Monitoring of Hydrogen Emission from Bacteria in Food, Animals and in the Blood of Humans Suffering from Lyme Disease by A Specific Hydrogen Sensor
Abstract
1. Introduction
2. Results
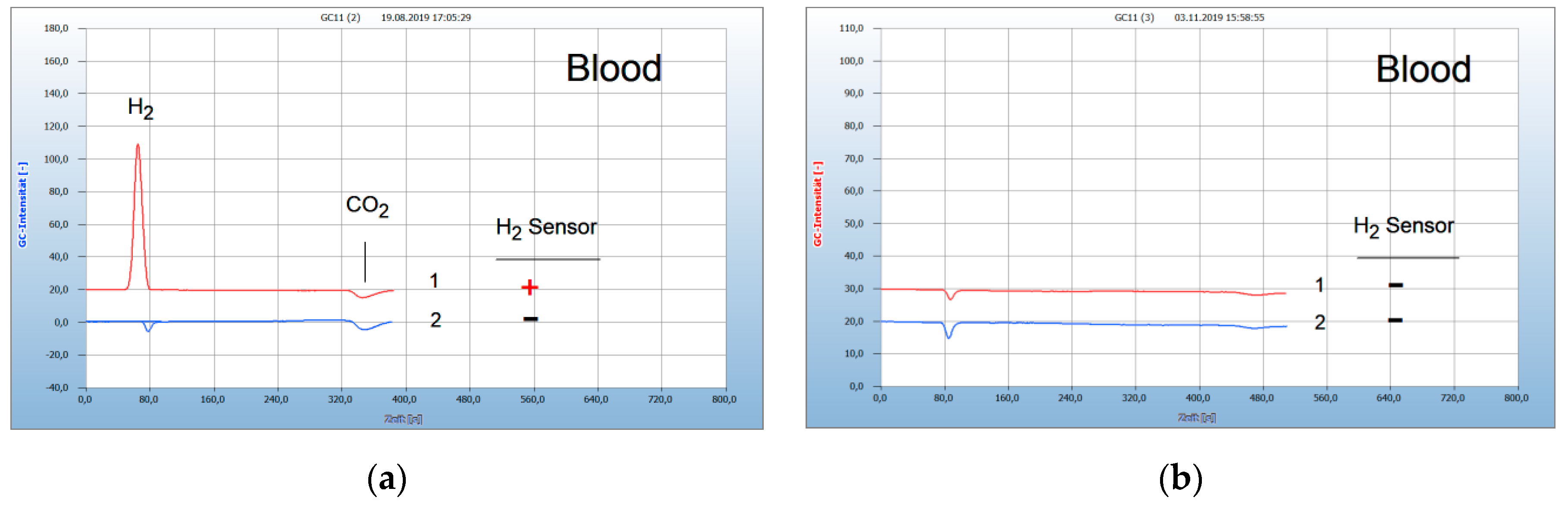
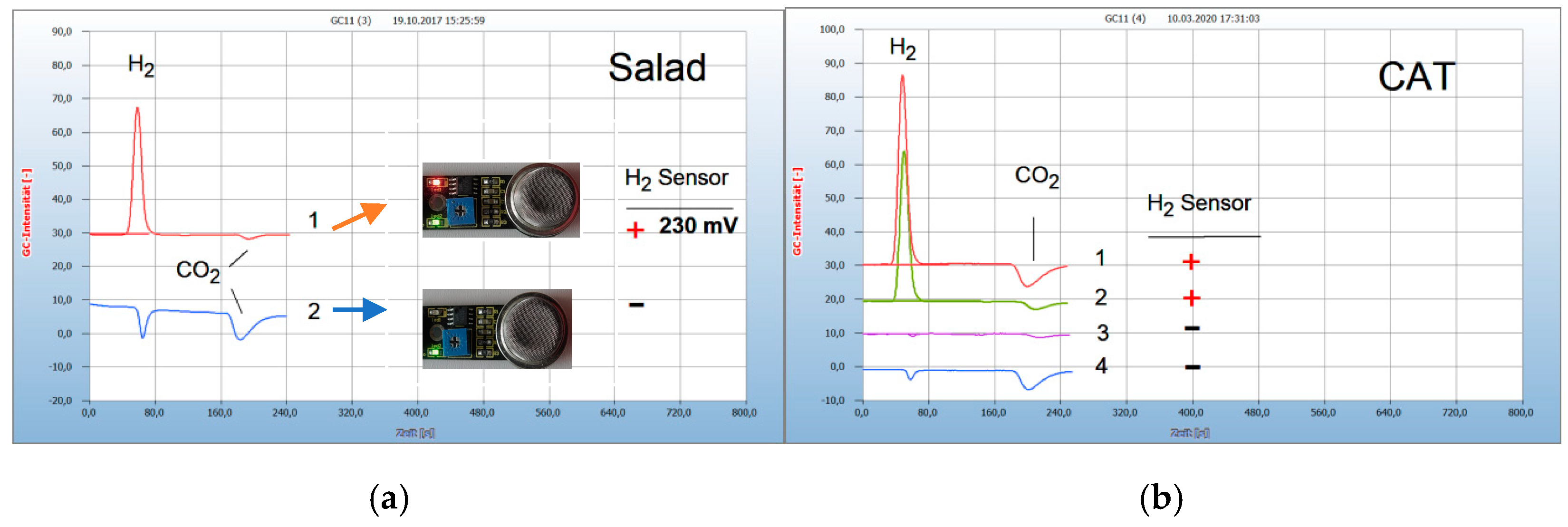
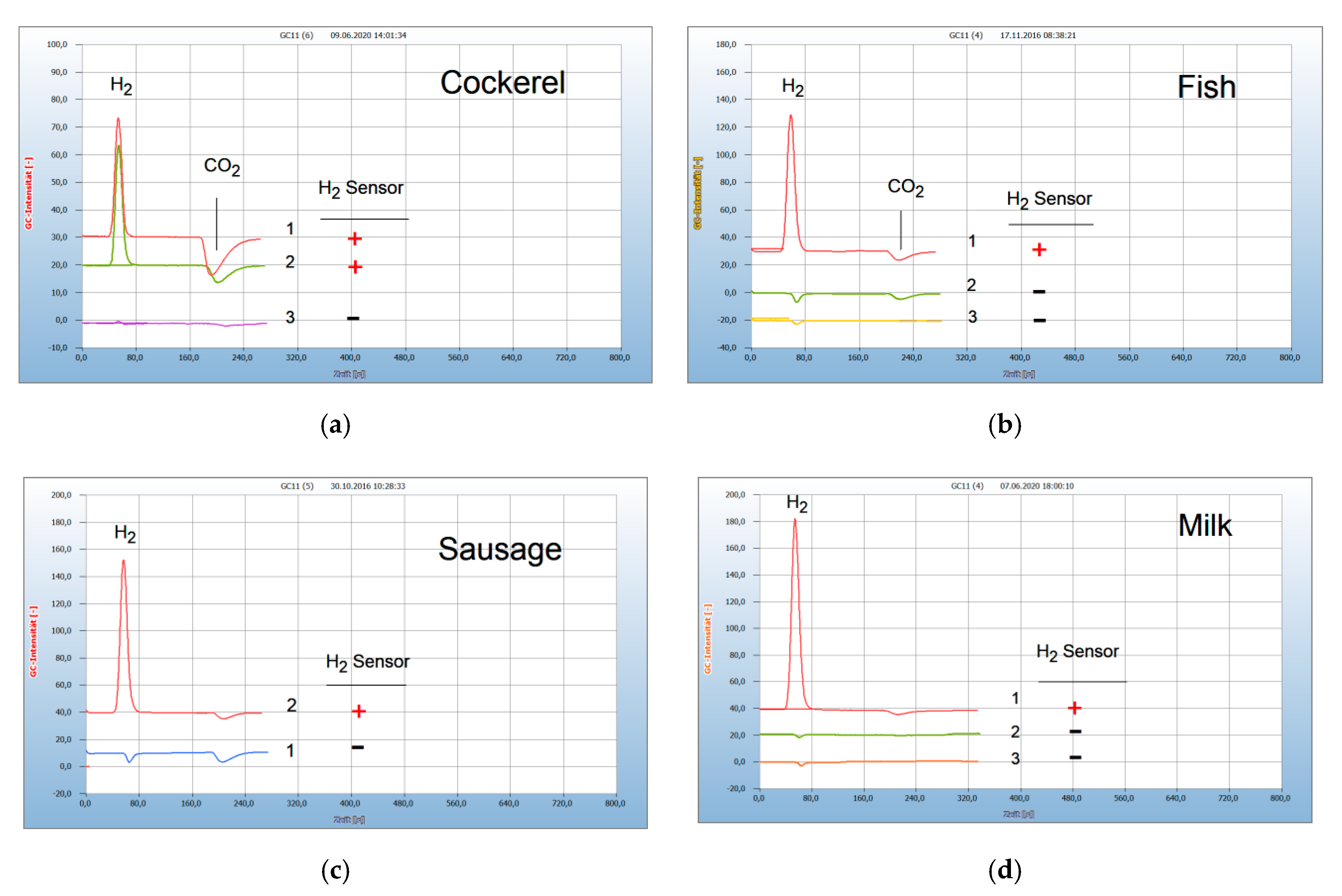
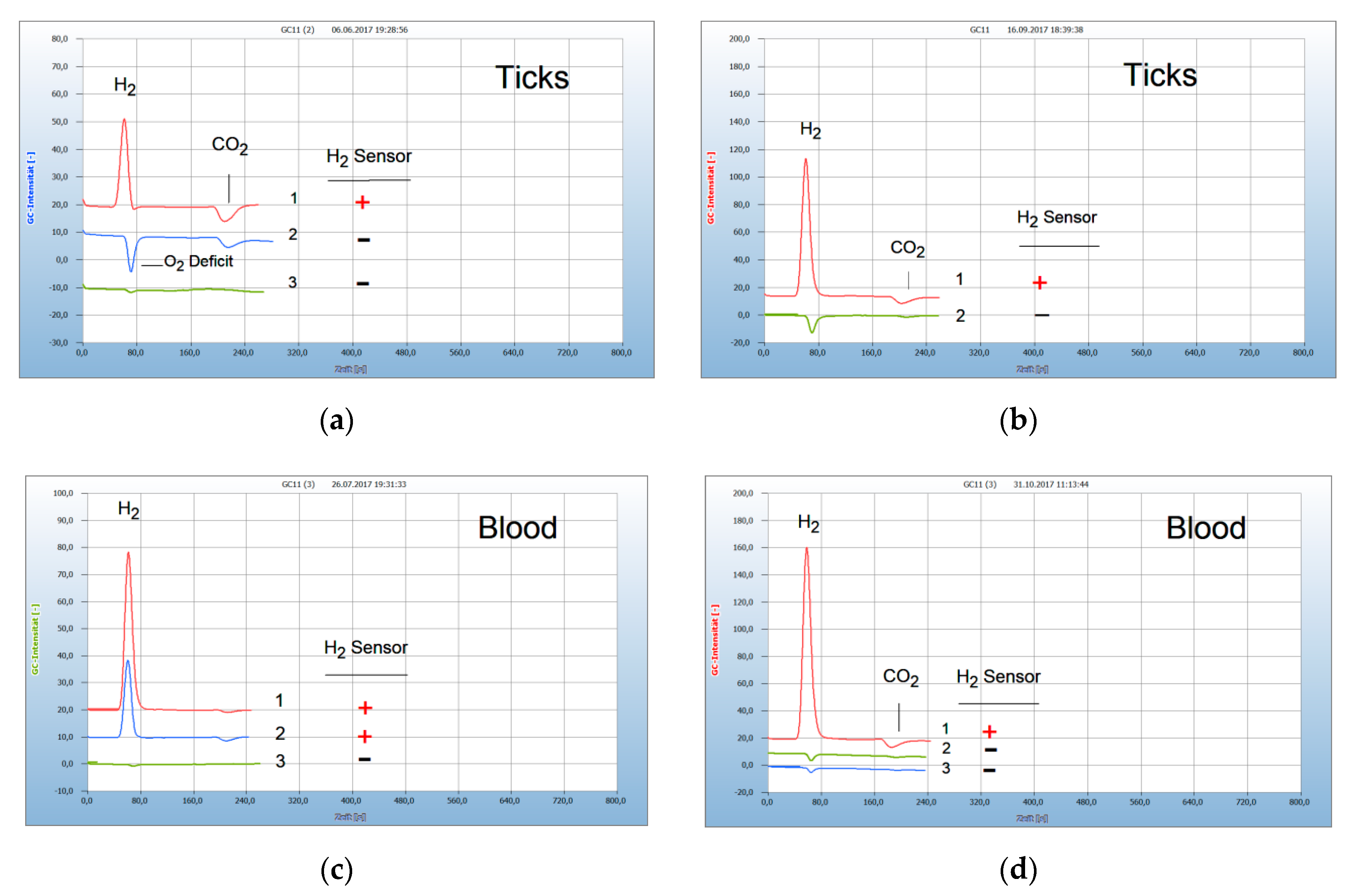
2.1. Interpretation of the Chromatograms
2.2. Method
2.3. Proof of Aerobes and Anaerobes by HS-GC and by the H2 Sensor Approach
2.4. Analysis of Food
2.5. Application for Lyme Disease
3. Discussion
4. Conclusions
5. Materials and Instrumentation
Author Contributions
Funding
Conflicts of Interest
References
- Bassette, R.; Claydon, T.J. Characterization of some bacteria by gas chromatographic analysis of head space vapors from milk cultures. J. Dairy Sci. 1965, 48, 775–779. [Google Scholar]
- Kolb, B.; Ettre, L.S. Static Headspace-Gas Chromatography: Theory and Practice, 2nd ed.; Wiley-Interscience: Hoboken, NJ, USA, 2006; pp. 286–291. [Google Scholar]
- Seifert, S.; Böhnel, H.; Gierke, S.; Heine, A.; Hoffman, D.; Sukop, U.; Boege, D. Identification of anaerobic bacteria by fatty acid pattern recognition. Int. Lab. 1986, 46–56. [Google Scholar]
