A Tale of Two Ends: Repurposing Metallic Compounds from Anti-Tumour Agents to Effective Antibacterial Activity
Abstract
:1. Introduction
2. Results
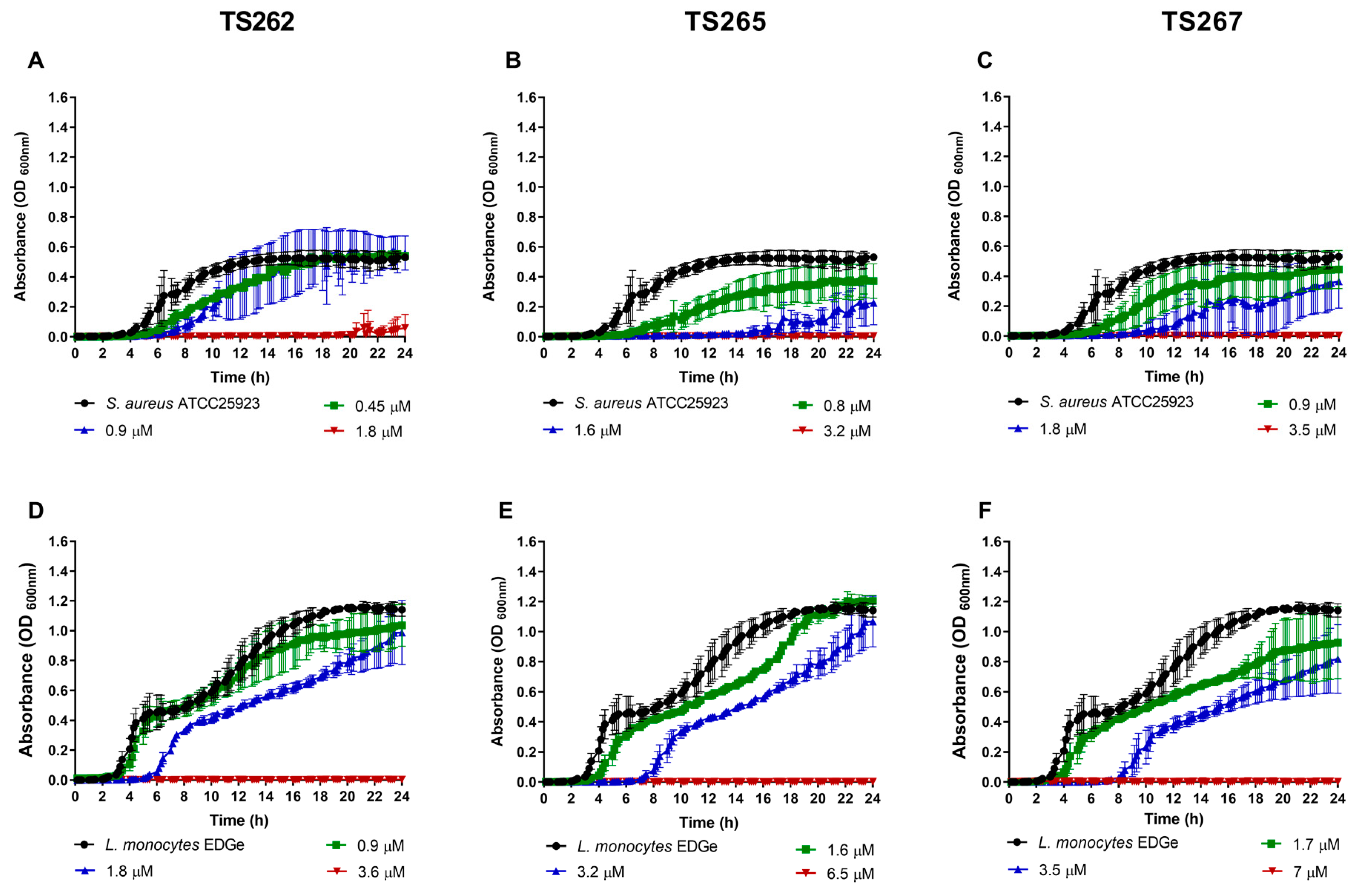
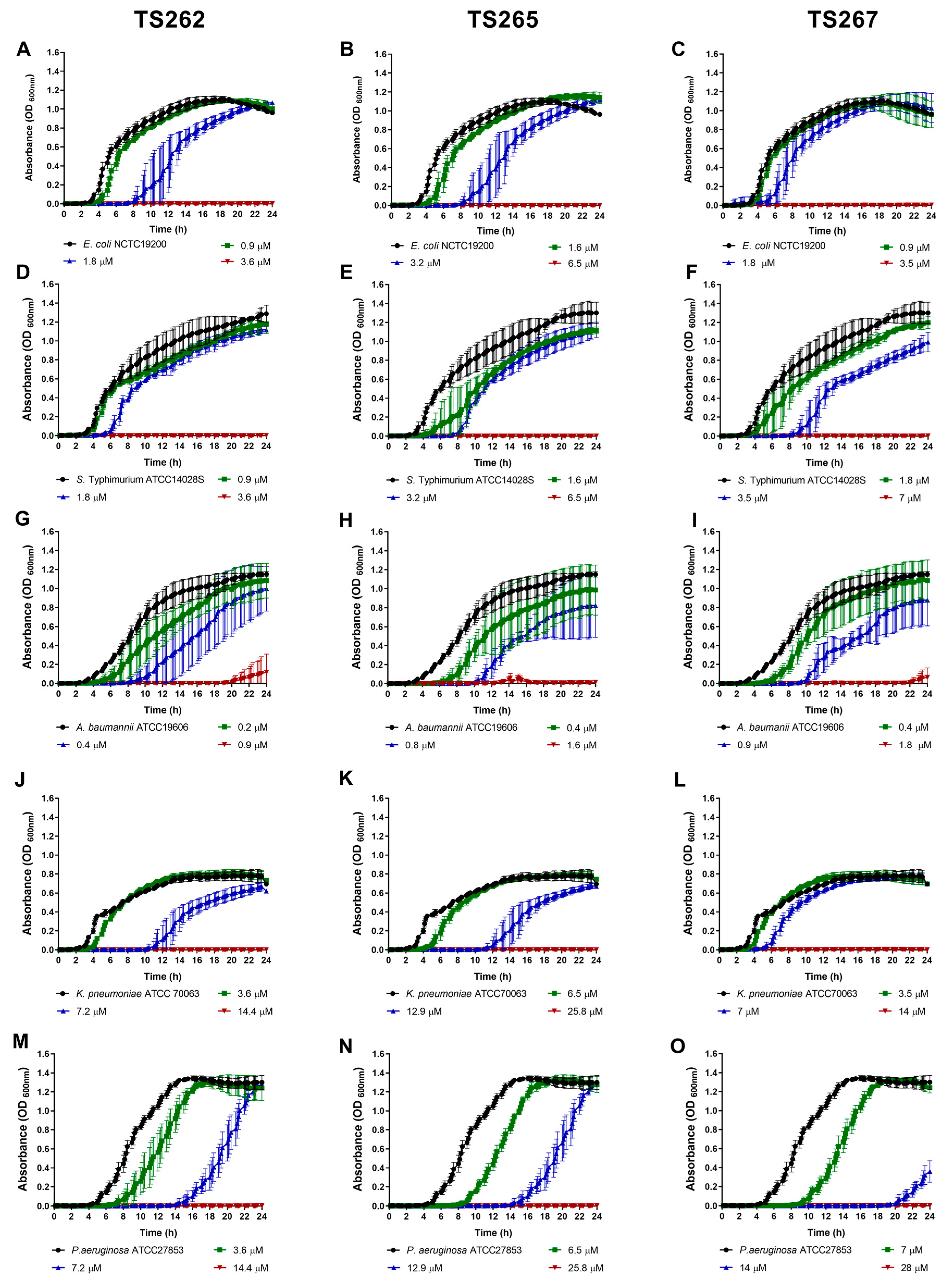
2.1. Antimicrobial Activity of the Anti-Tumour Agents
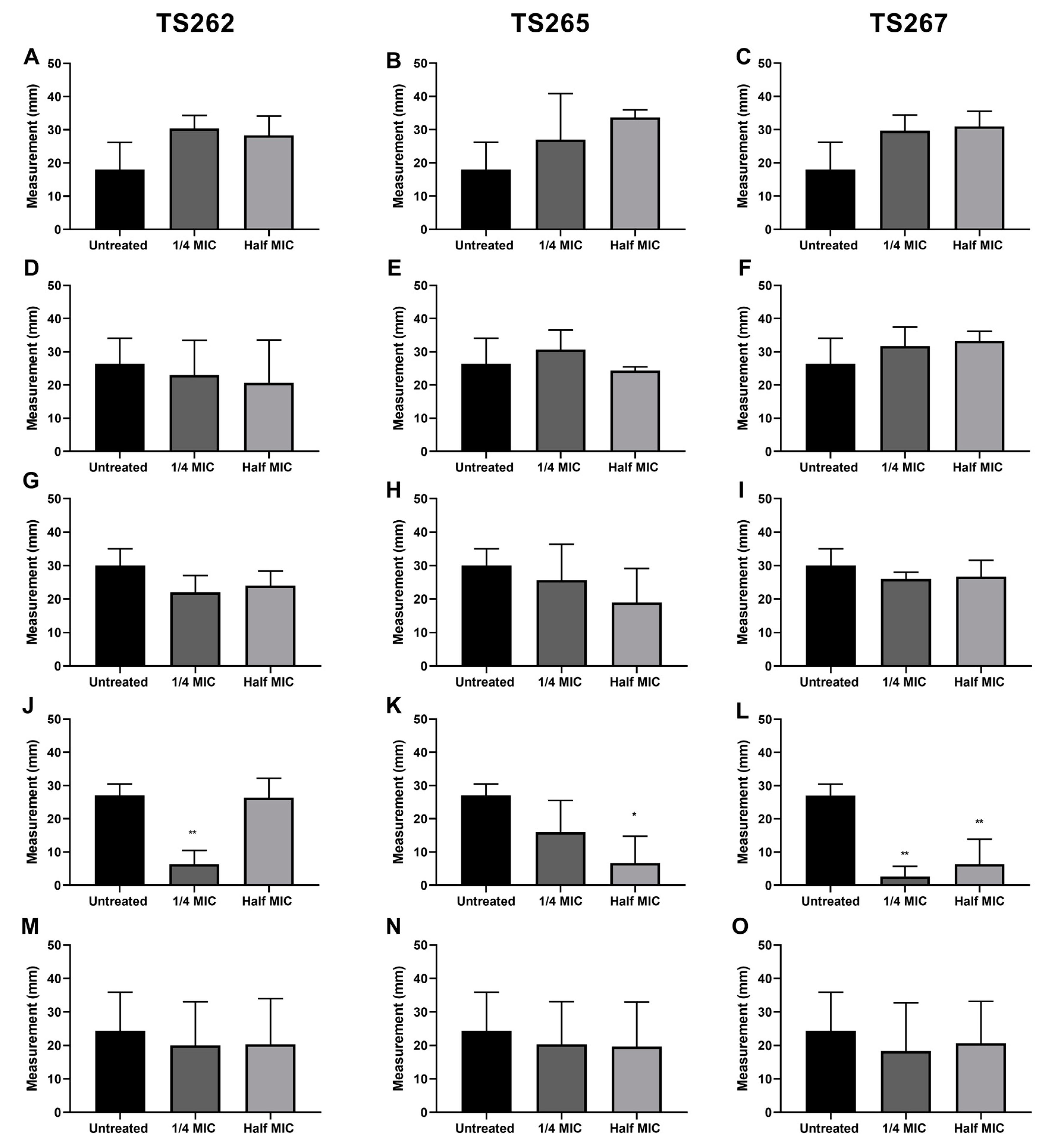
2.2. Effect on Bacterial Motility
2.3. Effect on Bacterial Membrane Permeability
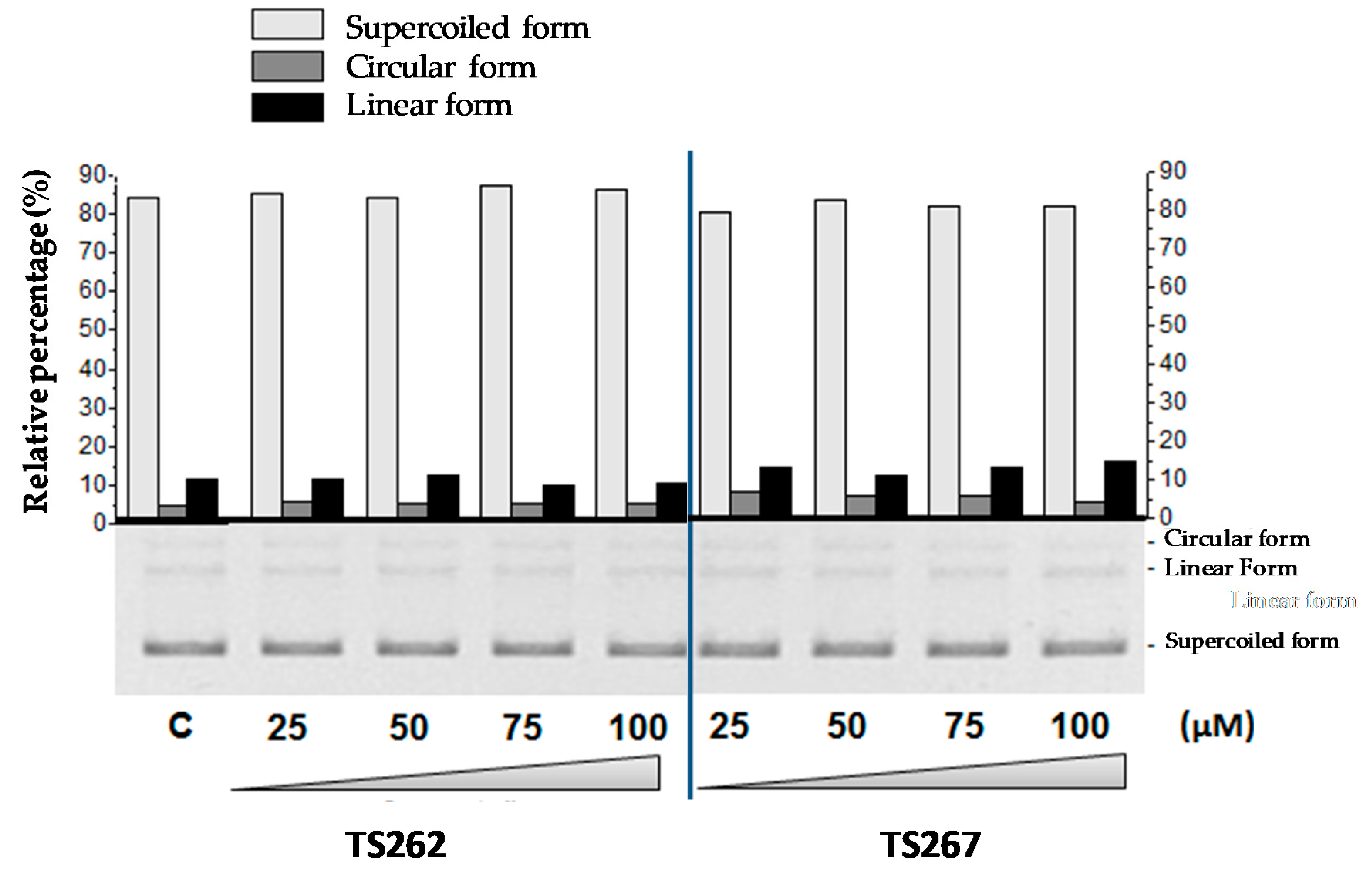
2.4. DNA–Metal Compound Interaction
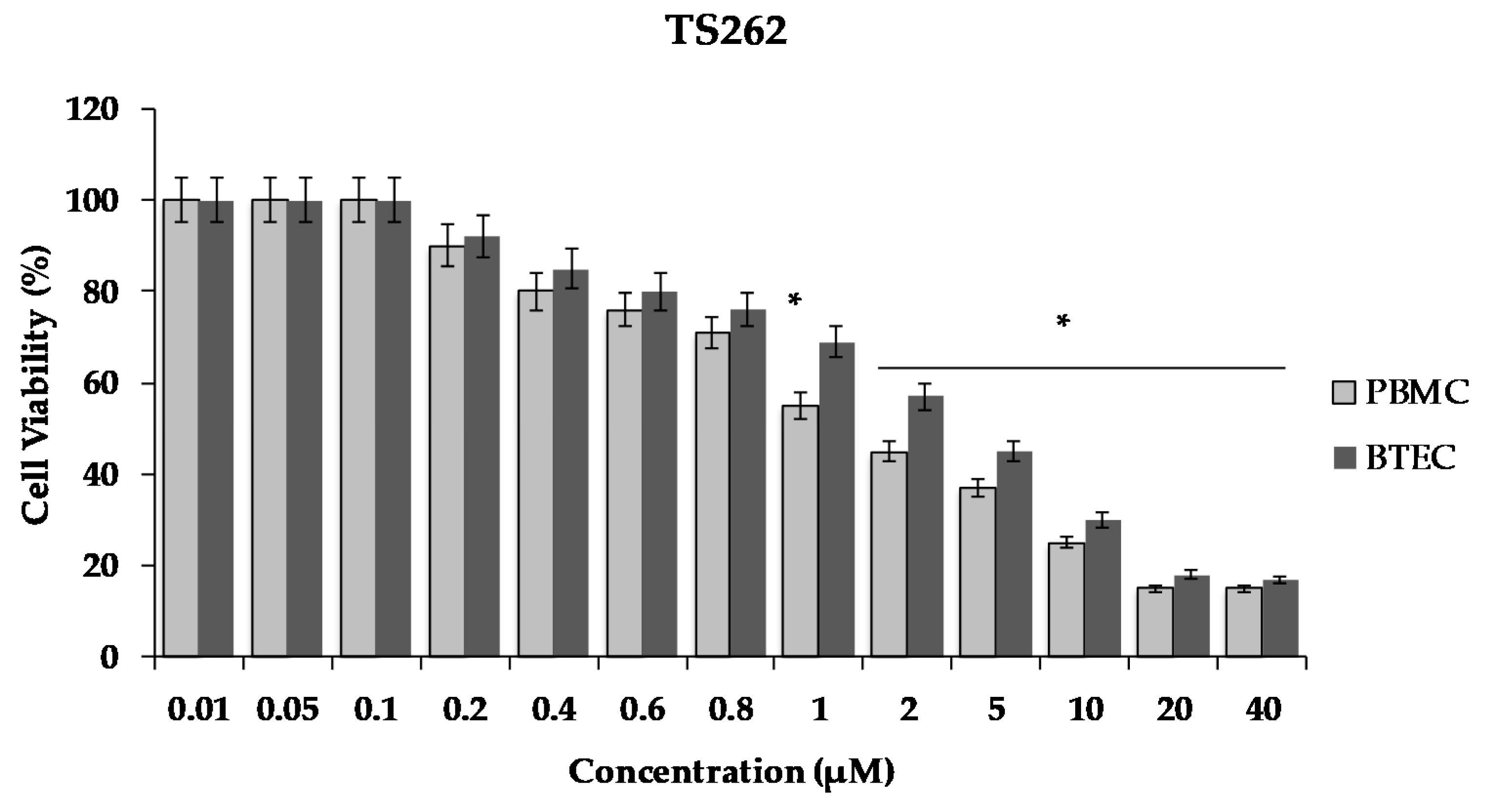
2.5. Cell Viability
3. Discussion
4. Materials and Methods
4.1. Compounds
4.2. Bacterial Strains
4.3. Antibacterial Activity
4.4. Bacterial Growth in the Presence of the Anti-Tumour Compounds
4.5. Motility Assays
4.6. Membrane Permeability
4.7. Electrophorectic Analysis of DNA-Metal Compound Interaction
4.8. Mammalian Cell Culture and Cell Viability
4.9. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- O’Neil, J.I.M. Antimicrobial Resistance: Tackling a crisis for the health and wealth of nations The Review on Antimicrobial Resistance Chaired. Rev. Antimicrob. Resist. 2014, 20, 1–16. [Google Scholar]
- Cassini, A.; Högberg, L.D.; Plachouras, D.; Quattrocchi, A.; Hoxha, A.; Simonsen, G.S.; Colomb-Cotinat, M.; Kretzschmar, M.E.; Devleesschauwer, B.; Cecchini, M.; et al. Attributable deaths and disability-adjusted life-years caused by infections with antibiotic-resistant bacteria in the EU and the European Economic Area in 2015: A population-level modelling analysis. Lancet Infect. Dis. 2019, 19, 56–66. [Google Scholar] [CrossRef] [Green Version]
