Detection of Carbapenem-Resistance Genes in Klebsiella Species Recovered from Selected Environmental Niches in the Eastern Cape Province, South Africa
Abstract
:1. Introduction
2. Materials and Methods
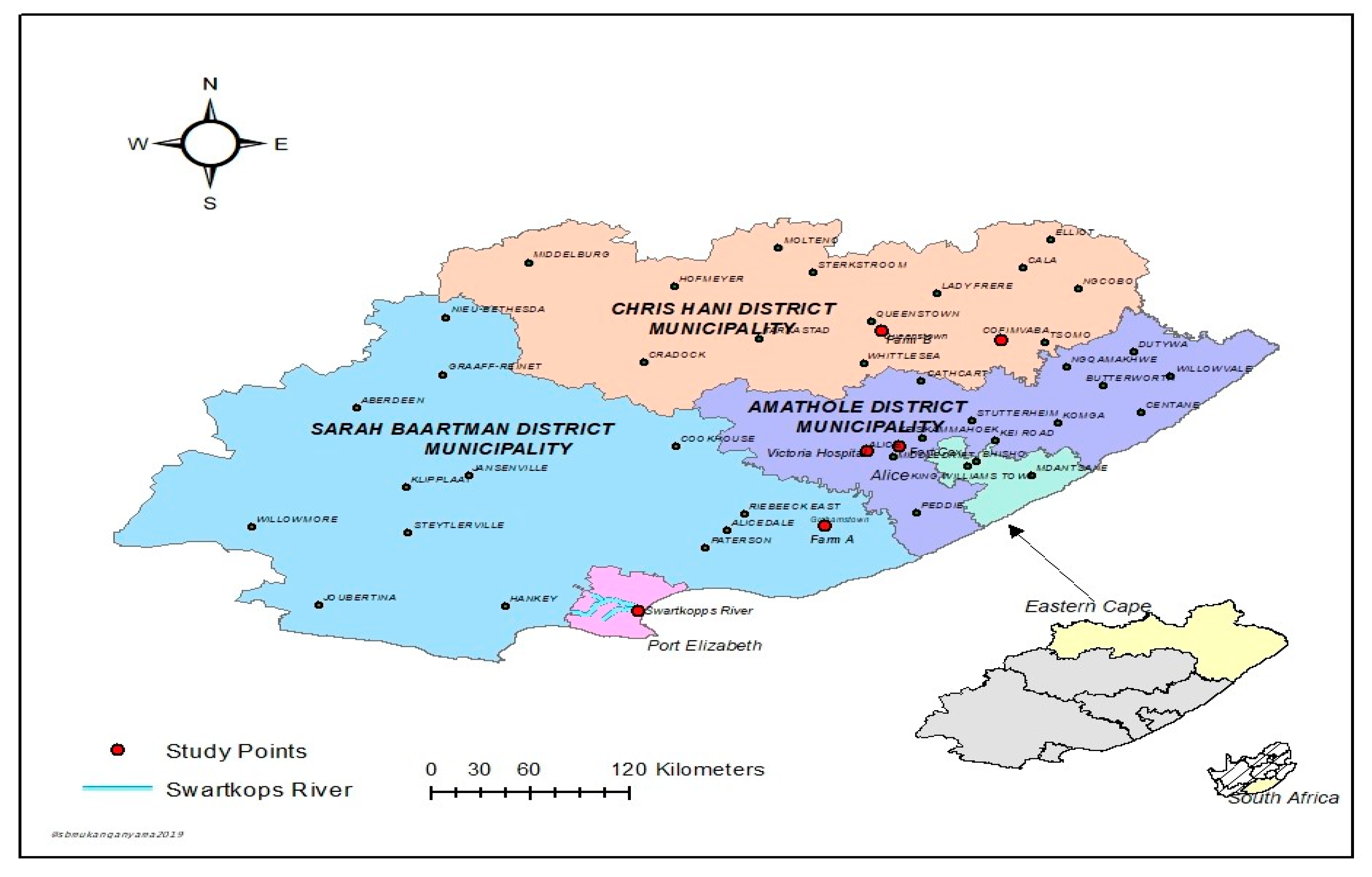
2.1. Description of Study Areas and Sample Collection
2.2. Sample Preparation and Processing
2.2.1. Processing of Vegetable Sample
2.2.2. Processing of Soil Sample
2.2.3. Processing of Water Samples
2.3. DNA Extraction
2.4. Molecular Identification of Presumptive Isolates
2.5. Antimicrobial Susceptibility Test of the Confirmed Klebsiella Species
2.6. Molecular Characterization of the Relevant Carbapenem Resistance Genes
3. Results
3.1. Identification and Characterization of Presumptive Klebsiella Spp. Isolates
3.2. Antimicrobial Susceptibility Patterns of the Confirmed Klebsiella Species
3.3. Molecular Characterization of the Relevant Carbapenem Resistance Genes
3.4. Percentage Occurrence of the Antimicrobial Resistance Genes in Klebsiella Species
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ventola, C.L. The antibiotic resistance crisis: Part 1: Causes and threats. Pharm. Ther. 2015, 40, 277–283. [Google Scholar]
- Muntean, M.M.; Muntean, A.A.; Popa, M.I. Carbapenemases in Enterobacteriaceae–overview and importance. Infect. Routine 2017, 49, 13–16. [Google Scholar]
- Bush, K. A resurgence of β-lactamase inhibitor combinations effective against multidrug-resistant Gram-negative pathogens. Int. J. Antimicrob. Agents 2015, 46, 483–493. [Google Scholar] [CrossRef] [PubMed]
- Laxminarayan, R.; Matsoso, P.; Pant, S.; Brower, C.; Røttingen, J.A.; Klugman, K.; Davies, S. Access to effective antimicrobials: A worldwide challenge. Lancet 2016, 387, 168–175. [Google Scholar] [CrossRef]
- Laudadio, E.; Cedraro, N.; Mangiaterra, G.; Citterio, B.; Mobbili, G.; Minnelli, C.; Bizzaro, D.; Biavasco, F.; Galeazzi, R. Natural Alkaloid Berberine Activity against Pseudomonas aeruginosa MexXY-Mediated Aminoglycoside Resistance: In Silico and in Vitro Studies. J. Nat. Prod. 2019, 82, 1935–1944. [Google Scholar] [CrossRef]
- Pormohammad, A.; Nasiri, M.J.; Azimi, T. Prevalence of antibiotic resistance in Escherichia coli strains simultaneously isolated from humans, animals, food, and the environment: A systematic review and meta-analysis. Infect. Drug Resist. 2019, 12, 1181–1197. [Google Scholar] [CrossRef] [Green Version]
- Walther-Rasmussen, J.; Høiby, N. OXA-type carbapenemases. J. Antimicrob. Chemother. 2006, 57, 373–383. [Google Scholar] [CrossRef] [Green Version]
- Walther-Rasmussen, J.; Høiby, N. Class A carbapenemases. J. Antimicrob. Chemother. 2007, 60, 470–482. [Google Scholar] [CrossRef] [Green Version]
- Aqel, A.A.; Giakkoupi, P.; Alzoubi, H.; Masalha, I.; Ellington, M.J.; Vatopoulos, A. Detection of OXA-48-like and NDM carbapenemases producing Klebsiella pneumoniae in Jordan: A pilot study. J. Infect. Public Health 2017, 10, 150–155. [Google Scholar] [CrossRef] [Green Version]
