Identification and Rational Design of a Novel Antibacterial Peptide Dermaseptin-AC from the Skin Secretion of the Red-Eyed Tree Frog Agalychnis callidryas
Abstract
1. Introduction
2. Results
2.1. Molecular Cloning of the Precusor Encoding a Novel Dermaseptin Peptide
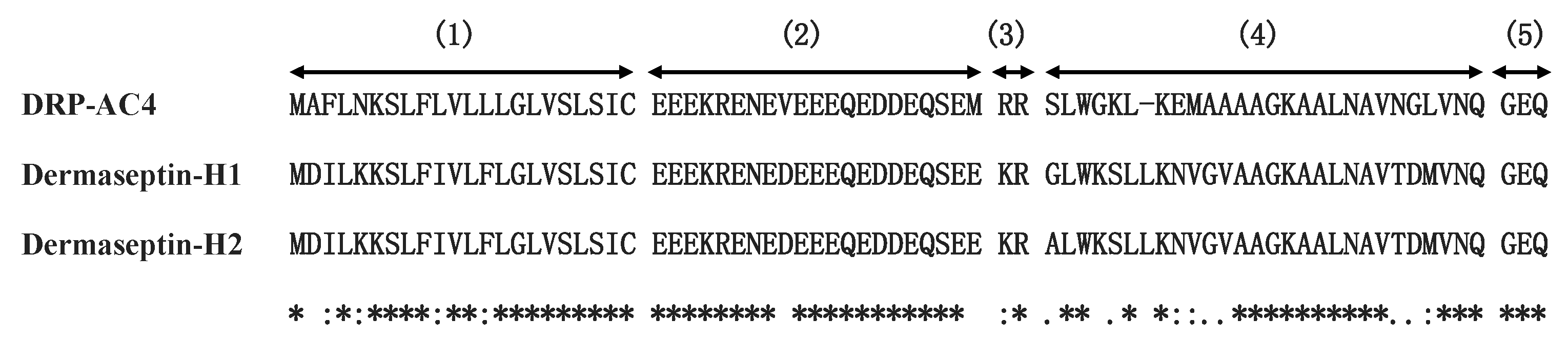
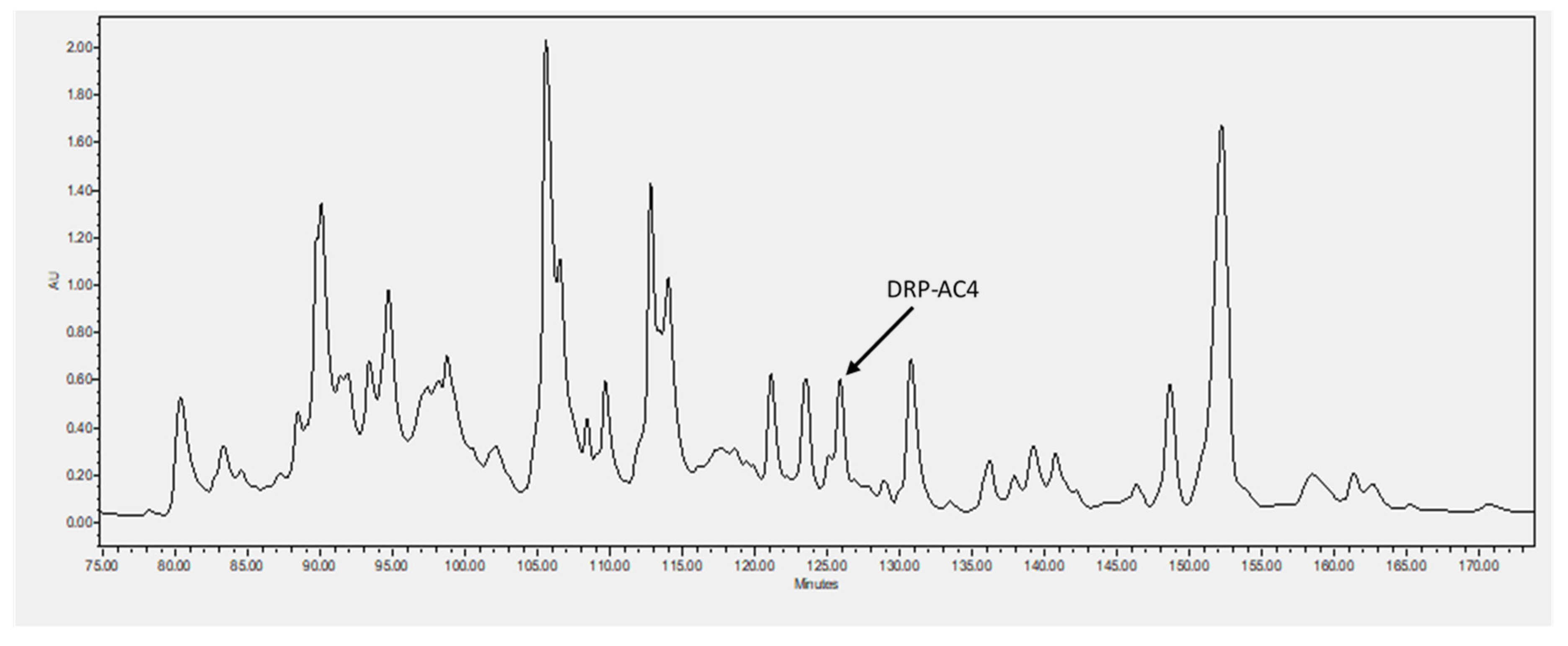
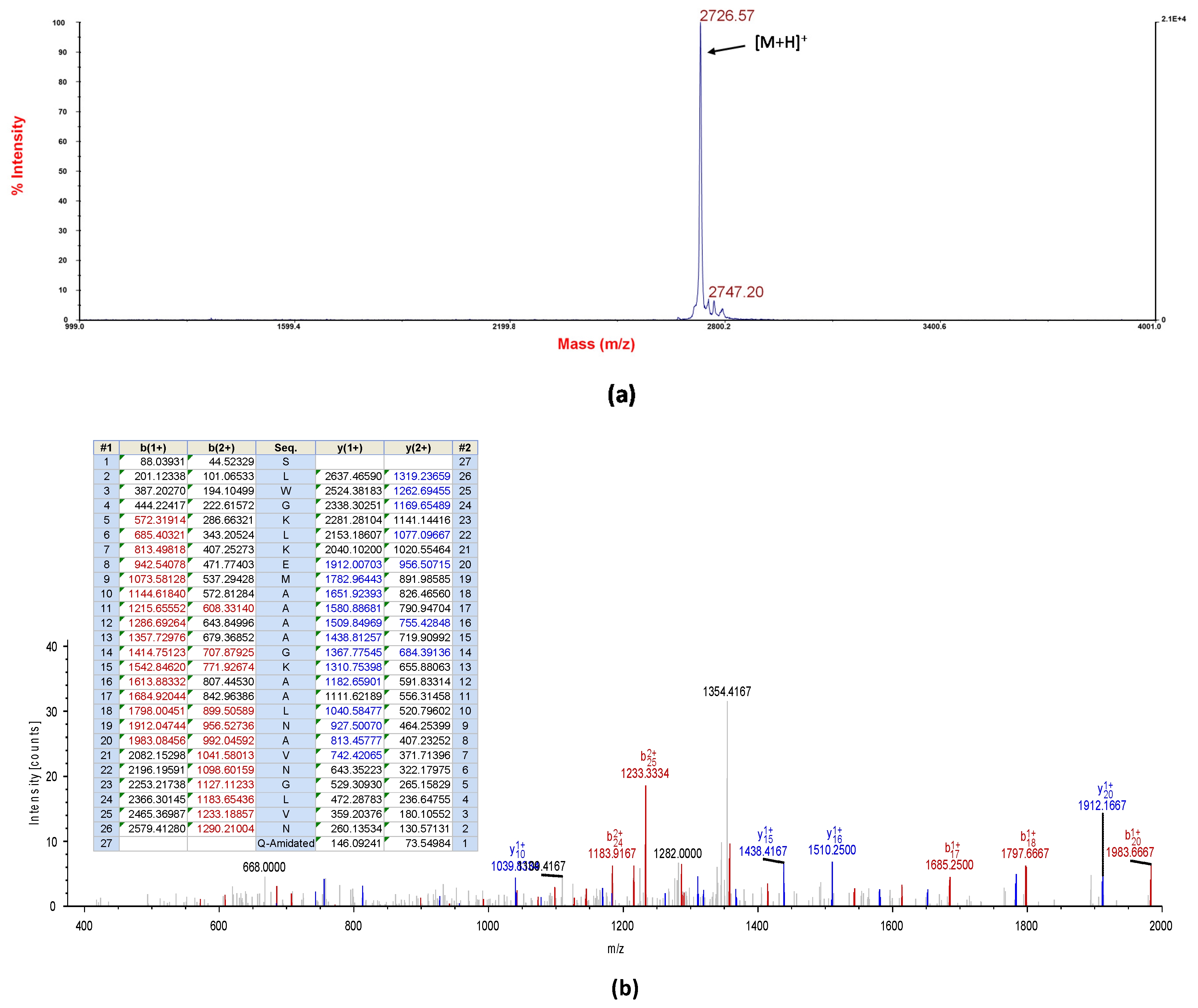
2.2. Identification and Structural Characterisation of DRP-AC4
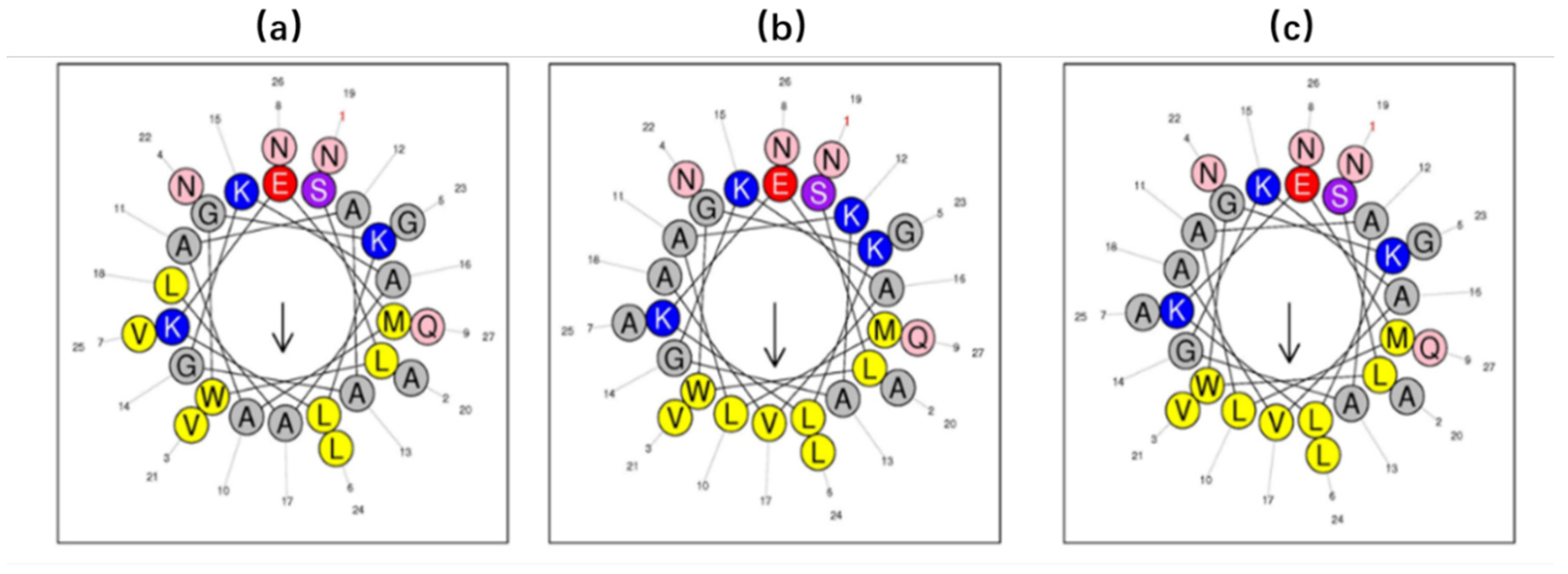
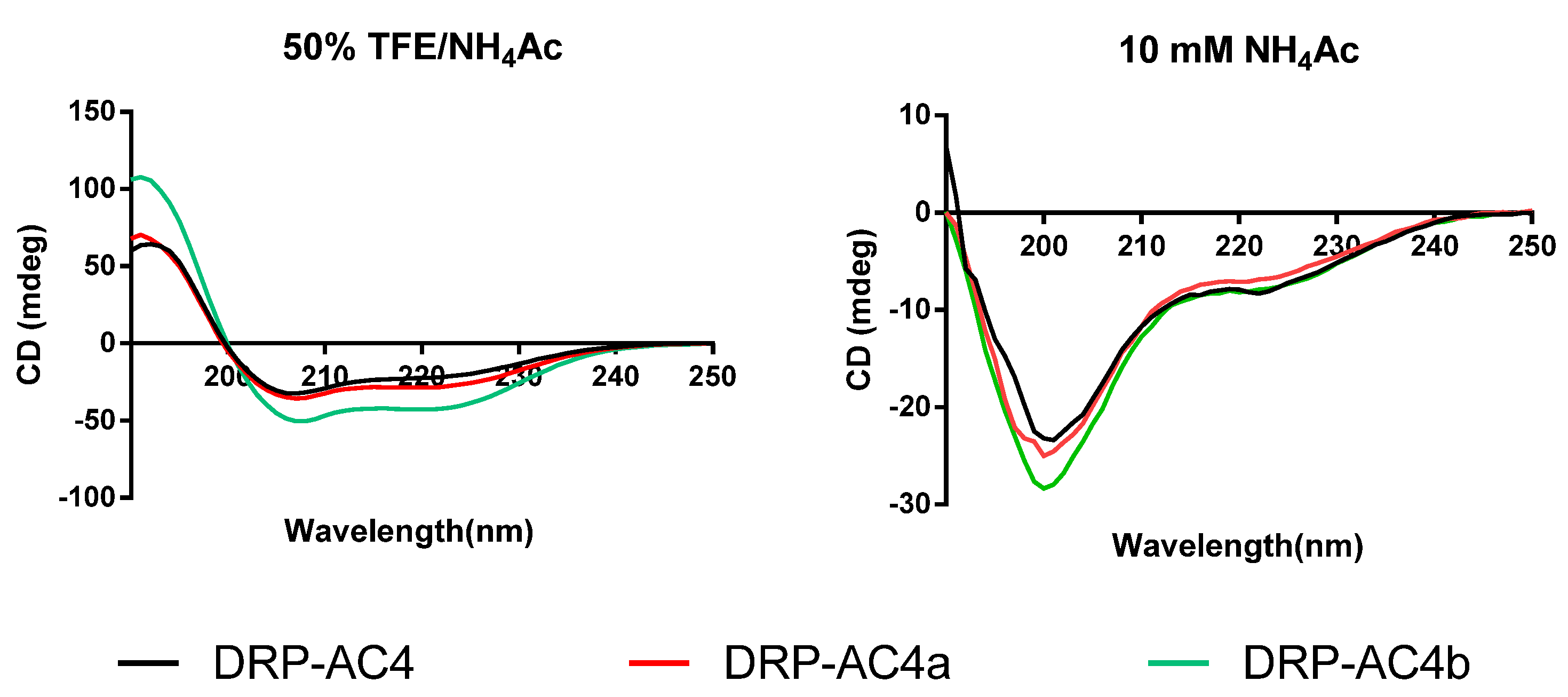
2.3. Prediction of Secondary Structure and Structural Analysis of DRP-AC4 and Its Analogues
2.4. Antimicrobial Assay of DRP-AC4 and Its Analogues
2.5. Anti-Biofilm Activity
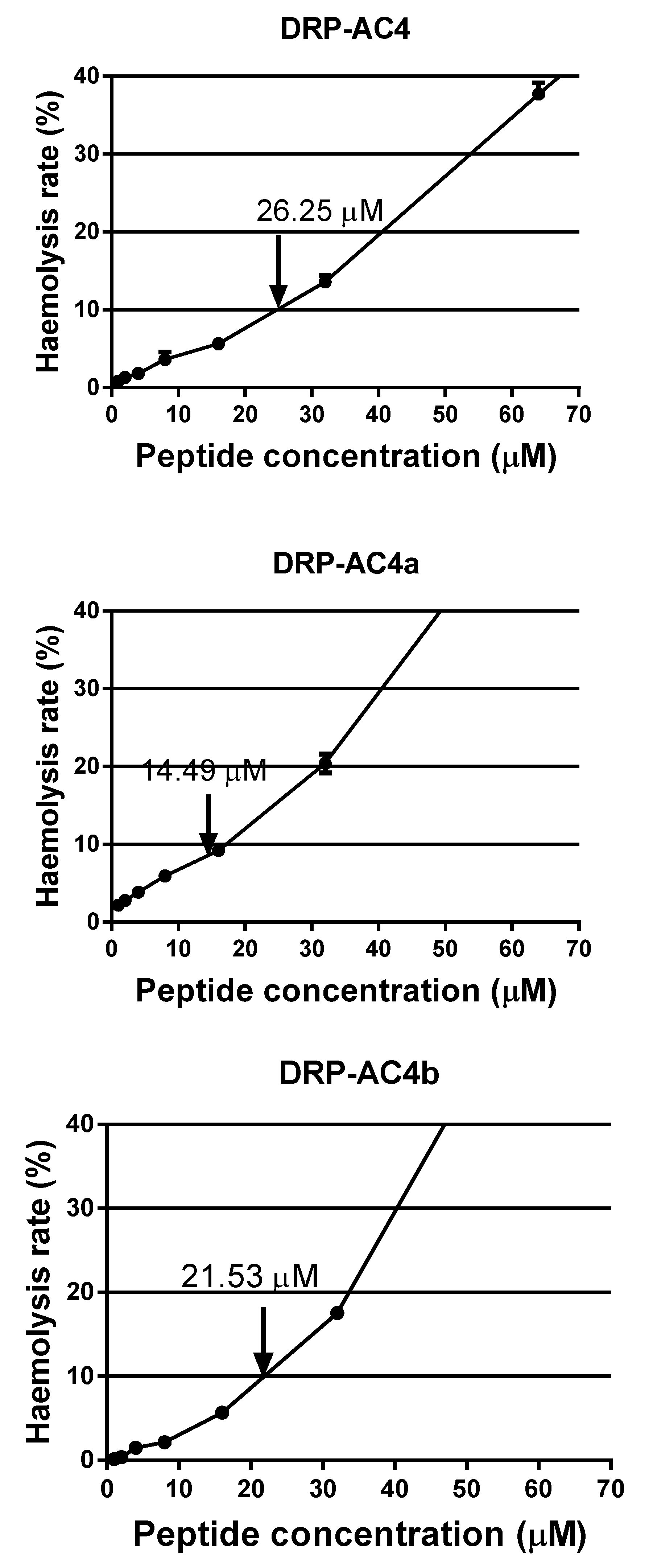
2.6. Haemolytic Activity
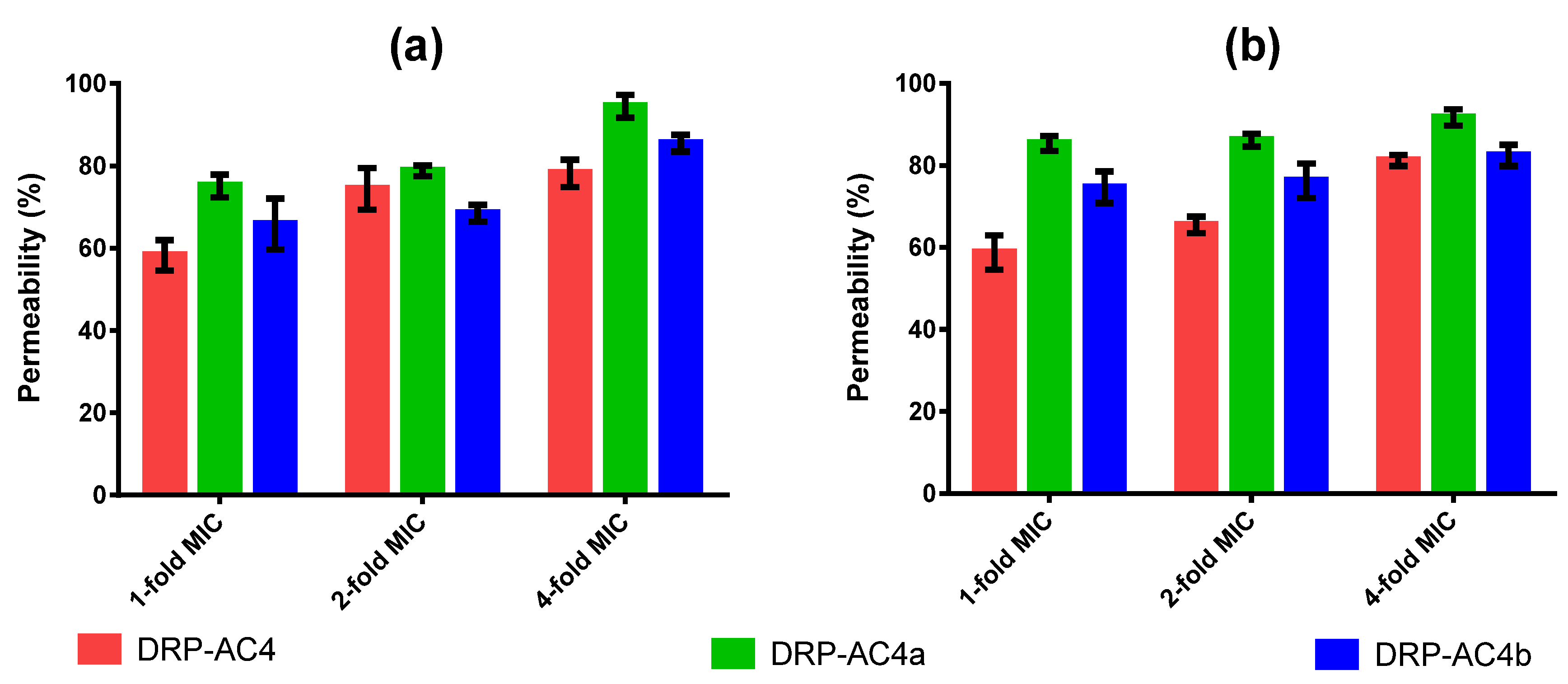
2.7. Permeabilisation Effects of Peptides on the Cell Membrane
2.8. Resistance Induction by Serial Passages in S. aureus
3. Discussion
4. Materials and Methods
4.1. Skin Secretion Harvesting
4.2. Identification of AMP Precursor Encoding cDNAs from the Skin Secretion
4.3. Identification and Structural Characterisation of the Predicted Peptide DRP-AC4 from the Skin Secretion of A. callidryas
4.4. Peptide Design and Synthesis
