Antiviral Action of Native and Methylated Lactoferrin and β-Lactoglobulin against Potato Virus Y (PVY) Infected into Potato Plants Grown in an Open Field
Abstract
:1. Introduction
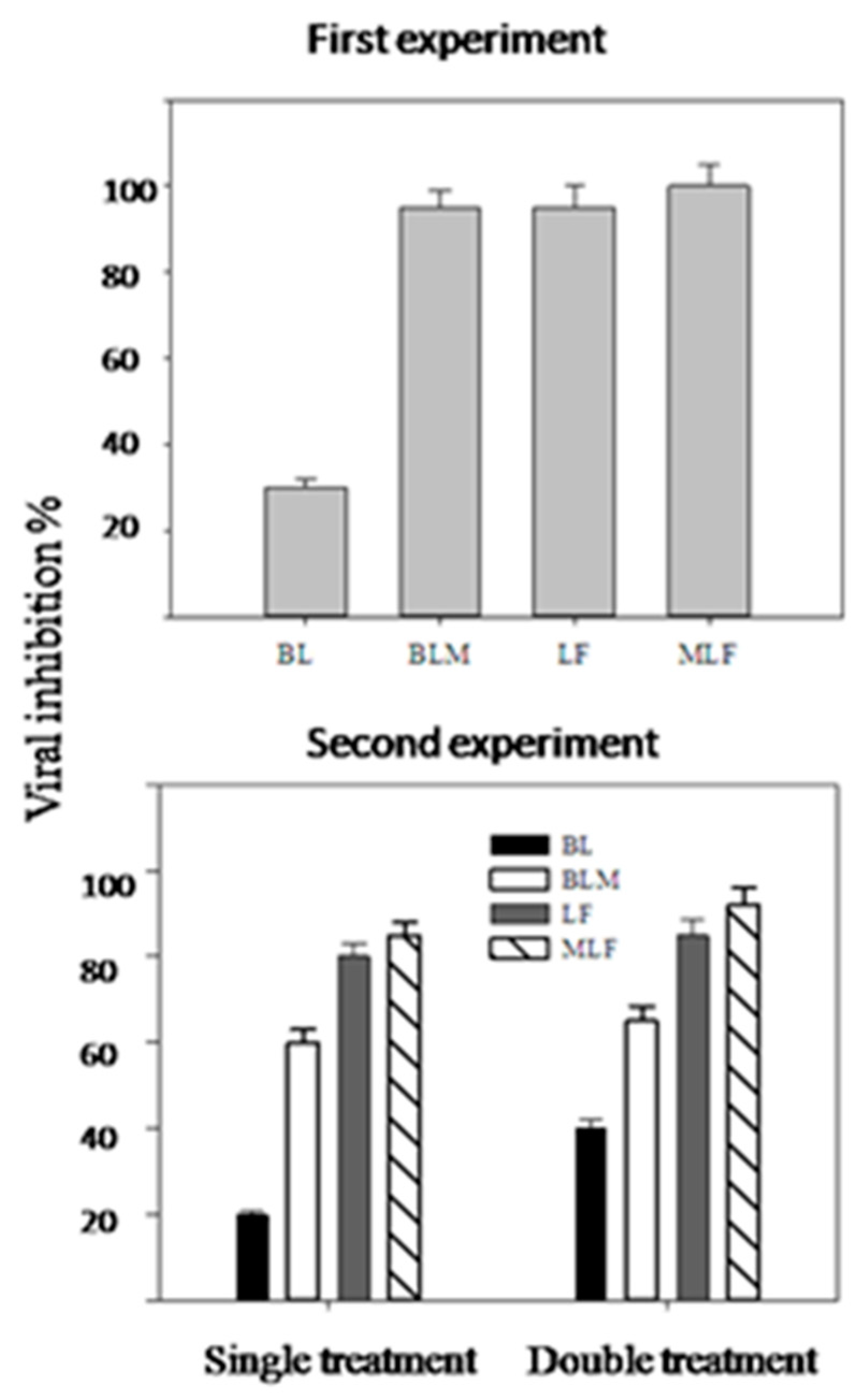
2. Results
3. Discussion
4. Materials and Methods
4.1. Tested Proteins
4.2. Virus and Plants
4.3. Preliminary Greenhouse Experiment
4.4. First Field Experiment
4.5. Second Field Experiment
4.6. Final Field Experiment
4.7. PVY Detection
4.7.1. Double Antibody Sandwich Enzyme Linked Immunosorbent Assay (DAS-ELISA) Assay
4.7.2. RT-PCR Detection of PVY
- PVYCPvBamH1: TCAAGGATCCGCAAATGACACAATTGATGCAGG
- PVYCPcEcoR1: AGAGAGAATTCATCACATGTTCTTGACTCC
4.7.3. Dot Blot Hybridization Test
4.7.4. Scanning Electron Microscopy (SEM)
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kerlan, C.; Moury, B. Potato Virus Y. In Encyclopedia of Virology; Mahy, B.W.J., van Regenmortel, M.H.V., Eds.; Academic Press: San Diego, CA, USA, 2008; pp. 287–296. [Google Scholar]
- Chikh-Ali, M.; Naidu, R.A.; Karasev, A.V. First report of Potato virus Y (PVY) strain PVYC associated with a tomato disease in Kenya. Plant Dis. 2016, 100, 864. [Google Scholar] [CrossRef]
- Munoz-Baena, L.; Gutierrez-Sanchez, P.A.; Marin-Montoya, M. Detection and genome sequencing of Potato virus y (PVY) infecting tomato in Antioquia, Colombia. Bioagro 2016, 28, 69–80. [Google Scholar]
- Parrado, J.; Bautista, J.; Romero, E.; Martínez, A.M.G.; Friaza, V.; Tejada, M. Production of a carob enzymatic extract: Potential use as a biofertilizer. Bioresour. Technol. 2008, 99, 2312–2318. [Google Scholar] [CrossRef]
- Gray, S.M.; Power, A.G. Anthropogenic influences on emergence of vector-borne plant viruses: The persistent problem of Potato virus Y. Curr. Opin. Virol. 2018, 33, 177–183. [Google Scholar] [CrossRef] [PubMed]
- Franc, G.D. Seed certification as a virus management tool. In Virus and Virus-Like Diseases of Potatoes and Production of Seed-Potatoes; Springer: Berlin/Heidelberg, Germany, 2001; pp. 407–420. [Google Scholar]
- Davidson, R.D.; Houser, A.J.; Sather, K.; Haslar, R. Controlling PVY in Seed: What Works and What Does Not. Am. J. Potato Res. 2013, 90, 28–32. [Google Scholar] [CrossRef]