- Lee, M.; Hesek, D.; Blázquez, B.; Lastochkin, E.; Boggess, B.; Fisher, J.F.; Mobashery, S. Catalytic spectrum of the penicillin-binding protein 4 of Pseudomonas aeruginosa, a nexus for the induction of β-lactam antibiotic resistance. J. Am. Chem. Soc. 2014, 137, 190–200. [Google Scholar] [CrossRef] [Green Version]
- Roberts, M.C. Antibiotic-resistant environmental bacteria and their role as reservoirs in disease. In Modeling the Transmission and Prevention of Infectious Disease; Springer: Cham, Switzerland, 2017; pp. 187–212. [Google Scholar]
- Chow, D.C.; Rice, K.; Huang, W.; Atmar, R.L.; Palzkill, T. Engineering specificity from broad to narrow: Design of a β-lactamase inhibitory protein (BLIP) variant that exclusively binds and detects KPC β-lactamase. ACS Infect. Dis. 2016, 2, 969–979. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yaghoubi, S.; Baseri, Z.; Rasti, A.; Gharani, M.; Erfani, Y. High prevalence of extended-spectrum β-lactamase (blaCTX-M-15) and New Delhi metallo-β-lactamase-1 (NDM-1) genes among high-level carbapenem resistance Klebsiella pneumonia: An alarm for our health system. Afr. J. Clin. Exp. Microbiol. 2019, 20, 72–82. [Google Scholar] [CrossRef]
- Waseem, H.; Williams, M.R.; Jameel, S.; Hashsham, S.A. Antimicrobial Resistance in the Environment. Water Environ. Res. 2018, 90, 865–884. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Proia, L.; Anzil, A.; Borrego, C.; Farrè, M.; Llorca, M.; Sanchis, J.; Bogaerts, P.; Balcázar, J.L.; Servais, P. Occurrence and persistence of carbapenemases genes in hospital and wastewater treatment plants and propagation in the receiving river. J. Hazard. Mater. 2018, 358, 33–43. [Google Scholar] [CrossRef]
- Khan, F.A.; Söderquist, B.; Jass, J. Prevalence and diversity of antibiotic resistance genes in Swedish aquatic environments impacted by household and hospital wastewater. Front. Microbiol. 2019, 10, 688. [Google Scholar] [CrossRef] [Green Version]
- Uyaguari, M.I.; Croxen, M.A.; Luo, Z.; Cronin, K.; Chan, M.; Baticados, W.N.; Nesbitt, M.J.; Li, S.; Miller, K.; Dooley, D.; et al. Antibiotic resistance genes in agriculture and urban influenced watersheds in southwestern British Columbia. BioRxiv 2017. [Google Scholar] [CrossRef] [Green Version]
- Stange, C.; Yin, D.; Xu, T.; Guo, X.; Schäfer, C.; Tiehm, A. Distribution of clinically relevant antibiotic resistance genes in Lake Tai, China. Sci. Total Environ. 2019, 655, 337–346. [Google Scholar] [CrossRef]
- Du Plessis, K.R.; Botha, A.; Joubert, L.; Bester, R.; Conradie, W.J.; Wolfaardt, G.M. Response of the microbial community to copper oxychloride in acidic sandy loam soil. J. Appl. Microbiol. 2005, 98, 901–909. [Google Scholar] [CrossRef] [Green Version]
- Da Silva, N.; Taniwaki, M.H.; Junqueira, V.C.; Silveira, N.; Okazaki, M.M.; Gomes, R.A.R. Microbiological Examination Methods of Food and Water: A Laboratory Manual; CRC Press: Boca Raton, FL, USA, 2018. [Google Scholar]