- Tyers, M.; Wright, G.D. Drug combinations: A strategy to extend the life of antibiotics in the 21st century. Nat. Rev. Genet. 2019, 17, 141–155. [Google Scholar] [CrossRef]
- Qadir, M.I.; Chauhdary, Z. Antibacterial Activity of Novel Strains of Bacteriophages: An Experimental Approach. Crit. Rev. Eukaryot. Gene Expr. 2018, 28, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Hua, L.; Hilliard, J.J.; Shi, Y.; Tkaczyk, C.; Cheng, L.I.; Yu, X.; Datta, V.; Ren, S.; Feng, H.; Zinsou, R.; et al. Assessment of an Anti-Alpha-Toxin Monoclonal Antibody for Prevention and Treatment of Staphylococcus aureus-Induced Pneumonia. Antimicrob. Agents Chemother. 2013, 58, 1108–1117. [Google Scholar] [CrossRef] [Green Version]
- Kotzampassi, K.; Giamarellos-Bourboulis, E.J. Probiotics for infectious diseases: More drugs, less dietary supplementation. Int. J. Antimicrob. Agents 2012, 40, 288–296. [Google Scholar] [CrossRef]
- Baptista, P.V.; McCusker, M.P.; Carvalho, A.; Ferreira, D.A.; Mohan, N.M.; Martins, M.; Fernandes, A.R. Nano-Strategies to Fight Multidrug Resistant Bacteria—“A Battle of the Titans”. Front. Microbiol. 2018, 9. [Google Scholar] [CrossRef] [Green Version]
