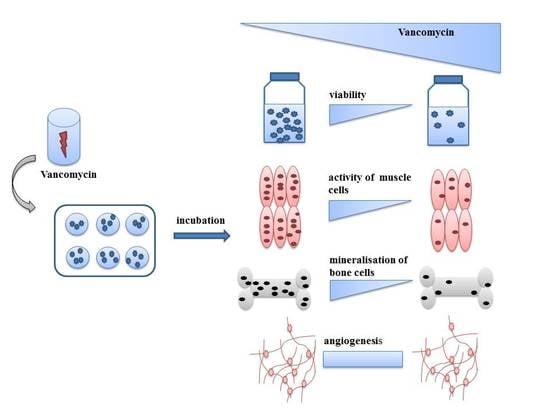
Toxic Effect of Vancomycin on Viability and Functionality of Different Cells Involved in Tissue Regeneration
Abstract
:1. Introduction
2. Results
2.1. Micobiology (Determination of the Minimal Inhibitory Concentration (MIC))
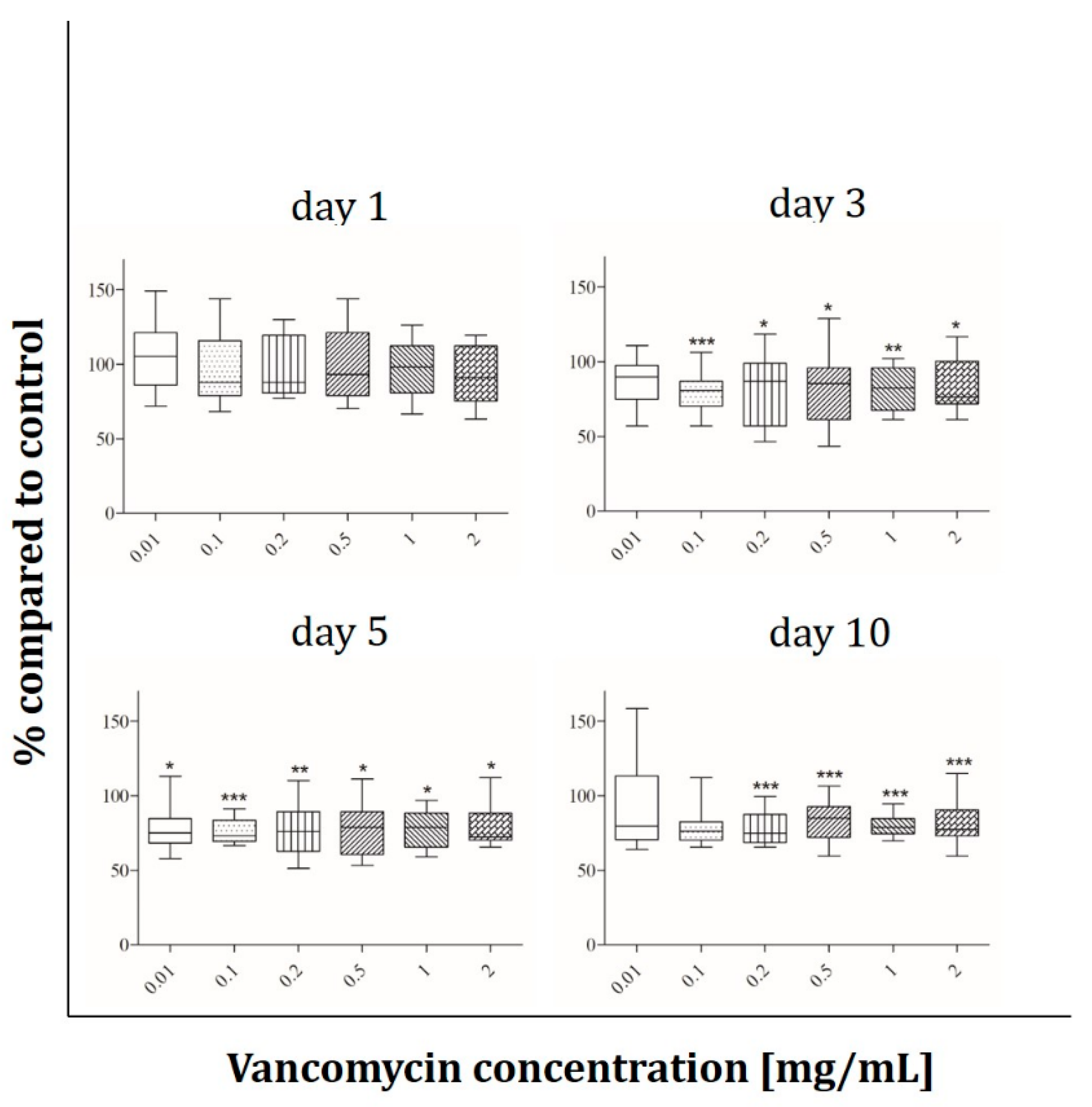
2.2. Cell Viability
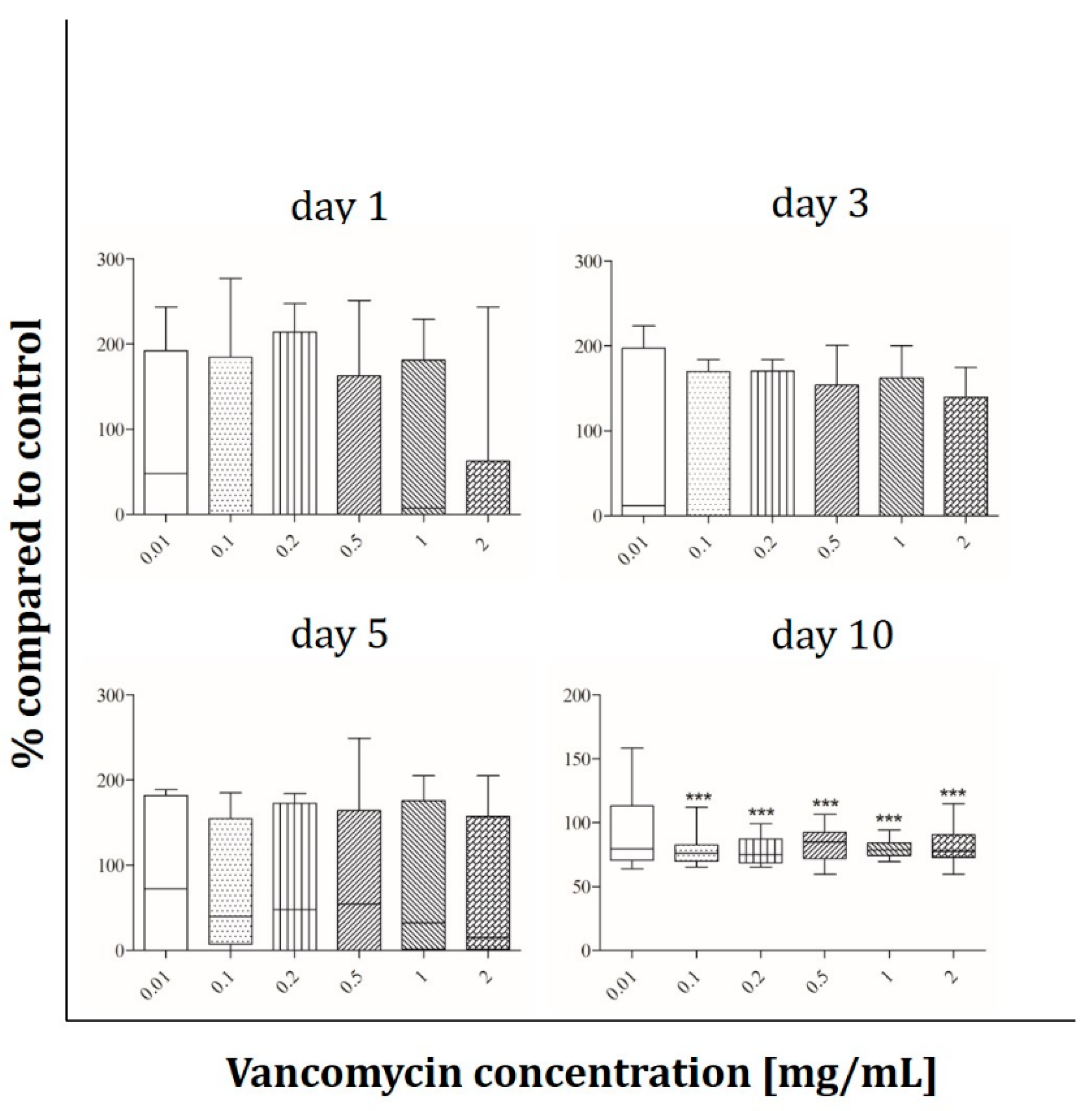
2.3. Angiogenesis Assay
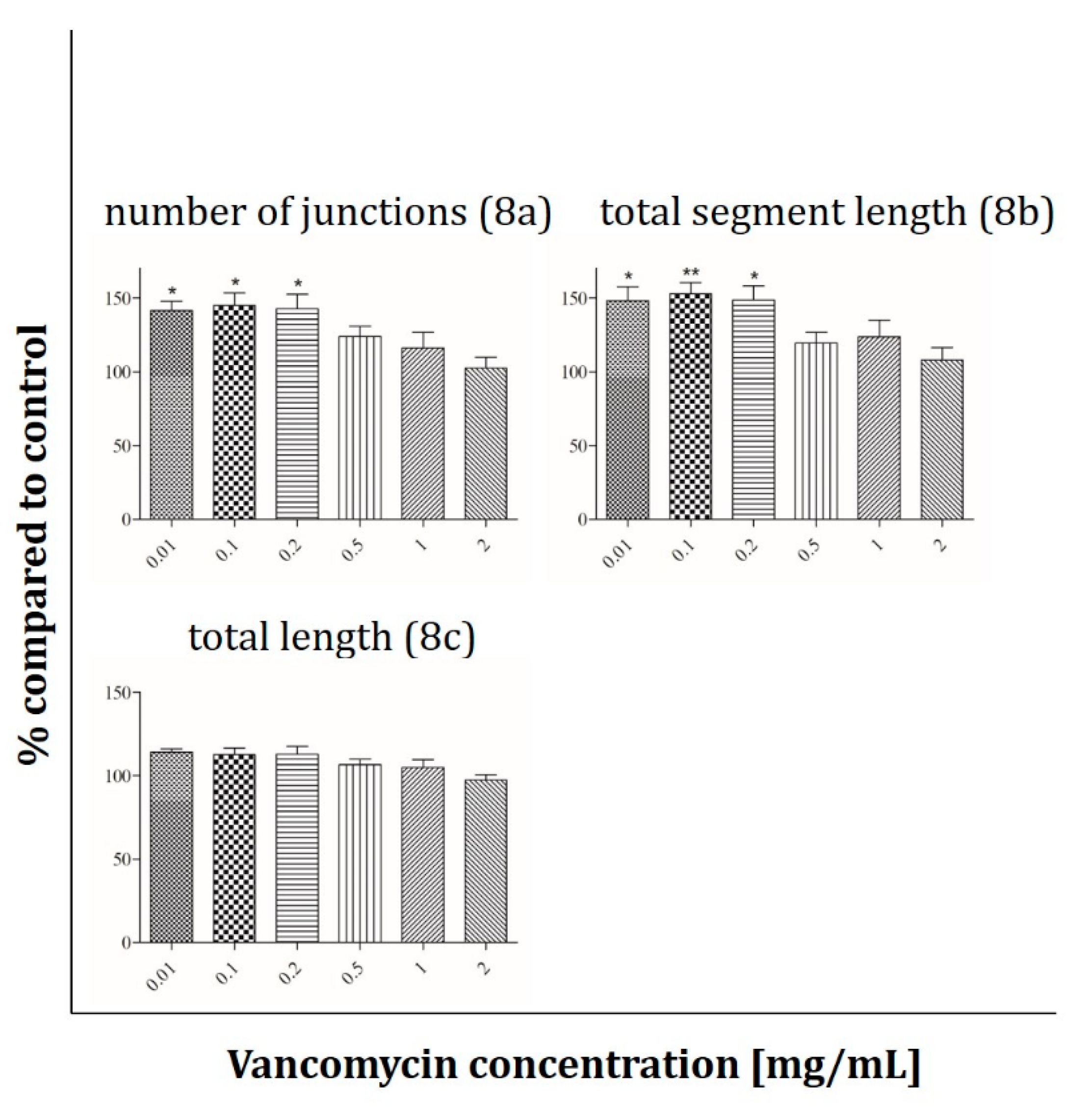
2.4. Immunofluorescence Analysis
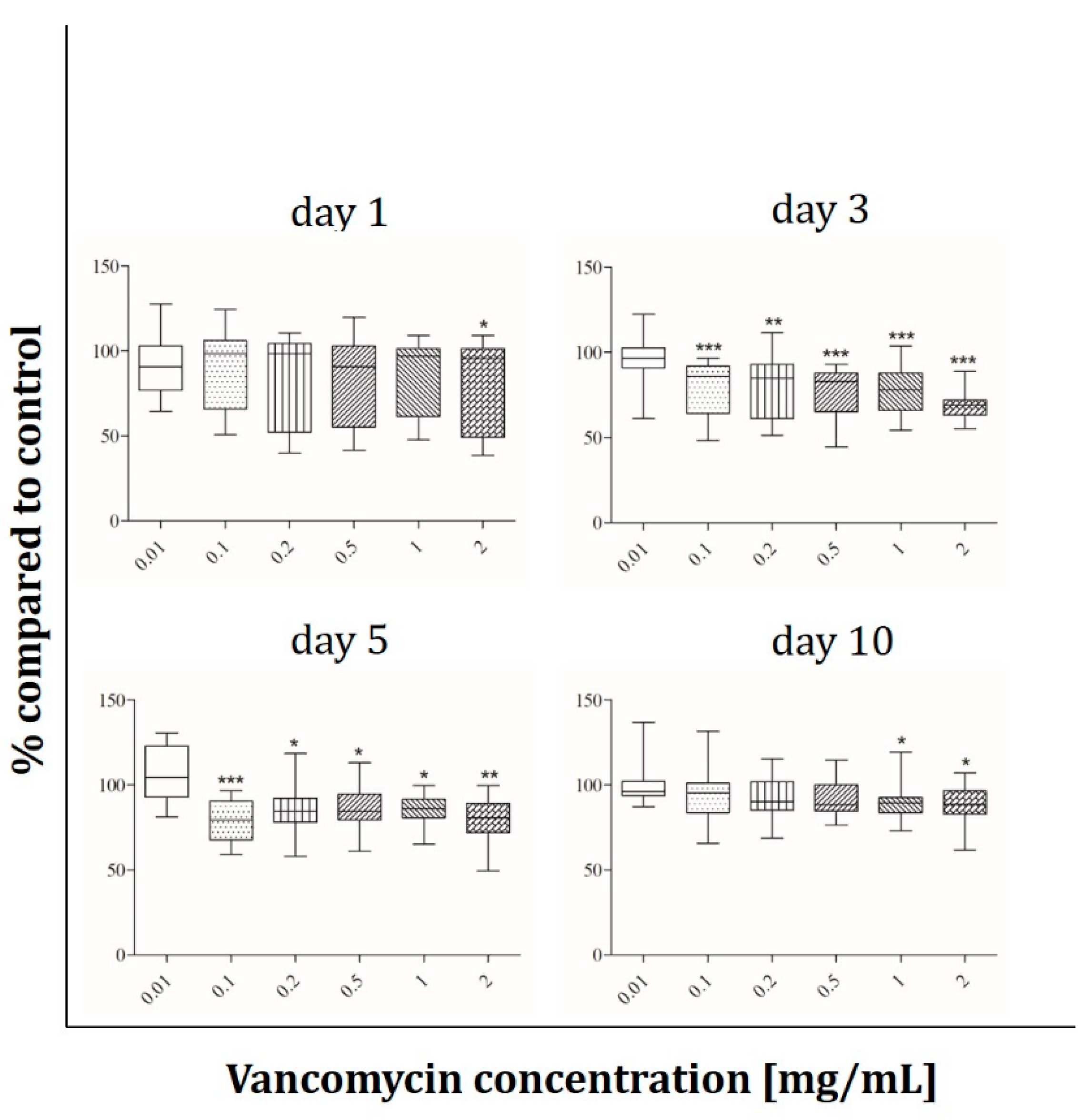
2.5. ALP Activity
3. Discussion
3.1. Microbiology (MIC Determination)
3.2. Cell Viability
3.3. Cell Functionality
4. Conclusion
5. Materials and Methods
5.1. Microbiology (MIC Determination)
5.2. Vancomycin
5.3. Cell Culture
5.4. Viability and functionality assays
5.4.1. AlamarBlue® Assay
5.4.2. Angiogenesis Assay
5.4.3. Immunofluorescence Analyses
5.4.4. ALP Activity
5.5. Statistical Analysis
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Yuan, K.; Chen, H.L. Obesity and surgical site infections risk in orthopedics: A meta-analysis. Int. J. Surg. 2013, 11, 383–388. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Horan, T.C.; Gaynes, R.P.; Martone, W.J.; Jarvis, W.R.; Emori, T.G. CDC definitions of nosocomial surgical site infections, 1992: A modification of CDC definitions of surgical wound infections. Infect. Control Hosp. Epidemiol. 1992, 13, 606–608. [Google Scholar] [CrossRef] [PubMed]
- Emori, T.G.; Gaynes, R.P. An overview of nosocomial infections, including the role of the microbiology laboratory. Clin. Microbiol. Rev. 1993, 6, 428–442. [Google Scholar] [CrossRef] [PubMed]
- Zahran, W.; Zein-Eldeen, A.; Hamam, S.; Sabal, E.M. Surgical site infections: Problem of multidrug-resistant bacteria. Menoufia Med. J. 2014, 30, 1005. [Google Scholar] [CrossRef]