- Moodley, V.; Naidoo, R.; Gubba, A.; Mafongoya, P. Development of Potato virus Y (PVY) resistant pepper (Capsicum annuum L.) lines using marker-assisted selection (MAS). Physiol. Mol. Plant Pathol. 2019, 105, 96–101. [Google Scholar] [CrossRef]
- Wang, J.; Zhu, Y.-K.; Wang, H.-Y.; Zhang, H.; Wang, K.-Y. Inhibitory effects of esterified whey protein fractions by inducing chemical defense against tobacco mosaic virus (TMV) in tobacco seedlings. Ind. Crop. Prod. 2012, 37, 207–212. [Google Scholar] [CrossRef]
- Wang, J.; Wang, H.-Y.; Xia, X.-M.; Li, P.-P.; Wang, K.-Y. Inhibitory effect of esterified lactoferin and lactoferin against tobacco mosaic virus (TMV) in tobacco seedlings. Pestic. Biochem. Physiol. 2013, 105, 62–68. [Google Scholar] [CrossRef]
- Osman, A.; Abbas, E.; Mahgoub, S.A.; Sitohy, M.Z. Inhibition of Penicillium digitatum in vitro and in postharvest orange fruit by a soy protein fraction containing mainly β-conglycinin. J. Gen. Plant Pathol. 2016, 82, 293–301. [Google Scholar] [CrossRef]
- Osman, A.; El-Araby, G.M.; Taha, H. Potential use as a bio-preservative from lupin protein hydrolysate generated by alcalase in food system. J. Appl. Biol. Biotechnol. 2016, 4, 76–81. [Google Scholar] [CrossRef]
- Sitohy, M.; Osman, A. Bioactive compounds in soybean proteins and its applications in food systems. In Sustainability of Agricultural Environment in Egypt: Part I; Springer: Cham, Switzerland, 2018; pp. 147–160. [Google Scholar]
- Osman, A.; Goda, H.A.; Abdel-Hamid, M.; Badran, S.M.; Otte, J. Antibacterial peptides generated by Alcalase hydrolysis of goat whey. LWT 2016, 65, 480–486. [Google Scholar] [CrossRef]
- Osman, A.; Goda, H.A.; Sitohy, M.Z. Storage stability of minced beef supplemented with chickpea legumin at 4 °C as a potential substitute for nisin. LWT 2018, 93, 434–441. [Google Scholar] [CrossRef]
- Osman, A.; Mahgoub, S.A.; El-Masry, R.; Al-Gaby, A.; Sitohy, M.Z. Extending the Technological Validity of Raw Buffalo Milk at Room Temperature by Esterified Legume Proteins. J. Food Process. Preserv. 2012, 38, 223–231. [Google Scholar] [CrossRef]
- Osman, A.O.; Mahgoub, S.A.; Sitohy, M.Z. Hindering milk quality storage deterioration by mild thermization combined with methylated chickpea protein. Int. Food Res. J. 2014, 21, 693–701. [Google Scholar]
- Sitohy, M.Z.; Mahgoub, S.A.; Osman, A. Controlling psychrotrophic bacteria in raw buffalo milk preserved at 4 °C with esterified legume proteins. LWT 2011, 44, 1697–1702. [Google Scholar] [CrossRef]