- Heiterfuß, S.; Heine, A.; Seifert, S. Identification of anaerobic bacteria by determination of non-volatile dicaboxylic acids after derivatization. J. Chromatog. Biomed. Appl. 1990, 532, 374–379. [Google Scholar]
- Tait, E.; Perry, J.D.; Stanforth, S.P.; Dean, J.R. Identification of volatile organic compounds produced by bacteria using HS-SPME-GC-MS. J. Chromatogr. Sci. 2014, 52, 363–373. [Google Scholar] [CrossRef] [PubMed]
- Kolb, B.; Riestere, L.; Bier, L.; Widenhorn, A.-M. Proof of bacteria and the activity of chemical and natural antibiotics by headspace gas chromatography. J. Anal. Sci. Technol. 2019, 10, 9. [Google Scholar] [CrossRef]
- Kolb, B.; Schneider, E.M.; Riesterer, L.; Bier, L.; Hein, T. Metabolic Profiling of Borrelia in Ticks and in Whole Blood Samples by Headspace Gas Chromatography—A Case Report. Am. J. Biomed. Sci. Res. 2019, 6, 330–347. [Google Scholar] [CrossRef]
- Alahi, M.E.E.; Mukhopadhyay, S.C. Detection Methodologies for Pathogen and Toxins: A Review. Sensors 2017, 17, 1885. [Google Scholar] [CrossRef] [PubMed]
