] [PubMed]
- Ward, J.S.; Morgan, R.; Lynam, J.M.; Fairlamb, I.J.S.; Moir, J.W.B. Toxicity of tryptophan manganese (i) carbonyl (Trypto-CORM), against Neisseria gonorrhoeae. MedChemComm 2017, 8, 346–352. [Google Scholar] [CrossRef] [PubMed]
- Simpson, P.V.; Nagel, C.; Bruhn, H.; Schatzschneider, U. Antibacterial and Antiparasitic Activity of Manganese (I) Tricarbonyl Complexes with Ketoconazole, Miconazole, and Clotrimazole Ligands. Organometallics 2015, 34, 3809–3815. [Google Scholar] [CrossRef]
- Low, M.L.; Maigre, L.; Dorlet, P.; Guillot, R.; Pagès, J.-M.; Crouse, K.A.; Policar, C.; Delsuc, N. Conjugation of a New Series of Dithiocarbazate Schiff Base Copper(II) Complexes with Vectors Selected to Enhance Antibacterial Activity. Bioconjugate Chem. 2014, 25, 2269–2284. [Google Scholar] [CrossRef]
- Yang, X.-Y.; Xu, J.-Y.; Meng, M.; Li, N.; Liu, C.-Y.; He, Q.-Y. Dirhodium (II) complex interferes with iron-transport system to exert antibacterial action against Streptococcus pneumoniae. J. Proteom. 2019, 194, 160–167. [Google Scholar] [CrossRef]
- Kalaivani, P.; Prabhakaran, R.; Ramachandran, E.; Dallemer, F.; Paramaguru, G.; Renganathan, R.; Poornima, P.; Vijaya Padma, V.; Natarajan, K. Influence of terminal substitution on structural, DNA, Protein binding, anticancer and antibacterial activities of palladium (ii) complexes containing 3-methoxy salicylaldehyde-4(N) substituted thiosemicarbazones. Dalton Trans. 2012, 41, 2486–2499. [Google Scholar] [CrossRef]