- Broex, E.C.; van Asselt, A.D.; Bruggeman, C.A.; van Tiel, F.H. Surgical site infections: How high are the costs? J. Hosp. Infect. 2009, 72, 193–201. [Google Scholar] [CrossRef] [PubMed]
- Mankin, H.J.; Hornicek, F.J.; Raskin, K.A. Infection in massive bone allografts. Clin. Orthop. Relat. Res. 2005, 432, 210–216. [Google Scholar] [CrossRef]
- Schroder, H.A.; Christoffersen, H.; Sorensen, T.S.; Lindequist, S. Fractures of the shaft of the tibia treated with Hoffmann external fixation. Arch. Orthop. Trauma. Surg. 1986, 105, 28–30. [Google Scholar] [CrossRef]
- Thakur, A.J.; Patankar, J. Open tibial fractures. Treatment by uniplanar external fixation and early bone grafting. J. Bone Jt. Surg. Br. Vol. 1991, 73, 448–451. [Google Scholar] [CrossRef]
- De Bastiani, G.; Aldegheri, R.; Renzi Brivio, L. Dynamic axial fixation. A rational alternative for the external fixation of fractures. Int. Orthop. 1986, 10, 95–99. [Google Scholar] [CrossRef]
- Masse, A.; Bruno, A.; Bosetti, M.; Biasibetti, A.; Cannas, M.; Gallinaro, P. Prevention of pin track infection in external fixation with silver coated pins: Clinical and microbiological results. J. Biomed. Mater. Res. 2000, 53, 600–604. [Google Scholar] [CrossRef]
- Weinstein, M.A.; McCabe, J.P.; Cammisa, F.P., Jr. Postoperative spinal wound infection: A review of 2391 consecutive index procedures. J. Spinal Disord. 2000, 13, 422–426. [Google Scholar] [CrossRef]
- Bratzler, D.W.; Dellinger, E.P.; Olsen, K.M.; Perl, T.M.; Auwaerter, P.G.; Bolon, M.K.; Fish, D.N.; Napolitano, L.M.; Sawyer, R.G.; Slain, D.; et al. Clinical practice guidelines for antimicrobial prophylaxis in surgery. Am. J. Health Syst. Pharm. 2013, 70, 195–283. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Romano, C.L.; Scarponi, S.; Gallazzi, E.; Romano, D.; Drago, L. Antibacterial coating of implants in orthopaedics and trauma: A classification proposal in an evolving panorama. J. Orthop. Surg. Res. 2015, 10, 157. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rutledge, B.; Huyette, D.; Day, D.; Anglen, J. Treatment of osteomyelitis with local antibiotics delivered via bioabsorbable polymer. Clin. Orthop. Relat. Res. 2003, 280–287. [Google Scholar] [CrossRef] [PubMed]
- Pan, C.; Zhou, Z.; Yu, X. Coatings as the useful drug delivery system for the prevention of implant-related infections. J. Orthop. Surg. Res. 2018, 13, 220. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rathbone, C.R.; Cross, J.D.; Brown, K.V.; Murray, C.K.; Wenke, J.C. Effect of various concentrations of antibiotics on osteogenic cell viability and activity. J. Orthop. Res. 2011, 29, 1070–1074. [Google Scholar] [CrossRef]
- Regis, D.; Sandri, A.; Samaila, E.; Benini, A.; Bondi, M.; Magnan, B. Release of gentamicin and vancomycin from preformed spacers in infected total hip arthroplasties: Measurement of concentrations and inhibitory activity in patients’ drainage fluids and serum. Sci. World J. 2013, 2013, 752184. [Google Scholar] [CrossRef] [Green Version]
- Hanssen, A.D. Local antibiotic delivery vehicles in the treatment of musculoskeletal infection. Clin. Orthop. Relat. Res. 2005, 91–96. [Google Scholar] [CrossRef]
- Aslam, B.; Wang, W.; Arshad, M.I.; Khurshid, M.; Muzammil, S.; Rasool, M.H.; Nisar, M.A.; Alvi, R.F.; Aslam, M.A.; Qamar, M.U.; et al. Antibiotic resistance: A rundown of a global crisis. Infect. Drug Resist. 2018, 11, 1645–1658. [Google Scholar] [CrossRef] [Green Version]
- Pelletier, M.H.; Malisano, L.; Smitham, P.J.; Okamoto, K.; Walsh, W.R. The compressive properties of bone cements containing large doses of antibiotics. J. Arthroplast. 2009, 24, 454–460. [Google Scholar] [CrossRef]