4.5. Physical and Chemical Property Predictions, Peptide Secondary Structure Predictions and CD Analyses
4.6. MIC and MBC Assays
4.7. Biofilm Assays
4.8. Haemolysis Assays
4.9. Bacterial Cell Membrane Permeability Assays
4.10. Resistance Induction by Serial Passages
4.11. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Jenssen, H.; Hamill, P.; Hancock, R.E.W. Peptide antimicrobial agents. Clin. Microbiol. Rev. 2006, 19, 491–511. [Google Scholar] [CrossRef] [PubMed]
- Lau, Q.Y.; Li, J.; Sani, M.-A.; Sinha, S.; Li, Y.; Ng, F.M.; Kang, C.; Bhattacharjya, S.; Separovic, F.; Verma, C.; et al. Elucidating the bactericidal mechanism of action of the linear antimicrobial tetrapeptide Brbr-Nh2. Biochim. Biophys. Acta Biomembr. 2018, 1860, 1517–1527. [Google Scholar] [CrossRef] [PubMed]
- Frieden, T. Antibiotic Resistance Threats in the United States; Centers for Disease Control and Prevention: Atlanta, GA, USA, 2013.
- Fry, D.E. Antimicrobial Peptides. Surg. Infect. 2018, 19, 804–811. [Google Scholar] [CrossRef] [PubMed]
- Lei, J.; Sun, L.; Huang, S.; Zhu, C.; Li, P.; He, J.; Mackey, V.; Coy, D.H.; He, Q. The antimicrobial peptides and their potential clinical applications. Am. J. Transl. Res. 2019, 11, 3919–3931. [Google Scholar] [PubMed]