- Jacobs, N.J.; Zeigler, W.L.; Reed, F.C.; Stukel, T.A.; Rice, E.W. Comparison of membrane filter, multiple-fermentation-tube, and presence-absence techniques for detecting total coliforms in small community water systems. Appl. Environ. Microbiol. 1986, 51, 1007–1012. [Google Scholar] [CrossRef] [Green Version]
- Jackson, D.P.; Lewis, F.A.; Taylor, G.R.; Boylston, A.W.; Quirke, P. Tissue extraction of DNA and RNA and analysis by the polymerase chain reaction. J. Clin. Pathol. 1990, 43, 499–504. [Google Scholar] [CrossRef]
- Ebomah, K.; Adefisoye, M.; Okoh, A. Pathogenic Escherichia coli Strains Recovered from Selected Aquatic Resources in the Eastern Cape, South Africa, and Its Significance to Public Health. Int. J. Environ. Res. Public Health 2018, 15, 1506. [Google Scholar] [CrossRef] [Green Version]
- Salloum, T.; Arabaghian, H.; Alousi, S.; Abboud, E.; Tokajian, S. Genome sequencing and comparative analysis of an NDM-1-producing Klebsiella pneumoniae ST15 isolated from a refugee patient. Pathog. Glob. Health 2017, 111, 166–175. [Google Scholar] [CrossRef]
- Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Susceptibility Testing; 27th Informational Supplement. CLSI Document M100-S27; Clinical and Laboratory Standards Institute: Wayne, NY, USA, 2017. [Google Scholar]
- Rahman, M.R.; Perisetti, A.; Coman, R.; Bansal, P.; Chhabra, R.; Goyal, H. Duodenoscope-associated infections: Update on an emerging problem. Dig. Dis. Sci. 2018, 64, 1–10. [Google Scholar] [CrossRef]
- Jacob, J.T.; Klein, E.; Laxminarayan, R.; Beldavs, Z.; Lynfield, R.; Kallen, A.J.; Ricks, P.; Edwards, J.; Srinivasan, A.; Fridkin, S.; et al. Vital signs: Carbapenem-resistant Enterobacteriaceae. Morb. Mortal. Wkly. Rep. 2013, 62, 165–170. [Google Scholar]
- Trivedi, M.; Patil, S.; Shettigar, H.; Bairwa, K.; Jana, S. Phenotypic and biotypic characterization of Klebsiella oxytoca: An impact of biofield treatment. Microb. Biochem. Technol. 2015, 7, 202–205. [Google Scholar]
- Siri, G.P.; Sithebe, N.P.; Ateba, C.N. Identification of Klebsiella species isolated from Modimola dam (Mafikeng) North West Province South Africa. Afr. J. Microbiol. Res. 2011, 5, 3958–3963. [Google Scholar]
- Sharma, M.; Chetia, P.; Puzari, M.; Neog, N.; Borah, A. Menace to the ultimate antimicrobials among common Enterobacteriaceae clinical isolates in part of North-East India. BioRxiv 2019. [Google Scholar] [CrossRef]
- Samanta, A.; Mahanti, A.; Chatterjee, S.; Joardar, S.N.; Bandyopadhyay, S.; Sar, T.K.; Mandal, G.P.; Dutta, T.K.; Samanta, I. Pig farm environment as a source of beta-lactamase or AmpC-producing Klebsiella pneumoniae and Escherichia coli. Ann. Microbiol. 2018, 68, 781–791. [Google Scholar] [CrossRef]
- Peneş, N.O.; Muntean, A.A.; Moisoiu, A.; Muntean, M.M.; Chirca, A.; Bogdan, M.A.; Popa, M.I. An overview of resistance profiles ESKAPE pathogens from 2010–2015 in a tertiary respiratory center in Romania. Rom. J. Morphol. Embryol. 2017, 58, 909–922. [Google Scholar]