- Parvizi, J.; Shohat, N.; Gehrke, T. Prevention of periprosthetic joint infection: New guidelines. Bone Jt. J. 2017, 99, 3–10. [Google Scholar] [CrossRef]
- Bistolfi, A.; Massazza, G.; Verne, E.; Masse, A.; Deledda, D.; Ferraris, S.; Miola, M.; Galetto, F.; Crova, M. Antibiotic-loaded cement in orthopedic surgery: A review. ISRN Orthop. 2011, 2011, 290851. [Google Scholar] [CrossRef] [PubMed]
- Anagnostakos, K.; Kelm, J. Enhancement of antibiotic elution from acrylic bone cement. J. Biomed. Mater. Res. Part B Appl. Biomater. 2009, 90, 467–475. [Google Scholar] [CrossRef] [PubMed]
- Musmar, S.M.; Ba’ba, H.; Owais, A. Adherence to guidelines of antibiotic prophylactic use in surgery: A prospective cohort study in North West Bank, Palestine. BMC Surg. 2014, 14, 69. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoeruek, E.; Spitzer, M.S.; Saygili, O.; Tatar, O.; Biedermann, T.; Yoeruek, E.; Bartz-Schmidt, K.U.; Szurman, P. Comparison of in vitro safety profiles of vancomycin and cefuroxime on human corneal endothelial cells for intracameral use. J. Cataract Refract. Surg. 2008, 34, 2139–2145. [Google Scholar] [CrossRef] [PubMed]
- Ince, A.; Schutze, N.; Karl, N.; Lohr, J.F.; Eulert, J. Gentamicin negatively influenced osteogenic function in vitro. Int. Orthop. 2007, 31, 223–228. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Drouet, M.; Chai, F.; Barthelemy, C.; Lebuffe, G.; Debaene, B.; Decaudin, B.; Odou, P. Influence of vancomycin infusion methods on endothelial cell toxicity. Antimicrob. Agents Chemother. 2015, 59, 930–934. [Google Scholar] [CrossRef] [Green Version]