- Starr, C.G.; Maderdrut, J.L.; He, J.; Coy, D.H.; Wimley, W.C. Pituitary adenylate cyclase-activating polypeptide is a potent broad-spectrum antimicrobial peptide: Structure-activity relationships. Peptides 2018, 104, 35–40. [Google Scholar] [CrossRef]
- Zanetti, M. Cathelicidins, multifunctional peptides of the innate immunity. J. Leukoc. Biol. 2004, 75, 39–48. [Google Scholar] [CrossRef]
- Wu, D.; Gao, Y.; Wang, L.; Xi, X.; Wu, Y.; Zhou, M.; Zhang, Y.; Ma, C.; Chen, T.; Shaw, C. A Combined Molecular Cloning and Mass Spectrometric Method to Identify, Characterize, and design frenatin peptides from the skin secretion of Litoria infrafrenata. Molecules 2016, 21, 1429. [Google Scholar] [CrossRef]
- Zairi, A.; Tangy, F.; Bouassida, K.; Hani, K. Dermaseptins and Magainins: Antimicrobial peptides from frogs’ skin—New sources for a promising spermicides microbicides—A mini review. J. Biomed. Biotechnol. 2009, 2009, 452567. [Google Scholar] [CrossRef]
- Shi, D.; Hou, X.; Wang, L.; Gao, Y.; Wu, D.; Xi, X.; Zhou, M.; Kwok, H.F.; Duan, J.; Chen, T.; et al. Two novel dermaseptin-like antimicrobial peptides with anticancer activities from the skin secretion of Pachymedusa dacnicolor. Toxins 2016, 8, 144. [Google Scholar] [CrossRef]
