In Vitro Activity of a Novel Siderophore-Cephalosporin, GT-1 and Serine-Type ?-Lactamase Inhibitor, GT-055, against Escherichia coli, Klebsiella pneumoniae and Acinetobacter spp. Panel Strains
Abstract
:1. Introduction
2. Results
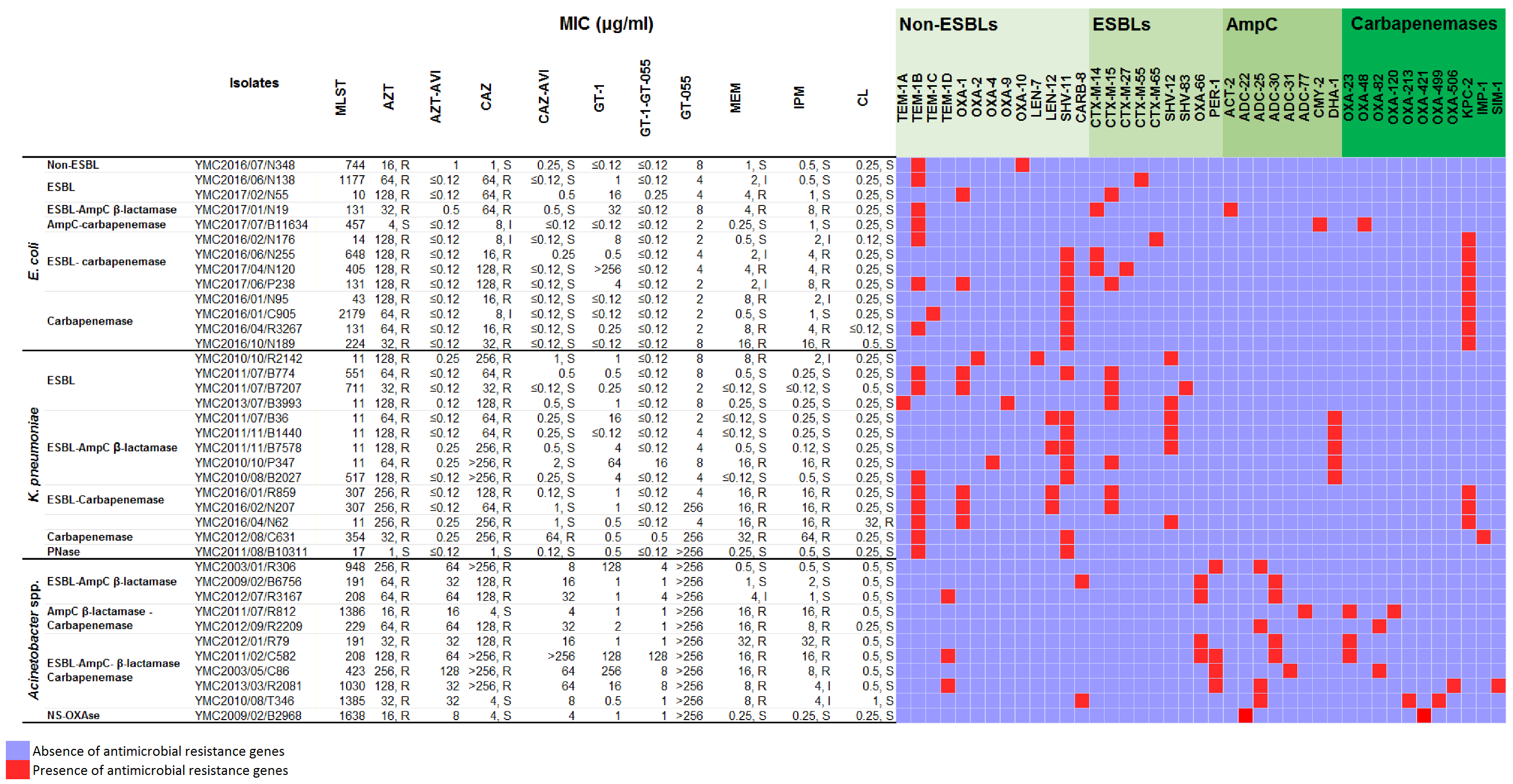
2.1. In Vitro Activity of GT-1 and GT-1/GT-055 against E. coli Panel Strains
2.2. In Vitro Activity of GT-1 and GT-1/GT-055 against K. pneumoniae Panel Strains
2.3. In Vitro Activity of GT-1 and GT-1/GT-055 against Acinetobacter spp. Panel Strains
3. Discussion
4. Materials and Methods
4.1. Isolates
4.2. Test Compounds
4.3. Antimicrobial Susceptibility Tests
4.4. DNA Extraction and Whole-Genome Sequencing
4.5. Sequence Assembly, Genome Annotation, MLST Determination and Resistome Analysis
4.6. Cloning
4.7. Analysis of Siderophore Uptake System and Porin Loss
4.8. Accession Numbers
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Tacconelli, E.; Carrara, E.; Savoldi, A.; Harbarth, S.; Mendelson, M.; Monnet, D.L.; Pulcini, C.; Kahlmeter, G.; Kluytmans, J.; Carmeli, Y.; et al. Discovery, research, and development of new antibiotics: The WHO priority list of antibiotic-resistant bacteria and tuberculosis. Lancet Infect. Dis. 2018, 18, 318–327. [Google Scholar] [CrossRef]
- Nguyen, L.P.; Pinto, N.A.; Vu, T.N.; Mai, H.; Pham, A.H.; Lee, H.; Cho, Y.L.; Byun, J.-H.; D’Souza, R.; Yong, D. Resistome profiles, plasmid typing, and whole-genome phylogenetic tree analyses of blaNDM-9 and mcr-1 co-harboring Escherichia coli ST617 from a patient without a history of farm exposure in Korea. Pathogens 2019, 8, 212. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aires, C.A.M.; da Conceição-Neto, O.C.; Tavares, E.; Oliveira, T.R.; Dias, C.F.; Montezzi, L.F.; Picão, R.C.; Albano, R.M.; Asensi, M.D.; Carvalho-Assef, A.P.D. Emergence of the plasmid-mediated mcr-1 gene in clinical KPC-2-producing Klebsiella pneumoniae sequence type 392 in Brazil. Antimicrob. Agents Chemother. 2017, 61, e00317-17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kohanski, M.A.; Dwyer, D.J.; Collins, J.J. How antibiotics kill bacteria: From targets to networks. Nat. Rev. Microbiol. 2010, 8, 423–435. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lopatkin, A.J.; Huang, S.; Smith, R.P.; Srimani, J.K.; Sysoeva, T.A.; Bewick, S.; Karig, D.K.; You, L. Antibiotics as a selective driver for conjugation dynamics. Nat. Microbiol. 2016, 1, 16044. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coyne, S.; Courvalin, P.; Périchon, B. Efflux-mediated antibiotic resistance in Acinetobacter spp. Antimicrob. Agents Chemother. 2011, 55, 947–953. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oh, S.-H.; Park, H.-S.; Kim, H.-S.; Yun, J.-Y.; Oh, K.; Cho, Y.-L.; Kwak, J.-H. Antimicrobial activities of LCB10-0200, a novel siderophore cephalosporin, against the clinical isolates of Pseudomonas aeruginosa and other pathogens. Int. J. Antimicrob. Agents 2017, 50, 700–706. [Google Scholar] [CrossRef]