- Mohan, N.M.; Zorgani, A.; Jalowicki, G.; Kerr, A.; Khaldi, N.; Martins, M. Unlocking NuriPep 1653 From Common Pea Protein: A Potent Antimicrobial Peptide to Tackle a Pan-Drug Resistant Acinetobacter baumannii. Front. Microbiol. 2019, 10, 2086. [Google Scholar] [CrossRef]
- Moroney, J.W.; Schlumbrecht, M.P.; Helgason, T.; Coleman, R.L.; Moulder, S.; Naing, A.; Bodurka, D.; Janku, F.; Hong, D.S.; Kurzrock, R. A phase I trial of liposomal doxorubicin, bevacizumab, and temsirolimus in patients with advanced gynecologic and breast malignancies. Clin. Cancer Res. 2011, 17, 6840–6846. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patra, M.; Gasser, G.; Metzler-Nolte, N. Small organometallic compounds as antibacterial agents. Dalton Trans. 2012, 41, 6350–6358. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patra, M.; Wenzel, M.; Prochnow, P.; Pierroz, V.; Gasser, G.; Bandow, J.E.; Metzler-Nolte, N. An organometallic structure-activity relationship study reveals the essential role of a Re(CO)3 moiety in the activity against gram-positive pathogens including MRSA. Chem. Sci. 2015, 6, 214–224. [Google Scholar] [CrossRef]
- Silva, T.F.S.; Smoleński, P.; Martins, L.M.; Da Silva, M.F.C.G.; Fernandes, A.R.; Luis, D.; Silva, A.; Santos, S.; Borralho, P.M.; Rodrigues, C.M.P.; et al. Cobalt and Zinc Compounds Bearing 1,10-Phenanthroline-5,6-dione or 1,3,5-Triaza-7-phosphaadamantane Derivatives—Synthesis, Characterization, Cytotoxicity, and Cell Selectivity Studies. Eur. J. Inorg. Chem. 2013, 2013, 3651–3658. [Google Scholar] [CrossRef] [Green Version]