- Kim, J.; Jacob, B.; Jang, M.; Kwak, C.; Lee, Y.; Son, K.; Lee, S.; Jung, I.D.; Jeong, M.S.; Kwon, S.-H.; et al. Development of a novel short 12-meric papiliocin-derived peptide that is effective against Gram-negative sepsis. Sci. Rep. 2019, 9, 3817. [Google Scholar] [CrossRef]
- Torres, M.D.T.; Sothiselvam, S.; Lu, T.K.; de la Fuente-Nunez, C. Peptide design principles for antimicrobial applications. J. Mol. Biol. 2019, 431, 3547–3567. [Google Scholar] [CrossRef] [PubMed]
- Navon-Venezia, S.; Feder, R.; Gaidukov, L.; Carmeli, Y.; Mor, A. Antibacterial properties of dermaseptin s4 derivatives with in vivo activity. Antimicrob. Agents Chemother. 2002, 46, 689–694. [Google Scholar] [CrossRef] [PubMed]
- Fleury, M.; Longeron, D. Combined resistivity and capillary pressure measurements using micropore membrane technique. J. Pet. Sci. Eng. 1998, 19, 73–79. [Google Scholar] [CrossRef]
- Amso, Z.; Hayouka, Z. Antimicrobial random peptide cocktails: A new approach to fight pathogenic bacteria. Chem. Commun. 2019, 55, 2007–2014. [Google Scholar] [CrossRef]