- Booysen, E.; Sadie-Van Gijsen, H.; Deane, S.M.; Ferris, W.; Dicks, L.M.T. The effect of vancomycin on the viability and osteogenic potential of bone-derived mesenchymal stem cells. Probiotics Antimicrob. Proteins 2019, 11, 1009–1014. [Google Scholar] [CrossRef]
- Edin, M.L.; Miclau, T.; Lester, G.E.; Lindsey, R.W.; Dahners, L.E. Effect of cefazolin and vancomycin on osteoblasts in vitro. Clin. Orthop. Relat. Res. 1996, 333, 245–251. [Google Scholar] [CrossRef]
- Kallala, R.; Graham, S.M.; Nikkhah, D.; Kyrkos, M.; Heliotis, M.; Mantalaris, A.; Tsiridis, E. In vitro and in vivo effects of antibiotics on bone cell metabolism and fracture healing. Expert Opin. Drug Saf. 2012, 11, 15–32. [Google Scholar] [CrossRef]
- Luo, S.; Jiang, T.; Yang, Y.; Yang, X.; Zhao, J. Combination therapy with vancomycin-loaded calcium sulfate and vancomycin-loaded PMMA in the treatment of chronic osteomyelitis. BMC Musculoskelet. Disord. 2016, 17, 502. [Google Scholar] [CrossRef] [Green Version]
- Strom, R.G.; Pacione, D.; Kalhorn, S.P.; Frempong-Boadu, A.K. Lumbar laminectomy and fusion with routine local application of vancomycin powder: Decreased infection rate in instrumented and non-instrumented cases. Clin. Neurol. Neurosurg. 2013, 115, 1766–1769. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, K.R.; Smith, J.G.; Abtahi, A.M.; Archer, K.R.; Spengler, D.M.; McGirt, M.J.; Devin, C.J. Reduced surgical site infections in patients undergoing posterior spinal stabilization of traumatic injuries using vancomycin powder. Spine J. Off. J. North Am. Spine Soc. 2011, 11, 641–646. [Google Scholar] [CrossRef] [PubMed]
- Kochanski, R.B.; Nazari, P.; Sani, S. The utility of vancomycin powder in reducing surgical site infections in deep brain stimulation surgery. Oper. Neurosurg. 2018, 15, 584–588. [Google Scholar] [CrossRef] [PubMed]