- Tehrani, K.H.M.E.; Martin, N.I. β-lactam/β-lactamase inhibitor combinations: An update. MedChemComm 2018, 9, 1439–1456. [Google Scholar] [CrossRef]
- Lomovskaya, O.; Sun, D.; Rubio-Aparicio, D.; Nelson, K.; Tsivkovski, R.; Griffith, D.C.; Dudley, M.N. Vaborbactam: Spectrum of beta-lactamase inhibition and impact of resistance mechanisms on activity in Enterobacteriaceae. Antimicrob. Agents Chemother. 2017, 61, e01443-17. [Google Scholar] [CrossRef] [Green Version]
- Lahiri, S.D.; Johnstone, M.R.; Ross, P.L.; McLaughlin, R.E.; Olivier, N.B.; Alm, R.A. Avibactam and class C β-lactamases: Mechanism of inhibition, conservation of the binding pocket, and implications for resistance. Antimicrob. Agents Chemother. 2014, 58, 5704–5713. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.; Shin, S.; Biek, D.; Cho, Y. Penicillin binding protein (PBP) activity of beta-lactamase inhibitor GT-055. In Posters of the Twenty Ninth European Congress of Clinical Microbiology & Infectious Diseases, Amsterdam, Netherlands; Poster 1187; European Society of Clinical Microbiology: Basel, Switzerland, 2019. [Google Scholar]
- Zimbler, D.L.; Arivett, B.A.; Beckett, A.C.; Menke, S.M.; Actis, L.A. Functional features of TonB energy transduction systems of Acinetobacter baumannii. Infect. Immun. 2013, 81, 3382–3394. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, J.; Zhang, H.; Liu, Y.-H.; Feng, Y. Towards understanding MCR-like colistin resistance. Trends Microbiol. 2018, 26, 794–808. [Google Scholar] [CrossRef] [PubMed]
- Andrews, S.C.; Robinson, A.K.; Rodríguez-Quiñones, F. Bacterial iron homeostasis. FEMS Microbiol. Rev. 2003, 27, 215–237. [Google Scholar] [CrossRef]
- Zughaier, S.M.; Cornelis, P. Editorial: Role of iron in bacterial pathogenesis. Front. Cell. Infect. Microbiol. 2018, 8, 344. [Google Scholar] [CrossRef] [Green Version]
- Möllmann, U.; Heinisch, L.; Bauernfeind, A.; Köhler, T.; Ankel-Fuchs, D. Siderophores as drug delivery agents: Application of the “Trojan Horse” strategy. Biometals 2009, 22, 615–624. [Google Scholar] [CrossRef] [PubMed]
- Eijkelkamp, B.A.; Hassan, K.A.; Paulsen, I.T.; Brown, M.H. Investigation of the human pathogen Acinetobacter baumannii under iron limiting conditions. BMC Genom. 2011, 12, 126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tooke, C.L.; Hinchliffe, P.; Bragginton, E.C.; Colenso, C.K.; Hirvonen, V.H.A.; Takebayashi, Y.; Spencer, J. β-lactamases and β-lactamase inhibitors in the 21st century. J. Mol. Biol. 2019, 431, 3472–3500. [Google Scholar] [CrossRef]