- Bartels, E.J.H.; Dekker, D.; Amiche, M. Dermaseptins, multifunctional antimicrobial peptides: A review of their pharmacology, effectivity, mechanism of action, and possible future directions. Front. Pharmacol. 2019, 10, 1421. [Google Scholar] [CrossRef]
- Liu, Y.; Du, Q.; Ma, C.; Xi, X.; Wang, L.; Zhou, M.; Burrows, J.F.; Chen, T.; Wang, H. Structure–activity relationship of an antimicrobial peptide, Phylloseptin-PHa: Balance of hydrophobicity and charge determines the selectivity of bioactivities. Drug Des. Devel. Ther. 2019, 13, 447–458. [Google Scholar] [CrossRef]
- Wiradharma, N.; Sng, M.Y.S.; Khan, M.; Ong, Z.-Y.; Yang, Y.-Y. Rationally designed α-helical broad-spectrum antimicrobial peptides with idealized facial amphiphilicity. Macromol. Rapid Commun. 2013, 34, 74–80. [Google Scholar] [CrossRef]
- Yasir, M.; Willcox, M.D.P.; Dutta, D. Action of Antimicrobial Peptides against Bacterial Biofilms. Materials 2018, 11, 2468. [Google Scholar] [CrossRef]
- Gaidukov, L.; Fish, A.; Mor, A. Analysis of Membrane-Binding Properties of Dermaseptin Analogues: Relationships between Binding and Cytotoxicity. Biochemistry 2003, 42, 12866–12874. [Google Scholar] [CrossRef]
- Shai, Y. Mechanism of the binding, insertion and destabilization of phospholipid bilayer membranes by α-helical antimicrobial and cell non-selective membrane-lytic peptides. Biochim. Biophys. Acta Biomembr. 1999, 1462, 55–70. [Google Scholar] [CrossRef]