- Kalaivani, P.; Prabhakaran, R.; Dallemer, F.; Poornima, P.; Vaishnavi, E.; Ramachandran, E.; Padma, V.V.; Renganathan, R.; Natarajan, K. DNA, protein binding, cytotoxicity, cellular uptake and antibacterial activities of new palladium (ii) complexes of thiosemicarbazone ligands: Effects of substitution on biological activity. Metallomics 2012, 4, 101–113. [Google Scholar] [CrossRef]
- Johnstone, T.C.; Alexander, S.M.; Lin, W.; Lippard, S.J. Effects of Monofunctional Platinum Agents on Bacterial Growth: A Retrospective Study. J. Am. Chem. Soc. 2014, 136, 116–118. [Google Scholar] [CrossRef] [Green Version]
- Patra, M.; Gasser, G.; Metzler-Nolte, N. Small organometallic compounds as antibacterial agents. Dalton Trans. 2012, 41, 6350–6358. [Google Scholar] [CrossRef] [Green Version]
- Frei, A.; Zuegg, J.; Elliott, A.G.; Baker, M.V.; Braese, S.; Brown, C.; Chen, F.; Dowson, C.G.; Dujardin, G.; Jung, N.; et al. Metal Complexes as a Promising Source for New Antibiotics. Chem. Sci. 2020. [Google Scholar] [CrossRef] [Green Version]
- Hansford, K.A.; Blaskovich, M.A.; Cooper, M.A. Chemical philanthropy: A path forward for antibiotic discovery? Future Med. Chem. 2016, 8, 925–929. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cooper, M.A. A community-based approach to new antibiotic discovery. Nat. Rev. Drug Discov. 2015, 14, 587–588. [Google Scholar] [CrossRef] [PubMed]
- Blaskovich, M.A.T.; Zuegg, J.; Elliott, A.G.; Cooper, M.A. Helping Chemists Discover New Antibiotics. ACS Infect. Dis. 2015, 1, 285–287. [Google Scholar] [CrossRef] [PubMed]
- Gianferrara, T.; Bratsos, I.; Alessio, E. A categorization of metal anticancer compounds based on their mode of action. Dalton Trans. 2009, 7588–7598. [Google Scholar] [CrossRef] [PubMed]