- Luís, D.V.; Silva, J.; Tomaz, A.I.; De Almeida, R.; Larguinho, M.; Baptista, P.V.; Martins, L.M.; Silva, T.F.S.; Borralho, P.; Rodrigues, C.M.P.; et al. Insights into the mechanisms underlying the antiproliferative potential of a Co(II) coordination compound bearing 1,10-phenanthroline-5,6-dione: DNA and protein interaction studies. JBIC J. Boil. Inorg. Chem. 2014, 19, 787–803. [Google Scholar] [CrossRef] [PubMed]
- Pedrosa, P.; Corvo, M.L.; Ferreira-Silva, M.; Martins, P.; Carvalheiro, M.C.; Costa, P.M.; Martins, C.; Martins, L.M.; Baptista, P.V.; Fernandes, A.R. Targeting Cancer Resistance via Multifunctional Gold Nanoparticles. Int. J. Mol. Sci. 2019, 20, 5510. [Google Scholar] [CrossRef] [Green Version]
- Fernandes, A.R.; Jesus, J.; Martins, P.; Figueiredo, S.; Rosa, D.; Martins, L.M.; Corvo, M.L.; Carvalheiro, M.C.; Costa, P.M.; Baptista, P.V. Multifunctional gold-nanoparticles: A nanovectorization tool for the targeted delivery of novel chemotherapeutic agents. J. Control. Release 2017, 245, 52–61. [Google Scholar] [CrossRef]
- Viganon, L.; Howe, O.L.; McCarron, P.; McCann, M.; Devereux, M. The Antibacterial Activity of Metal Complexes Containing 1, 10-phenanthroline: Potential as Alternatire Therapeutics in the Era of Antibiotic Resistance. Curr. Top. Med. Chem. 2017, 17, 1280–1302. [Google Scholar] [CrossRef]