- Shai, Y. Mode of action of membrane active antimicrobial peptides. Bioploymers 2002, 66, 236–248. [Google Scholar] [CrossRef] [PubMed]
- Schoutens, E.; Yourassowsky, E. Speed of bactericidal action of penicillin G, ampicillin, and carbenicillin on Bacteroides fragilis. Antimicrob. Agents Chemother. 1974, 6, 227–231. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Mohr, K.I. History of Antibiotics Research. In How to Overcome the Antibiotic Crisis: Facts, Challenges, Technologies and Future Perspectives; Stadler, M., Dersch, P., Eds.; Current Topics in Microbiology and Immunology; Springer International Publishing: Cham, Switzerland, 2016; pp. 237–272. ISBN 978-3-319-49284-1. [Google Scholar]
- Gautier, R.; Douguet, D.; Antonny, B.; Drin, G. HELIQUEST: A web server to screen sequences with specific -helical properties. Bioinformatics 2008, 24, 2101–2102. [Google Scholar] [CrossRef] [PubMed]
- Dolzani, L.; Milan, A.; Scocchi, M.; Lagatolla, C.; Bressan, R.; Benincasa, M. Sub-MIC effects of a proline-rich antibacterial peptide on clinical isolates of Acinetobacter baumannii. J. Med. Microbiol. 2019, 68, 1253–1265. [Google Scholar] [CrossRef]