- Sitohy, M.Z.; Mahgoub, S.A.; Osman, A.; El-Masry, R.; Al-Gaby, A. Extent and Mode of Action of Cationic Legume Proteins against Listeria monocytogenes and Salmonella enteritidis. Probiot. Antimicrob. Proteins 2013, 5, 195–205. [Google Scholar] [CrossRef]
- Sitohy, M.Z.; Osman, A. Enhancing Milk Preservation with Esterified Legume Proteins. Probiot. Antimicrob. Proteins 2011, 3, 48–56. [Google Scholar] [CrossRef]
- Sitohy, M.Z.; Osman, A.O.; Mahgoub, S.A. Bioactive proteins against pathogenic and spoilage bacteria. Funct. Foods Health Dis. 2014, 4, 451–462. [Google Scholar] [CrossRef]
- Abdel-Shafi, S.; Osman, A.; Enan, G.; El-Nemer, M.; Sitohy, M.Z. Antibacterial activity of methylated egg white proteins against pathogenic G(+) and G(-) bacteria matching antibiotics. SpringerPlus 2016, 5, 983. [Google Scholar] [CrossRef] [Green Version]
- Mahgoub, S.A.; Osman, A.; Sitohy, M.Z. Inhibition of Growth of Pathogenic Bacteria in Raw Milk by Legume Protein Esters. J. Food Prot. 2011, 74, 1475–1481. [Google Scholar] [CrossRef] [PubMed]
- Sitohy, M.Z.; Chobert, J.-M.; Gaudin, J.-C.; Haertlé, T. Esterified milk proteins inhibit DNA replication in vitro. Int. J. Biol. Macromol. 2001, 29, 259–266. [Google Scholar] [CrossRef]
- Sitohy, M.Z.; Chobert, J.M.; Haertlé, T. Study of the formation of complexes between DNA and esterified dairy proteins. Int. Dairy J. 2001, 11, 873–883. [Google Scholar] [CrossRef]
- Sitohy, M.Z.; Chobert, J.-M.; Gaudin, J.-C.; Renac, T.; Haertlé, T. When positively charged milk proteins can bind to dna. J. Food Biochem. 2002, 26, 511–532. [Google Scholar] [CrossRef]
- Sitohy, M.; Chobert, J.-M.; Schmidt, M.; Gozdzicka-Jozefiak, A.; Haertlé, T. Interactions between Esterified Whey Proteins (α-Lactalbumin and β-Lactoglobulin) and DNA Studied by Differential Spectroscopy. Protein J. 2001, 20, 633–640. [Google Scholar] [CrossRef]
- Sitohy, M.; Chobert, J.-M.; Haertlé, T. Esterified Whey Proteins Can ProtectLactococcus lactisagainst Bacteriophage Infection. Comparison with the Effect of Native Basic Proteins andl-Polylysines. J. Agric. Food Chem. 2005, 53, 3727–3734. [Google Scholar] [CrossRef]