- Frei, A. Metal Complexes, an Untapped Source of Antibiotic Potential? Antibiotics 2020, 9, 90. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lemire, J.A.; Harrison, J.J.; Cherak, S.J. Antimicrobial activity of metals: Mechanisms, molecular targets and applications. Nat. Rev. Microbiol. 2013, 11, 371–384. [Google Scholar] [CrossRef]
- Pathania, R.; Sharma, A.; Gupta, V.K. Efflux pump inhibitors for bacterial pathogens: From bench to bedside. Indian J. Med Res. 2019, 149, 129–145. [Google Scholar] [CrossRef]
- Mahamoud, A.; Chevalier, J.; Alibert-Franco, S.; Kern, W.V.; Pagès, J.-M. Antibiotic efflux pumps in Gram-negative bacteria: The inhibitor response strategy. J. Antimicrob. Chemother. 2007, 59, 1223–1229. [Google Scholar] [CrossRef] [PubMed]
- Meister, M.; Lowe, G.; Berg, H.C. The proton flux through the bacterial flagellar motor. Cell 1987, 49, 643–650. [Google Scholar] [CrossRef]
- Berridge, M.; Tan, A.S. Characterization of the Cellular Reduction of 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT): Subcellular Localization, Substrate Dependence, and Involvement of Mitochondrial Electron Transport in MTT Reduction. Arch. Biochem. Biophys. 1993, 303, 474–482. [Google Scholar] [CrossRef] [PubMed]