- Cannon, J.G.D.; Ho, A.L.; Mohole, J.; Pendharkar, A.V.; Sussman, E.S.; Cheshier, S.H.; Grant, G.A. Topical vancomycin for surgical prophylaxis in non-instrumented pediatric spinal surgeries. Child’s Nerv. Syst. 2019, 35, 107–111. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.; Chen, W.C.; Hsieh, P.H.; Chen, D.W.; Lee, M.S.; Shih, H.N.; Ueng, S.W. In vitro activities of daptomycin-, vancomycin-, and teicoplanin-loaded polymethylmethacrylate against methicillin-susceptible, methicillin-resistant, and vancomycin-intermediate strains of Staphylococcus aureus. Antimicrob. Agents Chemother. 2011, 55, 5480–5484. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, M.; Sung, B.; Kang, Y.J.; Kim, D.H.; Lee, Y.; Hwang, S.Y.; Yoon, J.H.; Yoo, M.A.; Kim, C.M.; Chung, H.Y.; et al. The combination of ursolic acid and leucine potentiates the differentiation of C2C12 murine myoblasts through the mTOR signaling pathway. Int. J. Mol. Med. 2015, 35, 755–762. [Google Scholar] [CrossRef] [Green Version]
- Farley, J.R.; Wergedal, J.E.; Baylink, D.J. Fluoride directly stimulates proliferation and alkaline phosphatase activity of bone-forming cells. Science 1983, 222, 330. [Google Scholar] [CrossRef]
- Penner, M.J.; Duncan, C.P.; Masri, B.A. The in vitro elution characteristics of antibiotic-loaded CMW and Palacos-R bone cements. J. Arthroplast. 1999, 14, 209–214. [Google Scholar] [CrossRef]
- Steinbrink, K. The case for revision arthroplasty using antibiotic-loaded acrylic cement. Clin. Orthop. Relat. Res. 1990, 261, 19–22. [Google Scholar] [CrossRef]
- Van de Belt, H.; Neut, D.; Schenk, W.; van Horn, J.R.; van Der Mei, H.C.; Busscher, H.J. Staphylococcus aureus biofilm formation on different gentamicin-loaded polymethylmethacrylate bone cements. Biomaterials 2001, 22, 1607–1611. [Google Scholar] [CrossRef]