- Ben Tanfous, F.; Alonso, C.A.; Achour, W.; Ruiz-Ripa, L.; Torres, C.; Ben Hassen, A. First description of KPC-2-producing Escherichia coli and ST15 OXA-48-positive Klebsiella pneumoniae in Tunisia. Microb. Drug Resist. 2017, 23, 365–375. [Google Scholar] [CrossRef]
- Abderrahim, A.; Djahmi, N.; Pujol, C.; Nedjai, S.; Bentakouk, M.C.; Kirane-Gacemi, D.; Dekhil, M.; Sotto, A.; Lavigne, J.P.; Pantel, A. First case of NDM-1-producing Klebsiella pneumoniae in Annaba University hospital, Algeria. Microb. Drug Resist. 2017, 23, 895–900. [Google Scholar] [CrossRef]
- Al-Agamy, M.H.; Aljallal, A.; Radwan, H.H.; Shibl, A.M. Characterization of carbapenemases, ESBLs, and plasmid-mediated quinolone determinants in carbapenem-insensitive Escherichia coli and Klebsiella pneumoniae in Riyadh hospitals. J. Infect. Public Health 2018, 11, 64–68. [Google Scholar] [CrossRef]
- Jansen, K.U.; Knirsch, C.; Anderson, A.S. The role of vaccines in preventing bacterial antimicrobial resistance. Nat. Med. 2018, 24, 10. [Google Scholar] [CrossRef]
- Henig, O.; Katz, D.E.; Marchaim, D. Multidrug-Resistant Gram-Negative Bacilli: Infection Prevention Considerations. In Infection Prevention; Springer: Cham, Switzerland, 2018; pp. 127–143. [Google Scholar]
- Arnett, H.; Scordo, K. Surveillance Screening to Reduce Carbapenem Resistance. J. Nurse Pract. 2019, 15, 434–437. [Google Scholar] [CrossRef]
- ElMahallawy, H.; Zafer, M.M.; Al-Agamy, M.; Amin, M.A.; Mersal, M.M.; Booq, R.Y.; Alyamani, E.; Radwan, S. Dissemination of ST101 blaOXA-48 producing Klebsiella pneumoniae at tertiary care setting. J. Infect. Dev. Ctries 2018, 12, 422–428. [Google Scholar] [CrossRef]
- Bell, J.M.; Turnidge, J.D.; Gales, A.C.; Pfaller, M.A.; Jones, R.N.; Sentry APAC Study Group. Prevalence of extended spectrum β-lactamase (ESBL)-producing clinical isolates in the Asia-Pacific region and South Africa: Regional results from SENTRY Antimicrobial Surveillance Program (1998–99). Diagn. Microbiol. Infect. Dis. 2002, 42, 193–198. [Google Scholar] [CrossRef]
- Usha, G.; Chunderika, M.; Prashini, M.; Willem, S.A.; Yusuf, E.S. Characterization of extended-spectrum β-lactamases in Salmonella spp. at a tertiary hospital in Durban, South Africa. Diagn. Microbiol. Infect. Dis. 2008, 62, 86–91. [Google Scholar] [CrossRef]
- Brink, A.; Coetzee, J.; Clay, C.; Corcoran, C.; Van Greune, J.; Deetlefs, J.D.; Nutt, L.; Feldman, C.; Richards, G.; Nordmann, P.; et al. The spread of carbapenem-resistant Enterobacteriaceae in South Africa: Risk factors for acquisition and prevention. S. Afr. Med. J. 2012, 102, 599–601. [Google Scholar] [CrossRef] [Green Version]
- Lamba, M.; Ahammad, S.Z. Sewage treatment effluents in Delhi: A key contributor of β-lactam resistant bacteria and genes to the environment. Chemosphere 2017, 188, 249–256. [Google Scholar] [CrossRef]