| Peptides | Length (aa) | Molecular Weight (g/mol) | Net Charge at PH7 | Hydrophobicity (<H>) | Hydrophobic Moment (<µH>) |
|---|---|---|---|---|---|
| DRP-AC4 | 27 | 2725.18 | 3 | 0.341 | 0.404 |
| DRP-AC4a | 27 | 2782.27 | 4 | 0.293 | 0.526 |
| DRP-AC4b | 27 | 2725.18 | 3 | 0.341 | 0.492 |
| Peptides | Amino Acid Sequence | % of α-Helix in 50% TFE/(v/v) a |
|---|---|---|
| DRP-AC4 | SLWGKLKEMAAAAGKAALNAVNGLVNQ-NH2 | 22.4 |
| DRP-AC4a | SLWGKLKEMLAKAGKAVANAVNGLANQ-NH2 | 26.9 |
| DRP-AC4b | SLWGKLKEMLAAAGKAVANAVNGLANQ-NH2 | 38.8 |
| MIC/MBC (μM) | |||
|---|---|---|---|
| Microorganisms | DRP-AC4 | DRP-AC4a | DRP-AC4b |
| S. aureus | 8/32 | 8/16 | 8/32 |
| E. coli | 8/8 | 8/8 | 8/16 |
| C. albicans | 64/128 | 16/32 | 64/128 |
| P. aeruginosa | 64/128 | 32/128 | 32/128 |
| E. faecalis | 32/64 | 8/32 | 32/32 |
| K. pneumoniae | 32/32 | 8/16 | 32/64 |
| MRSA | 32/64 | 8/32 | 16/32 |
| GM | 26.25 | 14.49 | 21.53 |
| MBIC/MBEC (μM) | |||
|---|---|---|---|
| Microorganisms | DRP-AC4 | DRP-AC4a | DRP-AC4b |
| S. aureus | 32/>256 | 16/64 | 32/256 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gong, Z.; Pei, X.; Ren, S.; Chen, X.; Wang, L.; Ma, C.; Xi, X.; Chen, T.; Shaw, C.; Zhou, M. Identification and