- Hsieh, P.H.; Chang, Y.H.; Chen, S.H.; Ueng, S.W.; Shih, C.H. High concentration and bioactivity of vancomycin and aztreonam eluted from Simplex cement spacers in two-stage revision of infected hip implants: A study of 46 patients at an average follow-up of 107 days. J. Orthop. Res. 2006, 24, 1615–1621. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.T.; Ng, K.J.; Lin, Y.; Kao, M.C. Red man syndrome following the use of vancomycin-loaded bone cement in the primary total knee replacement: A case report. Medicine 2018, 97, e13371. [Google Scholar] [CrossRef] [PubMed]
- Amerstorfer, F.; Fischerauer, S.; Sadoghi, P.; Schwantzer, G.; Kuehn, K.D.; Leithner, A.; Glehr, M. Superficial vancomycin coating of bone cement in orthopedic revision surgery: A safe technique to enhance local antibiotic concentrations. J. Arthroplast. 2017, 32, 1618–1624. [Google Scholar] [CrossRef]
- Hanssen, A.D.; Spangehl, M.J. Practical applications of antibiotic-loaded bone cement for treatment of infected joint replacements. Clin. Orthop. Relat. Res. 2004, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Moreno, J.; Merino, V.; Nacher, A.; Rodrigo, J.L.; Climente, M.; Merino-Sanjuan, M. Antibiotic-loaded bone cement as prophylaxis in total joint replacement. Orthop. Surg. 2017, 9, 331–341. [Google Scholar] [CrossRef]
- Gogia, J.S.; Meehan, J.P.; di Cesare, P.E.; Jamali, A.A. Local antibiotic therapy in osteomyelitis. Semin. Plast. Surg. 2009, 23, 100–107. [Google Scholar] [CrossRef] [Green Version]
- Hadaway, L.; Chamallas, S.N. Vancomycin: New perspectives on an old drug. J. Infus. Nurs. 2003, 26, 278–284. [Google Scholar] [CrossRef]
- Yatsunami, J.; Turuta, N.; Wakamatsu, K.; Hara, N.; Hayashi, S. Clarithromycin is a potent inhibitor of tumor-induced angiogenesis. Res. Exp. Med. 1997, 197, 189–197. [Google Scholar] [CrossRef]