- Ng, C.; Tay, M.; Tan, B.; Le, T.H.; Haller, L.; Chen, H.; Koh, T.H.; Barkham, T.; Thompson, J.R.; Gin, K.Y.H. Characterization of metagenomes in urban aquatic compartments reveals high prevalence of clinically relevant antibiotic resistance genes in wastewaters. Front. Microbiol. 2017, 8, 2200. [Google Scholar] [CrossRef] [Green Version]
- Said, L.B.; Jouini, A.; Klibi, N.; Dziri, R.; Alonso, C.A.; Boudabous, A.; Slama, K.B.; Torres, C. Detection of extended-spectrum beta-lactamase (ESBL)-producing Enterobacteriaceae in vegetables, soil and water of the farm environment in Tunisia. Int. J. Food Microbiol. 2015, 203, 86–92. [Google Scholar] [CrossRef]
- Naidoo, S.; Olaniran, A. Treated wastewater effluent as a source of microbial pollution of surface water resources. Int. J. Environ. Res. Public Health 2014, 11, 249–270. [Google Scholar] [CrossRef] [Green Version]
- Mairi, A.; Pantel, A.; Sotto, A.; Lavigne, J.P.; Touati, A. OXA-48-like carbapenemases producing Enterobacteriaceae in different niches. Eur. J. Clin. Microbiol. Infect. Dis. 2018, 37, 587–604. [Google Scholar] [CrossRef]
- Hoang, C.Q.; Nguyen, H.D.; Vu, H.Q.; Nguyen, A.T.; Pham, B.T.; Tran, T.L.; Nguyen, H.T.; Dao, Y.M.; Nguyen, T.S.; Nguyen, D.A.; et al. Emergence of New Delhi Metallo-Beta-Lactamase (NDM) and Klebsiella pneumoniae Carbapenemase (KPC) Production by Escherichia coli and Klebsiella pneumoniae in Southern Vietnam and Appropriate Methods of Detection: A Cross-Sectional Study. Biomed. Res. Int. 2019. [Google Scholar] [CrossRef] [Green Version]






| Target Strain | Target Gene | Primer Sequence (5′→3′) | Amplicon Size (bp) | PCR Cycling Condition | References |
|---|---|---|---|---|---|
| Klebsiella genus | gyrA | F: CGC GTA CTA TAC GCC ATG AAC GTA R: ACC GTT GAT CAC TTC GGT CAGG | 441 | Initial denaturation of 5 min at 94 °C followed by 35 cycles, denaturation at 94 °C for 30 s, annealing at 55 °C for 45 s, extension at 72 °C for 45 s and final extension at 72 °C for 10 min. | Salloum et al. [24] |
| Klebsiella pneumonia | magA | F: ATT TGA AGA GGT TGC AAA CGAT R: TTC ACT CTG AAG TTT TCT TGT GTTC | 130 | Initial denaturation of 5 min at 94 °C followed by 35 cycles, denaturation at 94 °C for 30 s, annealing at 55 °C for 30 s, extension at 72 °C for 40 s and final extension at 72 °C for 10 min. | Salloum et al. [24] |
| Klebsiella oxytoca | pehX | F: GAT ACG GAG TAT GCC TTT ACG GTG R: TAG CCT TTA TCA AGC GGA TAC TGG | 343 | Initial denaturation of 5 min at 94 °C followed by 35 cycles, denaturation at 94 °C for 30 s, annealing at 55 °C for 30 s, extension at 72 °C for 40 s and final extension at 72 °C for 10 min. | Salloum et al. [24] |
| Target Gene | Primer Sequence (5′→3′) | Amplicon Size (bp) | PCR Cycling Condition |
|---|---|---|---|
| blaNDM-1 | F: AAA ACG GCA AGA AAA AGC AG R: AAA ACG GCA AGA AAA AGC AG | 439 | Initial denaturation of 5 min at 95 °C followed by 35 cycles, denaturation at 95 °C for 1 min, annealing at 52 °C for 1 min, extension at 72 °C for 1 min and final extension at 72 °C for 5 min. |