- Lantos, P.M.; Wormser, G.P. Chronic coinfections in patients diagnosed with chronic Lyme disease: A systematic review. Am. J. Med. 2014, 127, 1105–1110. [Google Scholar] [CrossRef] [PubMed]
- Adeolu, M.; Gupta, R.S. A phylogenomic and molecular marker based proposal for the division of the genus Borrelia into two genera: The emended genus Borrelia containing only the members of the relapsing fever Borrelia, and the genus Borreliella gen. nov. containing the members of the Lyme disease Borrelia (Borrelia burgdorferi sensu lato complex). Antonie Van Leeuwenhoek 2014, 105, 1049–1072. [Google Scholar] [CrossRef] [PubMed]
- Winslow, C.; Coburn, J. Recent discoveries and advancements in research on the Lyme disease spirochete Borrelia burgdorferi. F1000Research 2019, 8. [Google Scholar] [CrossRef] [PubMed]
- Patini, R.; Mangino, G.; Martellacci, L.; Quaranta, G.; Masucci, L.; Gallenzi, P. The Effect of Different Antibiotic Regimens on Bacterial Resistance: A Systematic Review. Antibiotics 2020, 9, 22. [Google Scholar] [CrossRef] [PubMed]
- Harris, J.C.; Cottrell, S.L.; Plummer, D.; Lloyd, D. Antimicrobial properties of Allium sativum (garlic). Appl. Microbiol. Biotechnol. 2001, 57, 282–286. [Google Scholar] [CrossRef] [PubMed]