- Hemarajata, P.; Humphries, R.M. Ceftazidime/avibactam resistance associated with L169P mutation in the omega loop of KPC-2. J. Antimicrob. Chemother. 2019, 74, 1241–1243. [Google Scholar] [CrossRef] [PubMed]
- Venditti, C.; Nisii, C.; Ballardini, M.; Meledandri, M.; Di Caro, A. Identification of L169P mutation in the omega loop of KPC-3 after a short course of ceftazidime/avibactam. J. Antimicrob. Chemother. 2019, 74, 2466–2467. [Google Scholar] [CrossRef] [PubMed]
- Dsouza, R.; Pinto, N.A.; Hwang, I.; Cho, Y.; Yong, D.; Choi, J.; Lee, K.; Chong, Y. Panel strain of Klebsiella pneumoniae for beta-lactam antibiotic evaluation: Their phenotypic and genotypic characterization. PeerJ 2017, 5, e2896. [Google Scholar] [CrossRef] [Green Version]
- D’Souza, R.; Pinto, N.A.; Nguyen, L.P.; Higgins, P.G.; Vu, T.N.; Byun, J.-H.; Cho, Y.L.; Choi, J.R.; Yong, D. Phenotypic and genotypic characterization of Acinetobacter spp. panel strains: A cornerstone to facilitate antimicrobial development. Front. Microbiol. 2019, 10, 559. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Magiorakos, A.-P.; Srinivasan, A.; Carey, R.B.; Carmeli, Y.; Falagas, M.E.; Giske, C.G.; Harbarth, S.; Hindler, J.F.; Kahlmeter, G.; Olsson-Liljequist, B.; et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: An international expert proposal for interim standard definitions for acquired resistance. Clin. Microbiol. Infect. 2012, 18, 268–281. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- CLSI. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria that Grow Aerobically M7-A10; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2015; ISBN 1562389874. [Google Scholar]
- CLSI. Performance Standards for Antimicrobial Susceptibility Testing Twenty-eighth Informational Supplement M100-S28; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2018; ISBN 1-562388-39-8. [Google Scholar]
- Oh, S.; Kwak, J.; Lee, J.; Han, H.; Biek, D.; Oh, K.; Cho, Y. Serum and iron effects on the in vitro activity of siderophore cephalosporin GT-1. In Posters of American Society for Microbiology (ASM) Microbe 2018; American Society for Microbiology: Washington, DC, USA, 2018. [Google Scholar]
- Bankevich, A.; Nurk, S.; Antipov, D.; Gurevich, A.A.; Dvorkin, M.; Kulikov, A.S.; Lesin, V.M.; Nikolenko, S.I.; Pham, S.; Prjibelski, A.D.; et al. SPAdes: A new genome assembly algorithm and its applications to single-cell sequencing. J. Comput. Biol. 2012, 19, 455–477. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aziz, R.K.; Bartels, D.; Best, A.A.; DeJongh, M.; Disz, T.; Edwards, R.A.; Formsma, K.; Gerdes, S.; Glass, E.M.; Kubal, M.; et al. The RAST Server: Rapid annotations using subsystems technology. BMC Genom. 2008, 9, 75. [Google Scholar] [CrossRef] [Green Version]
- Zankari, E.; Hasman, H.; Cosentino, S.; Vestergaard, M.; Rasmussen, S.; Lund, O.; Aarestrup, F.M.; Larsen, M.V. Identification of acquired antimicrobial resistance genes. J. Antimicrob. Chemother. 2012, 67, 2640–2644. [Google Scholar] [CrossRef]