- Dougan, S.J.; Habtemariam, A.; McHale, S.E.; Parsons, S.; Sadler, P.J. Catalytic organometallic anticancer complexes. Proc. Natl. Acad. Sci. USA 2008, 105, 11628–11633. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coverdale, J.P.C.; Romero-Canelón, I.; Sanchez-Cano, C.; Clarkson, G.J.; Habtemariam, A.; Wills, M.; Sadler, P.J. Asymmetric transfer hydrogenation by synthetic catalysts in cancer cells. Nat. Chem. 2018, 10, 347. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Banerjee, S.; Qiu, K.; Zhang, P.; Blacque, O.; Malcomson, T.; Paterson, M.J.; Clarkson, G.J.; Staniforth, M.; Stavros, V.G.; et al. Targeted photoredox catalysis in cancer cells. Nature Chem. 2019. [Google Scholar] [CrossRef]
- Soldevila-Barreda, J.J.; Romero-Canelón, I.; Habtemariam, A.; Sadler, P.J. Transfer hydrogenation catalysis in cells as a new approach to anticancer drug design. Nat. Commun. 2015, 6, 6582. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Li, H.; Sun, H. Metalloproteomics for Unveiling the Mechanism of Action of Metallodrugs. Inorg. Chem. 2019, 58, 13673–13685. [Google Scholar] [CrossRef]







| Aba | Pab | Ecc | Kpd | Sae | Eff | Ecg | Hph | |
|---|---|---|---|---|---|---|---|---|
| Aur i | 47 | 377 | 189 | 377 | 0.04 | 0.09–0.2 | 24 | n.d. |
| 1 | 4–17 | >547 | 4–9 | 34 | 0.3–0.5 | 0.3–0.5 | 9 | n.d. |
| 2 | 9–17 | >547 | 4 | 34 | 0.3 | 0.3–0.5 | 9 | n.d. |
| 3 | 3–6 | 23–91 | 3 | 11 | 0.3 | 0.3 | 1–6 | n.d. |
| 4 | 8–16 | >503 | 8–16 | 31 | 0.5 | 0.2–0.5 | 8–31 | n.d. |