| blaKPC | F: AAA ACG GCA AGA AAA AGC AG R: AAA ACG GCA AGA AAA AGC AG | 340 | Initial denaturation of 5 min at 95 °C followed by 35 cycles, denaturation at 95 °C for 1 min, annealing at 56 °C for 1 min, extension at 72 °C for 1 min and final extension at 72 °C for 5 min. |
| blaOXA-48-like | F: TTGG TGGC ATCG ATTA TCGG R: GAGC ACTT CTTT TGTG ATGG C | 597 | Initial denaturation of 5 min at 95 °C followed by 35 cycles, denaturation at 95 °C for 1 min, annealing at 56 °C for 1 min, extension at 72 °C for 1 min and final extension at 72 °C for 5 min. |
| Sample Types | Number of Confirmed K. pneumonia (cfu/mL) | Number of Confirmed K. oxytoca (cfu/mL) | ||||
|---|---|---|---|---|---|---|
| Amathole DM | Chris Hani DM | Sarah Baartman DM | Amathole DM | Chris Hani DM | Sarah Baartman DM | |
| Hospital effluent | 21 | 22 | - | 11 | 3 | - |
| WWTP final effluent | 10 | 25 | - | 2 | 2 | - |
| Surface water | 6 | 26 | - | 19 | 8 | 1 |
| Irrigation water | 3 | 5 | 14 | 9 | 1 | 7 |
| Farm soil | 9 | 19 | 10 | 8 | 11 | 7 |
| Vegetables | 3 | 8 | 1 | 9 | 10 | 7 |
| Carbapenem-Resistance Genes | Number of Isolates | Percentage Occurrence (%) |
|---|---|---|
| blaNDM-1 | 73/173 | 42 |
| blaKPC | 17/173 | 10 |
| blaOXA-48-like | 10/173 | 6 |
| Total | 100 | 58% |
| Patterns of Multiple Resistance Genes | Number of Isolates | Total | |||
|---|---|---|---|---|---|
| K. pneumoniae (%) | K. oxytoca (%) | Unidentified Species (%) | Sample Types | ||
| blaNDM-1, blaKPC, blaOXA-48-like | 0 | 0 | 1 | River water | 1 |
| blaNDM-1, blaKPC | 2 | 2 | 8 | Hospital effluent, farm soil, WWTP, river water | 12 |
| blaKPC, blaOXA-48-like | 1 | 1 | 4 | Farm soil | 6 |
| blaNDM-1, blaOXA-48-like | 0 | 1 | 0 | Vegetable | 1 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ebomah, K.E.; Okoh, A.I. Detection of Carbapenem-Resistance Genes in Klebsiella Species Recovered from Selected Environmental Niches in the Eastern Cape Province, South Africa. Antibiotics 2020, 9, 425. https://doi.org/10.3390/antibiotics9070425
Ebomah KE, Okoh AI. Detection of Carbapenem-Resistance Genes in Klebsiella Species Recovered from Selected Environmental Niches in the Eastern Cape Province, South Africa. Antibiotics. 2020; 9(7):425. https://doi.org/10.3390/antibiotics9070425
Chicago/Turabian StyleEbomah, Kingsley Ehi, and Anthony Ifeanyi Okoh. 2020. "Detection of Carbapenem-Resistance Genes in Klebsiella Species Recovered from Selected Environmental Niches in the Eastern Cape Province, South Africa" Antibiotics 9, no. 7: 425. https://doi.org/10.3390/antibiotics9070425
APA StyleEbomah, K. E., & Okoh, A. I. (2020). Detection of Carbapenem-Resistance Genes in Klebsiella Species Recovered from Selected Environmental Niches in the Eastern Cape Province, South Africa. Antibiotics, 9(7), 425. https://doi.org/10.3390/antibiotics9070425