Rational Design of a Novel Antibacterial Peptide Dermaseptin-AC from the Skin Secretion of the Red-Eyed Tree Frog Agalychnis callidryas. Antibiotics 2020, 9, 243. https://doi.org/10.3390/antibiotics9050243
Gong Z, Pei X, Ren S, Chen X, Wang L, Ma C, Xi X, Chen T, Shaw C, Zhou M. Identification and Rational Design of a Novel Antibacterial Peptide Dermaseptin-AC from the Skin Secretion of the Red-Eyed Tree Frog Agalychnis callidryas. Antibiotics. 2020; 9(5):243. https://doi.org/10.3390/antibiotics9050243
Chicago/Turabian StyleGong, Zijian, Xinjie Pei, Shen Ren, Xiaoling Chen, Lei Wang, Chengbang Ma, Xinping Xi, Tianbao Chen, Chris Shaw, and Mei Zhou. 2020. "Identification and Rational Design of a Novel Antibacterial Peptide Dermaseptin-AC from the Skin Secretion of the Red-Eyed Tree Frog Agalychnis callidryas" Antibiotics 9, no. 5: 243. https://doi.org/10.3390/antibiotics9050243
APA StyleGong, Z., Pei, X., Ren, S., Chen, X., Wang, L., Ma, C., Xi, X., Chen, T., Shaw, C., & Zhou, M. (2020). Identification and Rational Design of a Novel Antibacterial Peptide Dermaseptin-AC from the Skin Secretion of the Red-Eyed Tree Frog Agalychnis callidryas. Antibiotics, 9(5), 243. https://doi.org/10.3390/antibiotics9050243