- Cannon, C.L.; Hogue, L.A.; Vajravelu, R.K.; Capps, G.H.; Ibricevic, A.; Hindi, K.M.; Kascatan-Nebioglu, A.; Walter, M.J.; Brody, S.L.; Youngs, W.J. In Vitro and Murine Efficacy and Toxicity Studies of Nebulized SCC1, a Methylated Caffeine-Silver(I) Complex, for Treatment of Pulmonary Infections. Antimicrob. Agents Chemother. 2009, 53, 3285–3293. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kazemizadeh, A.R.; Shajari, N.; Shapouri, R.; Adibpour, N.; Teimuri-Mofrad, R.; Dinmohammadi, P. One-pot, four-component synthesis of 1,3,4-oxadiazole derivatives containing a ferrocene unit and their antimicrobial activity. Appl. Organomet. Chem. 2015, 30, 148–153. [Google Scholar] [CrossRef]
- Frei, A.; Zuegg, J.; Elliott, A.G.; Baker, M.V.; Bräse, S.; Brown, C.; Chen, F.; Dowson, C.G.; Dujardin, G.; Jung, N.; et al. Metal complexes as a promising source for new antibiotics. Chem. Sci. 2020, 11, 2627–2639. [Google Scholar] [CrossRef] [Green Version]
- Torres-Urquidy, O.; Bright, K.R. Efficacy of multiple metals against copper-resistant bacterial strains. J. Appl. Microbiol. 2012, 112, 695–704. [Google Scholar] [CrossRef]
- Lima de Silva, A.A.; de Carvalho, M.A.; de Souza, S.A.; Dias, P.M.; da Silva Filho, R.G.; de Meirelles Saramago, C.S.; de Melo Bento, C.A. Heavy metal tolerance (Cr, Ag AND Hg) in bacteria isolated from sewage. Braz. J. Microbiol. 2012, 43, 1620–1631. [Google Scholar] [CrossRef] [Green Version]