| 5 | 24 | 189 | 47 | 189 | 0.04–0.09 | 0.09 | 12 | 0.3 |
| 6 | 15–29 | 464 | 116 | 464 | 0.02 | 0.05–0.1 | 4–7 | 0.35 |
| Compound | # a | Metal | G(+) b | G(−) c | Media d | Target/MoA e | Cytotoxicity f | In vivog |
|---|---|---|---|---|---|---|---|---|
| AgNO3 [113] | 1 | Ag | Yes | Yes | LB | TCA cycle | Yes (Yes) | Yes (human) |
| Aur [15] | 1 | Au | Yes | No | CAMBH | Trx inhibition | Yes (Yes) | Yes (mouse) |
| 1–6 [16] | 6 | Au | Yes | Yes | CAMBH | Trx inhibition | Yes (some) | n.d. |
| Ganite [50] | 1 | Ga | No | Yes | MH; HS | Fe-metabolism [61] | Yes (No) | Yes (human) |
| Ga(DFO) [53] | 1 | Ga | n.d. | Yes | TSB | Fe-metabolism [61] | Yes (No) | Yes (rabbit) |
| Ga(PPIX) [51] | 1 | Ga | Yes | Yes | LB, MHB, DMHB, RPMI-HS | Fe-metabolism [61] Cytochrome [114] | Yes (No) | Yes (sheep) |
| CBS [64] | 1 | Bi | Yes | Yes | TSB | Multiple targets and MBLs [64] | Yes (No) | Yes |
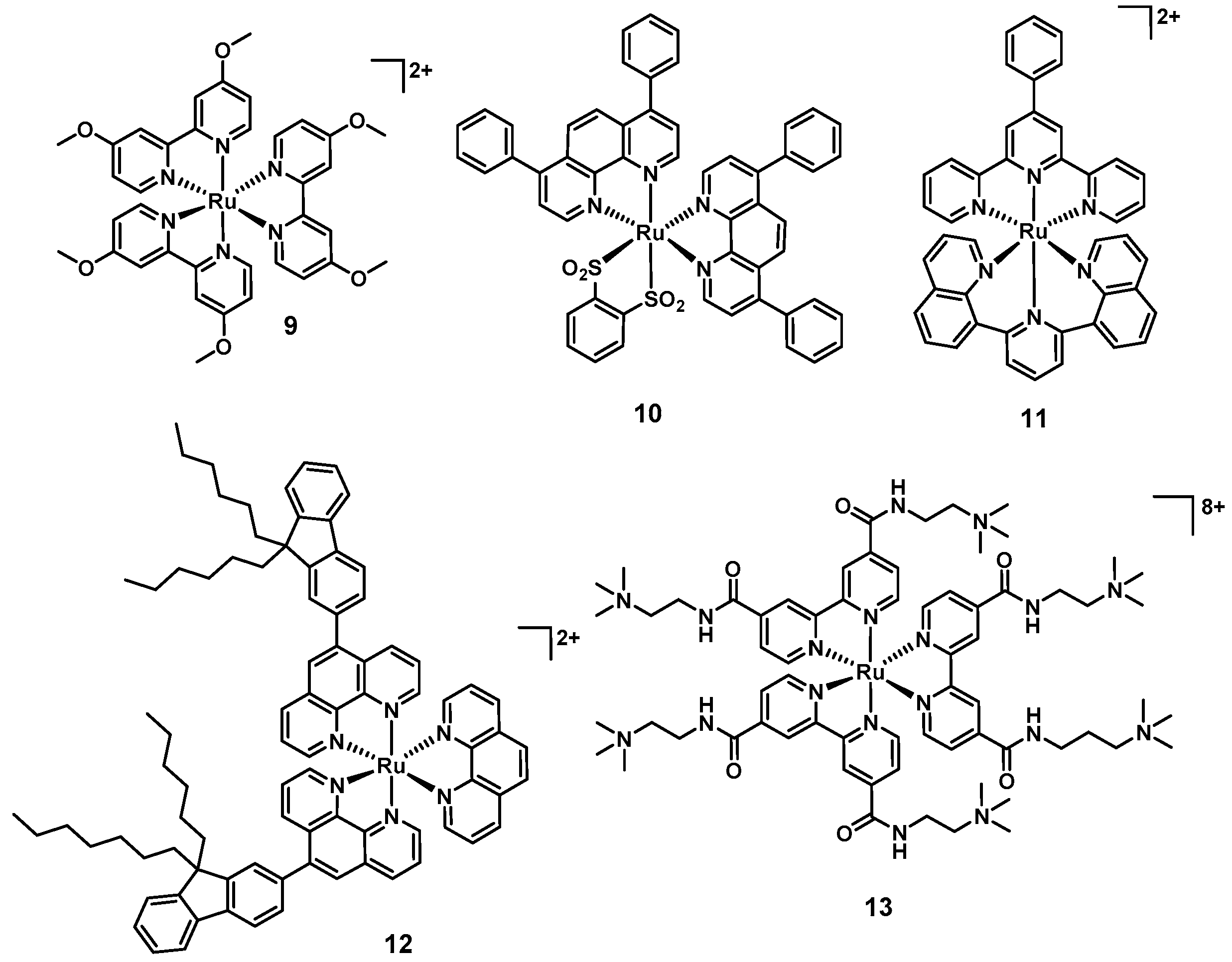
| 7 [70] | 10 | Ru | Yes | n.d. | DFH | n.d. | Yes (Yes) | Yes (mouse) |
| 8 [73] | 3 | Ru | Yes | No | LB, BHI | DNA intercalation | n.d. | Yes (fungi) |
| 9 [78] | 3 | Ru | Yes | Yes | MH | PDT | n.d. | n.d. |
| 10 [79] | 1 | Ru | Yes | No | MH | PDT | Yes (No) | n.d. |
| 11 [79] | 1 | Ru | Yes | Yes | MH | PDT | Yes (No) | n.d. |
| 12 [80] | 17 | Ru | Yes | Yes | LB | PDT | Yes (Some) | n.d. |
| 13 [81] | 3 | Ru | Yes | Yes | LB | PDT | Yes (No) | n.d. |
| 14 [82] | 1 | Ru | No | No | TSB | Light-triggered isoniazid release | Yes (No) | n.d |
| 15 [83] | 2 | Ru | Yes | Yes | CAMBH | β-lactamase | n.d. | n.d. |
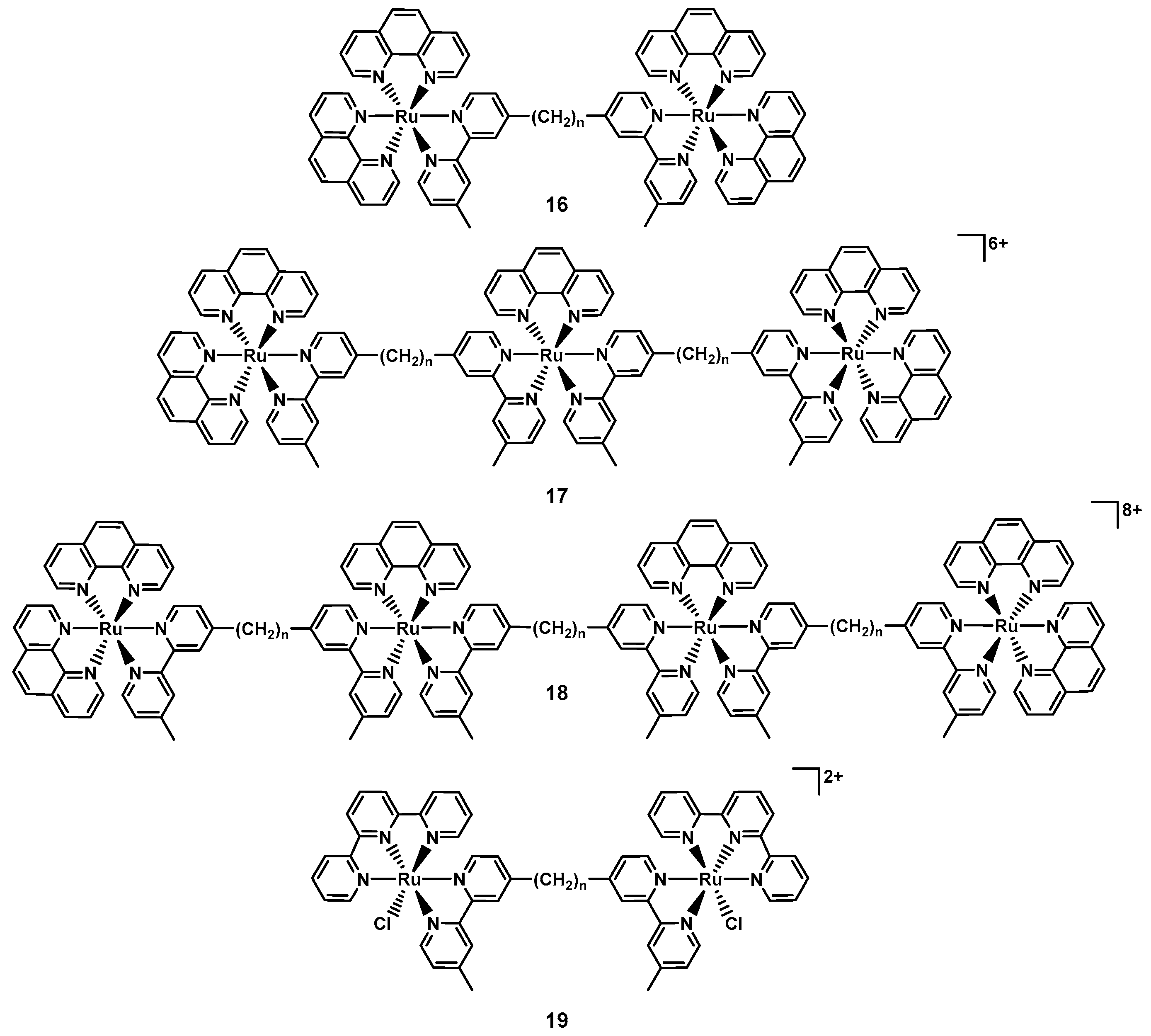