- Carattoli, A.; Zankari, E.; García-Fernández, A.; Voldby Larsen, M.; Lund, O.; Villa, L.; Møller Aarestrup, F.; Hasman, H. In silico detection and typing of plasmids using PlasmidFinder and plasmid multilocus sequence typing. Antimicrob. Agents Chemother. 2014, 58, 3895–3903. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Langmead, B. Aligning short sequencing reads with Bowtie. Curr. Protoc. Bioinform. 2010, 32, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Koebnik, R. TonB-dependent trans-envelope signalling: The exception or the rule? Trends Microbiol. 2005, 13, 343–347. [Google Scholar] [CrossRef] [PubMed]
- Braun, V.; Braun, M. Iron transport and signaling in Escherichia coli. FEBS Lett. 2002, 529, 78–85. [Google Scholar] [CrossRef] [Green Version]
- Stephens, D.L.; Choe, M.D.; Earhart, C.F. Escherichia coli periplasmic protein FepB binds ferrienterobactin. Microbiology 1995, 141, 1647–1654. [Google Scholar] [CrossRef] [Green Version]
- Pierce, J.R.; Earhart, C.F. Escherichia coli K-12 envelope proteins specifically required for ferrienterobactin uptake. J. Bacteriol. 1986, 166, 930–936. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shea, C.M.; McIntosh, M.A. Nucleotide sequence and genetic organization of the ferric enterobactin transport system: Homology to other periplasmic binding protein-dependent systems in Escherichia coli. Mol. Microbiol. 1991, 5, 1415–1428. [Google Scholar] [CrossRef] [PubMed]
- Molloy, M.P.; Herbert, B.R.; Slade, M.B.; Rabilloud, T.; Nouwens, A.S.; Williams, K.L.; Gooley, A.A. Proteomic analysis of the Escherichia coli outer membrane. Eur. J. Biochem. 2000, 267, 2871–2881. [Google Scholar] [CrossRef] [PubMed]
- Garcia, E.C.; Brumbaugh, A.R.; Mobley, H.L.T. Redundancy and specificity of Escherichia coli iron acquisition systems during urinary tract infection. Infect. Immun. 2011, 79, 1225–1235. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dorsey, C.W.; Tomaras, A.P.; Connerly, P.L.; Tolmasky, M.E.; Crosa, J.H.; Actis, L.A. The siderophore-mediated iron acquisition systems of Acinetobacter baumannii ATCC 19606 and Vibrio anguillarum 775 are structurally and functionally related. Microbiology 2004, 150, 3657–3667. [Google Scholar] [CrossRef] [Green Version]
- Mihara, K.; Tanabe, T.; Yamakawa, Y.; Funahashi, T.; Nakao, H.; Narimatsu, S.; Yamamoto, S. Identification and transcriptional organization of a gene cluster involved in biosynthesis and transport of acinetobactin, a siderophore produced by Acinetobacter baumannii ATCC 19606T. Microbiology 2004, 150, 2587–2597. [Google Scholar] [CrossRef] [Green Version]