- Peters, K.; Pazos, M.; Edoo, Z.; Hugonnet, J.E.; Martorana, A.M.; Polissi, A.; VanNieuwenhze, M.S.; Arthur, M.; Vollmer, W. Copper inhibits peptidoglycan LD-transpeptidases suppressing β-lactam resistance due to bypass of penicillin-binding proteins. Proc. Natl. Acad. Sci. USA 2018, 115, 10786–10791. [Google Scholar] [CrossRef] [Green Version]
- Giovanella, P.; Cabral, L.; Bento, F.M.; Gianello, C.; Camargo, F.A.O. Mercury (II) removal by resistant bacterial isolates and mercuric (II) reductase activity in a new strain of Pseudomonas sp. B50A. New Biotechnol. 2016, 33, 216–223. [Google Scholar] [CrossRef]
- Abou-Shanab, R.A.I.; Van Berkum, P.; Angle, J. Heavy metal resistance and genotypic analysis of metal resistance genes in gram-positive and gram-negative bacteria present in Ni-rich serpentine soil and in the rhizosphere of Alyssum murale. Chemosphere 2007, 68, 360–367. [Google Scholar] [CrossRef]
- Mindlin, S.; Kholodii, G.; Gorlenko, Z.; Minakhina, S.; Minakhin, L.; Kalyaeva, E.; Kopteva, A.; Petrova, M.; Yurieva, O.; Nikiforov, V.; et al. Mercury resistance transposons of Gram-negative environmental bacteria and their classification. Res. Microbiol. 2001, 152, 811–822. [Google Scholar] [CrossRef]
- Wayne, P.A. Clinical and Laboratory Standards Institute Performance standards for antimicrobial susceptibility testing: Twenty-third informational supplement. In CLSI Document M100-S23; 2013; ISBN 1562388657. Available online: https://www.facm.ucl.ac.be/intranet/CLSI/CLSI-M100S23-susceptibility-testing-2013-no-protection.pdf (accessed on 7 April 2020).