| 16 [85] | 26 | Ru | Yes | Yes | CAMBH | RNA, ribosome | Yes (Some) | n.d. |
| 17–18 [89] | 14 | Ru | Yes | Yes | CAMBH | Bacterial membrane | Yes (Some) | n.d. |
| 19 [91] | 3 | Ru | Yes | Yes | CAMBH | Bacterial membrane | Yes (Some) | n.d. |
| 20 [92] | 4 | Ru | Yes | Yes | CAMBH CDM | Bacterial membrane, DNA | Yes (No) | Yes (moth) |
| 21 [96] | 5 | Ir | Yes | No | LB | Not studied | Yes (Yes) | n.d. |
| 22 [97] | 6 | Ir | Yes | Yes | MH | Binds DNA | n.d. | n.d. |
| 23 [99] | 3 | Ir | n.d. | Yes | LB | Not studied | n.d. | n.d. |
| 24 [101] | 16 | Ir | Yes | n.d. | MH | Not studied | Yes (No) | n.d. |
| 25 [102] | 8 | Ir | Yes | n.d. | MH | Not studied | Yes (No) | Yes (mouse) |
| 26 [103] | 14 | Ir | Yes | Yes | CAMBH | Biguanine ligand release | Yes (Some) | n.d. |
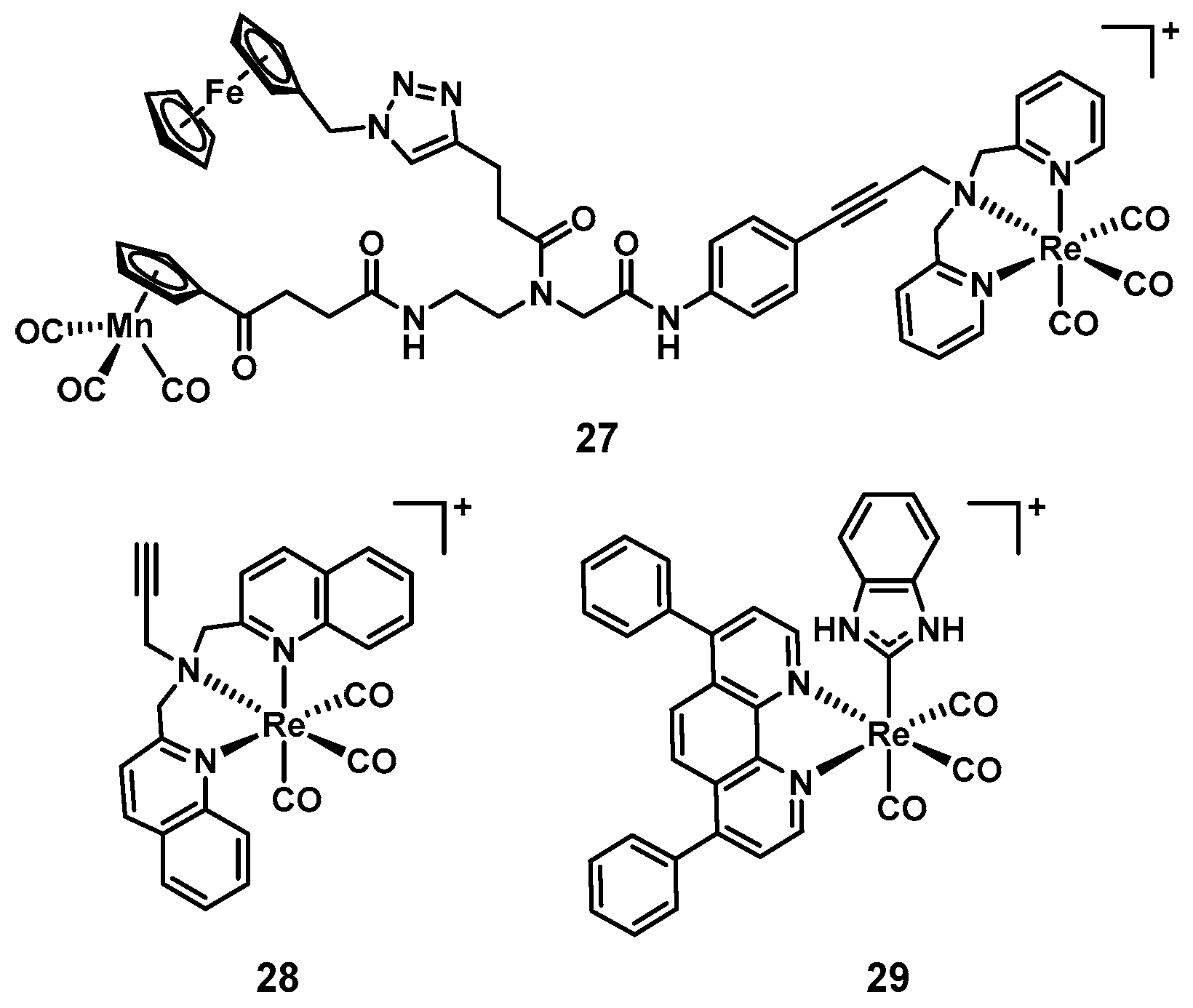
| 27 [108] | 13 | Re | Yes | No | MH | Cell wall synthesis, respiration | Yes (Some) | n.d. |
| 28 [110] | 3 | Re | Yes | Yes | CAMBH | PDT | Yes (Some) | n.d. |
| 29 [111] | 10 | Re | Yes | No | MH | Not studied | n.d. | n.d. |
© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Frei, A. Metal Complexes, an Untapped Source of Antibiotic Potential? Antibiotics 2020, 9, 90. https://doi.org/10.3390/antibiotics9020090
Frei A. Metal Complexes, an Untapped Source of Antibiotic Potential? Antibiotics. 2020; 9(2):90. https://doi.org/10.3390/antibiotics9020090
Chicago/Turabian StyleFrei, Angelo. 2020. "Metal Complexes, an Untapped Source of Antibiotic Potential?" Antibiotics 9, no. 2: 90. https://doi.org/10.3390/antibiotics9020090