- Stjernberg, L.; Berglund, J. Garlic as an insect repellent. JAMA 2000, 284, 829–831. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Zhang, S.; Shi, W.; Zubcevik, N.; Miklossy, J.; Zhang, Y. Selective Essential Oils from Spice or Culinary Herbs Have High Activity against Stationary Phase and Biofilm Borrelia burgdorferi. Front. Med. 2017, 4, 169. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Shi, W.; Miklossy, J.; Tauxe, G.M.; McMeniman, C.J.; Zhang, Y. Identification of Essential Oils with Strong Activity against Stationary Phase Borrelia burgdorferi. Antibiotics 2018, 7, 89. [Google Scholar] [CrossRef] [PubMed]
- Diagnostik und Therapie der Lyme-Borreliose, Leitlinien. Deutsche Borreliose-Gesellschaft.e.V.Jena. December 2010, Germany. Available online: www.borreliose-gesellschaft.de/Texte/Leitlinien.pdf (accessed on 1 December 2010).
- Marques, A.R. Laboratory diagnosis of Lyme disease: Advances and challenges. Infect. Dis. Clin. 2015, 29, 295–307. [Google Scholar] [CrossRef] [PubMed]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kolb, B.; Riesterer, L.; Widenhorn, A.-M.; Bier, L. Monitoring of Hydrogen Emission from Bacteria in Food, Animals and in the Blood of Humans Suffering from Lyme Disease by A Specific Hydrogen Sensor. Antibiotics 2020, 9, 427. https://doi.org/10.3390/antibiotics9070427
Kolb B, Riesterer L, Widenhorn A-M, Bier L. Monitoring of Hydrogen Emission from Bacteria in Food, Animals and in the Blood of Humans Suffering from Lyme Disease by A Specific Hydrogen Sensor. Antibiotics. 2020; 9(7):427. https://doi.org/10.3390/antibiotics9070427
Chicago/Turabian StyleKolb, Bruno, Lorina Riesterer, Anna-Maria Widenhorn, and Leona Bier. 2020. "Monitoring of Hydrogen Emission from Bacteria in Food, Animals and in the Blood of Humans Suffering from Lyme Disease by A Specific Hydrogen Sensor" Antibiotics 9, no. 7: 427. https://doi.org/10.3390/antibiotics9070427
APA StyleKolb, B., Riesterer, L., Widenhorn, A.-M., & Bier, L. (2020). Monitoring of Hydrogen Emission from Bacteria in Food, Animals and in the Blood of Humans Suffering from Lyme Disease by A Specific Hydrogen Sensor. Antibiotics, 9(7), 427. https://doi.org/10.3390/antibiotics9070427