- Hofmann, A.; Ritz, U.; Hessmann, M.H.; Schmid, C.; Tresch, A.; Rompe, J.D.; Meurer, A.; Rommens, P.M. Cell viability, osteoblast differentiation, and gene expression are altered in human osteoblasts from hypertrophic fracture non-unions. Bone 2008, 42, 894–906. [Google Scholar] [CrossRef]
- Hofmann, A.; Ritz, U.; Verrier, S.; Eglin, D.; Alini, M.; Fuchs, S.; Kirkpatrick, C.J.; Rommens, P.M. The effect of human osteoblasts on proliferation and neo-vessel formation of human umbilical vein endothelial cells in a long-term 3D co-culture on polyurethane scaffolds. Biomaterials 2008, 29, 4217–4226. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are available from the authors. |







© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Braun, J.; Eckes, S.; Rommens, P.M.; Schmitz, K.; Nickel, D.; Ritz, U. Toxic Effect of Vancomycin on Viability and Functionality of Different Cells Involved in Tissue Regeneration. Antibiotics 2020, 9, 238. https://doi.org/10.3390/antibiotics9050238
Braun J, Eckes S, Rommens PM, Schmitz K, Nickel D, Ritz U. Toxic Effect of Vancomycin on Viability and Functionality of Different Cells Involved in Tissue Regeneration. Antibiotics. 2020; 9(5):238. https://doi.org/10.3390/antibiotics9050238
Chicago/Turabian StyleBraun, Joy, Stefanie Eckes, Pol Maria Rommens, Katja Schmitz, Daniela Nickel, and Ulrike Ritz. 2020. "Toxic Effect of Vancomycin on Viability and Functionality of Different Cells Involved in Tissue Regeneration" Antibiotics 9, no. 5: 238. https://doi.org/10.3390/antibiotics9050238
APA StyleBraun, J., Eckes, S., Rommens, P. M., Schmitz, K., Nickel, D., & Ritz, U. (2020). Toxic Effect of Vancomycin on Viability and Functionality of Different Cells Involved in Tissue Regeneration. Antibiotics, 9(5), 238. https://doi.org/10.3390/antibiotics9050238