- Wang, Q.; Zhao, Y.; McClelland, M.; Harshey, R.M. The RcsCDB Signaling System and Swarming Motility in Salmonella enterica Serovar Typhimurium: Dual Regulation of Flagellar and SPI-2 Virulence Genes. J. Bacteriol. 2007, 189, 8447–8457. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodrigues, L.; Viveiros, M.; Aínsa, J.A. Measuring efflux and permeability in mycobacteria. Methods Mol. Biol. 2015, 1285, 227–239. [Google Scholar] [CrossRef] [PubMed]
- Barroco, C.A.; Roma-Rodrigues, C.; Raposo, L.R.; Brás, C.; Diniz, M.; Caço, J.; Costa, P.M.; Santos-Sanches, I.; Fernandes, A.R. Streptococcus dysgalactiae subsp. dysgalactiae isolated from milk of the bovine udder as emerging pathogens: In vitro and in vivo infection of human cells and zebrafish as biological models. Microbiologyopen 2018, 8, e00623. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pedrosa, P.; Mendes, R.; Cabral, R.; Martins, L.M.D.R.S.; Baptista, P.V.; Fernandes, A.R. Combination of chemotherapy and Au-nanoparticle photothermy in the visible light to tackle doxorubicin resistance in cancer cells. Sci. Rep. 2018, 8, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Bacteria | TS262 | TS265 | TS267 | |||
|---|---|---|---|---|---|---|
| MIC | MBC | MIC | MBC | MIC | MBC | |
| µM | ||||||
| Staphylococcus aureus ATCC25923 | 1.8 | 1.8 | 3.2 | 3.2 | 3.5 | 3.5 |
| Listeria monocytogenes EGDe | 3.6 | 3.6 | 6.5 | 6.5 | 7.0 | 7.0 |
| Escherichia coli NCTC12900 | 3.6 | 3.6 | 6.5 | 6.5 | 3.5 | 3.5 |
| Salmonella Typhimurium ATCC14028S | 3.6 | 3.6 | 6.5 | 6.5 | 7.0 | 7.0 |
| Acinetobacter baumannii ATCC19606 | 0.9 | 1.8 | 1.6 | 1.6 | 1.8 | 1.8 |
| Klebsiella pneumoniae ATCC70063 | 14.4 | 14.4 | 25.8 | 25.8 | 14 | 14 |
| Pseudomonas aeruginosa ATCC27853 | 14.4 | 14.4 | 25.8 | 25.8 | 14 | 28 |
| Bacterial Strains | Lag Phases (in hours) | ||||||
|---|---|---|---|---|---|---|---|
| Control | TS 262 | TS 265 | TS 267 | ||||
| ¼MIC | ½MIC | ¼MIC | ½MIC | ¼MIC | ½MIC | ||
| S. aureus ATCC25923 | 4.75 | 6 | 7.5 | 7.5 | 16.5 | 6.5 | 11.5 |
| L. monocytogenes EGDe | 3.25 | 3.2 | 6 | 4 | 7.3 | 3.75 | 8.25 |
| E . coli NCTC12900 | 3 | 4.5 | 8.25 | 4.5 | 8.5 | 4 | 5.25 |
| S. Typhimurium ATCC14028S | 3.75 | 4 | 6 | 5 | 8 | 4 | 9 |
| A. baumannii ATCC19606 | 3.75 | 5.25 | 9 | 7.25 | 10.5 | 6 | 10.25 |
| K. pneumoniae ATCC70063 | 3 | 4.25 | 11.25 | 5 | 12 | 4 | 5.5 |
| P. aeruginosa ATCC27853 | 5 | 8 | 15.25 | 9 | 15 | - | 10 |
| Compound | Chemical Formula | Molecular Weight (g mol−1) | Solvent |
|---|---|---|---|
| TS262 | C24H12Cl2N4O4Zn | 556.67 | H2O |
| TS265 | C24H15BClCoF4N4O5 | 620.59 | H2O |
| TS267 | C18H18BClF4N5O3PZn | 570.99 | H2O |
| DION (phendione) | C12H6N2O2 | 210.19 | H2O |
| PTA (1,3,5-Triaza-7-phosphaadamantane) | C6H12N3P | 157.15 | H2O |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alves Ferreira, D.; Martins, L.M.D.R.S.; Fernandes, A.R.; Martins, M. A Tale of Two Ends: Repurposing Metallic Compounds from Anti-Tumour Agents to Effective Antibacterial Activity. Antibiotics 2020, 9, 321. https://doi.org/10.3390/antibiotics9060321
Alves Ferreira D, Martins LMDRS, Fernandes AR, Martins M. A Tale of Two Ends: Repurposing Metallic Compounds from Anti-Tumour Agents to Effective Antibacterial Activity. Antibiotics. 2020; 9(6):321. https://doi.org/10.3390/antibiotics9060321
Chicago/Turabian StyleAlves Ferreira, Daniela, Luísa M. D. R. S. Martins, Alexandra R. Fernandes, and Marta Martins. 2020. "A Tale of Two Ends: Repurposing Metallic Compounds from Anti-Tumour Agents to Effective Antibacterial Activity" Antibiotics 9, no. 6: 321. https://doi.org/10.3390/antibiotics9060321
APA StyleAlves Ferreira, D., Martins, L. M. D. R. S., Fernandes, A. R., & Martins, M. (2020). A Tale of Two Ends: Repurposing Metallic Compounds from Anti-Tumour Agents to Effective Antibacterial Activity. Antibiotics, 9(6), 321. https://doi.org/10.3390/antibiotics9060321