- Fiester, S.E.; Nwugo, C.C.; Penwell, W.F.; Neary, J.M.; Beckett, A.C.; Arivett, B.A.; Schmidt, R.E.; Geiger, S.C.; Connerly, P.L.; Menke, S.M.; et al. Role of the carboxy terminus of SecA in iron acquisition, protein translocation, and virulence of the bacterial pathogen Acinetobacter baumannii. Infect. Immun. 2015, 83, 1354–1365. [Google Scholar] [CrossRef] [Green Version]
- Echenique, J.R.; Arienti, H.; Tolmasky, M.E.; Read, R.R.; Staneloni, R.J.; Crosa, J.H.; Actis, L.A. Characterization of a high-affinity iron transport system in Acinetobacter baumannii. J. Bacteriol. 1992, 174, 7670–7679. [Google Scholar] [CrossRef] [Green Version]
- Moynié, L.; Luscher, A.; Rolo, D.; Pletzer, D.; Tortajada, A.; Weingart, H.; Braun, Y.; Page, M.G.P.; Naismith, J.H.; Köhler, T. Structure and function of the PiuA and PirA siderophore-drug receptors from Pseudomonas aeruginosa and Acinetobacter baumannii. Antimicrob. Agents Chemother. 2017, 61, e02531-16. [Google Scholar] [CrossRef] [Green Version]
- Hantke, K.; Nicholson, G.; Rabsch, W.; Winkelmann, G. Salmochelins, siderophores of Salmonella enterica and uropathogenic Escherichia coli strains, are recognized by the outer membrane receptor IroN. Proc. Natl. Acad. Sci. USA 2003, 100, 3677–3682. [Google Scholar] [CrossRef] [Green Version]
- Hancock, V.; Ferrières, L.; Klemm, P. The ferric yersiniabactin uptake receptor FyuA is required for efficient biofilm formation by urinary tract infectious Escherichia coli in human urine. Microbiology 2008, 154, 167–175. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Penwell, W.F.; DeGrace, N.; Tentarelli, S.; Gauthier, L.; Gilbert, C.M.; Arivett, B.A.; Miller, A.A.; Durand-Reville, T.F.; Joubran, C.; Actis, L.A. Discovery and characterization of new hydroxamate siderophores, baumannoferrin A and B, produced by Acinetobacter baumannii. ChemBioChem 2015, 16, 1896–1904. [Google Scholar] [CrossRef] [PubMed]
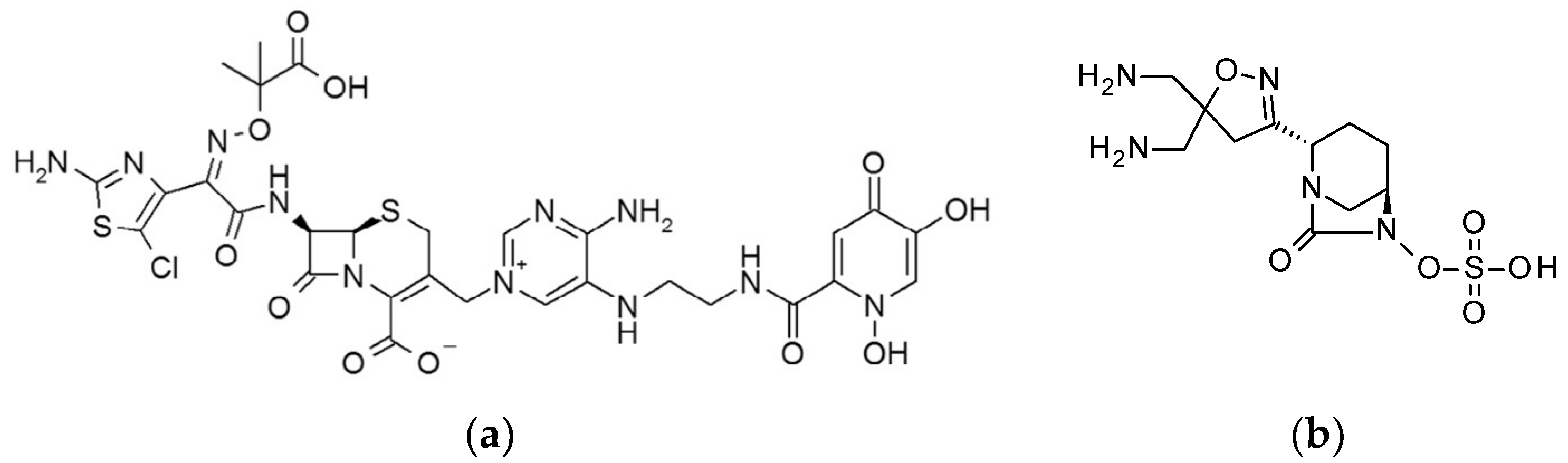

| Strain | GT-1 MIC (μg/mL) | Fold Change |
|---|---|---|
| DH5α+ ZpUC19::blaDHA-1 | 0.5 | ↑ 16-fold |
| DH5α+ ZpUC19::blaPER-1 | 32 | ↑ 1024-fold |
| DH5α+ ZpUC19 | 0.03125 | − |
| No. | Gene Code | Gene Name | Membrane Position | Reference |
|---|---|---|---|---|
| 1 | tonB | Ton complex subunit B | Inner membrane | [32] |
| 2 | exbB | Biopolymer transport subunit B | Inner membrane | [32] |
| 3 | exbD | Biopolymer transport subunit D | Inner membrane | [32] |
| 4 | tonB3 | Ton complex subunit B | N/D | [12] |
| 5 | exbB3 | Biopolymer transport subunit B3 | N/D | [12] |
| 6 | exbD3 | Biopolymer transport subunit D3 | N/D | [12] |
| 7 | fepA | Ferric enterobactin outer membrane transporter | Outer membrane | [33] |
| 8 | fepB | Ferric enterobactin-binding periplasmic protein | Periplasm | [34] |
| 9 | fepC | Ferric enterobactin transport ATP-binding protein | Inner membrane | [35] |
| 10 | fepD | Ferric enterobactin transport system permease protein | Inner membrane | [36] |
| 11 | fecA | Ferric citrate outer membrane transporter | Inner membrane | [33] |
| 12 | fiu | Catecholate siderophore receptor | Outer membrane | [37] |
| 13 | cirA | Ferric dihyroxybenzoylserine outer membrane transporter | Outer membrane | [32] |
| 14 | iutA | Ferric aerobactin receptor | Outer membrane | [38] |
| 15 | fhuA | Ferrichrome outer membrane transporter receptor | Outer membrane | [33] |
| 16 | bauA | Ferric acinetobactin receptor | Outer membrane | [39] |
| 17 | bauB | Ferric acinetobactin transport system periplasmic binding protein | Inner membrane | [40] |
| 18 | bauC | ABC-type enterochelin transport system, permease component | Inner membrane | [41] |
| 19 | bauD | Ferric acinetobactin transport system permease | Inner membrane | [41] |
| 20 | bauE | ABC-type enterochelin transport system ATPase component | N/D | [42] |
| 21 | pirA | Ferric enterobactin receptor | Outer membrane | [43] |
| 22 | piuA | Hydroxamate-type ferrisiderophore receptor | Outer membrane | [43] |
| 23 | iroN | Salmochelin uptake receptor IroN | Outer membrane | [44] |
| 24 | fyuA | Yersiniabactin uptake receptor | Outer membrane | [45] |
| 25 | bfnH | Baumannoferrin uptake receptor | Outer membrane | [46] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nguyen, L.P.; Pinto, N.A.; Vu, T.N.; Lee, H.; Cho, Y.L.; Byun, J.-H.; D’Souza, R.; Yong, D. In Vitro Activity of a Novel Siderophore-Cephalosporin, GT-1 and Serine-Type ?-Lactamase Inhibitor, GT-055, against Escherichia coli, Klebsiella pneumoniae and Acinetobacter spp. Panel Strains. Antibiotics 2020, 9, 267. https://doi.org/10.3390/antibiotics9050267
Nguyen LP, Pinto NA, Vu TN, Lee H, Cho YL, Byun J-H, D’Souza R, Yong D. In Vitro Activity of a Novel Siderophore-Cephalosporin, GT-1 and Serine-Type ?-Lactamase Inhibitor, GT-055, against Escherichia coli, Klebsiella pneumoniae and Acinetobacter spp. Panel Strains. Antibiotics. 2020; 9(5):267. https://doi.org/10.3390/antibiotics9050267
Chicago/Turabian StyleNguyen, Le Phuong, Naina Adren Pinto, Thao Nguyen Vu, Hyunsook Lee, Young Lag Cho, Jung-Hyun Byun, Roshan D’Souza, and Dongeun Yong. 2020. "In Vitro Activity of a Novel Siderophore-Cephalosporin, GT-1 and Serine-Type ?-Lactamase Inhibitor, GT-055, against Escherichia coli, Klebsiella pneumoniae and Acinetobacter spp. Panel Strains" Antibiotics 9, no. 5: 267. https://doi.org/10.3390/antibiotics9050267
APA StyleNguyen, L. P., Pinto, N. A., Vu, T. N., Lee, H., Cho, Y. L., Byun, J.-H., D’Souza, R., & Yong, D. (2020). In Vitro Activity of a Novel Siderophore-Cephalosporin, GT-1 and Serine-Type ?-Lactamase Inhibitor, GT-055, against Escherichia coli, Klebsiella pneumoniae and Acinetobacter spp. Panel Strains. Antibiotics, 9(5), 267. https://doi.org/10.3390/antibiotics9050267