- Sitohy, M.; Chobert, J.-M.; Karwowska, U.; Goździcka-Józefiak, A.; Haertlé, T. Inhibition of Bacteriophage M13 Replication with Esterified Milk Proteins. J. Agric. Food Chem. 2006, 54, 3800–3806. [Google Scholar] [CrossRef]
- Sitohy, M.Z.; Dalgalarrondo, M.; Nowoczin, M.; Besse, B.; Billaudel, S.; Haertlé, T.; Chobert, J.M. The effect of bovine whey proteins on the ability of poliovirus and Coxsackie virus to infect Vero cell cultures. Int. Dairy J. 2008, 18, 658–668. [Google Scholar] [CrossRef]
- Abdelbacki, A.M.; Taha, S.; Sitohy, M.Z.; Dawood, A.I.A.; Abdel-Hamid, M.; Rezk, A.A. Inhibition of Tomato Yellow Leaf Curl Virus (TYLCV) using whey proteins. Virol. J. 2010, 7, 26. [Google Scholar] [CrossRef] [Green Version]
- Chobert, J.-M.; Sitohy, M.; Billaudel, S.; Dalgalarrondo, M.; Haertlé, T. Anticytomegaloviral Activity of Esterified Milk Proteins and L-Polylysines. J. Mol. Microbiol. Biotechnol. 2007, 13, 255–258. [Google Scholar] [CrossRef]
- Sitohy, M.Z.; Besse, B.; Billaudel, S.; Haertlé, T.; Chobert, J.-M. Antiviral Action of Methylated β-Lactoglobulin on the Human Influenza Virus A Subtype H3N2. Probiot. Antimicrob. Proteins 2010, 2, 104–111. [Google Scholar] [CrossRef]
- Taha, S.; Mehrez, M.A.; Sitohy, M.Z.; Dawood, A.G.I.A.; Abdel-Hamid, M.; Kilany, W.H. Effectiveness of esterified whey proteins fractions against Egyptian Lethal Avian Influenza A (H5N1). Virol. J. 2010, 7, 330. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sitohy, M.; Scanu, M.; Besse, B.; Mollat, C.; Billaudel, S.; Haertlé, T.; Chobert, J.M. Influenza virus A subtype H1N1 is inhibited by methylated β-lactoglobulin. J. Dairy Res. 2010, 77, 411–418. [Google Scholar] [CrossRef] [PubMed]
- Sitohy, M. Antiviral activity of esterified α-lactalbumin and β-lactoglobulin against herpes simplex virus type 1. Comparison with the effect of acyclovir and L-polylysines. J. Agric. Food Chem. 2007, 55, 10214–10220. [Google Scholar] [CrossRef] [PubMed]
- Sojar, H.T.; Hamada, N.; Genco, R.J. Structures involved in the interaction of Porphyromonas gingivalisfimbriae and human lactoferrin. Febs Lett. 1998, 422, 205–208. [Google Scholar] [CrossRef] [Green Version]
- Osman, A.; Daidamony, G.; Sitohy, M.; Khalifa, M.; Enan, G. Soybean glycinin basic subunit inhibits methicillin resistant-vancomycin intermediate Staphylococcus aureus (MRSA-VISA) in vitro. Int. J. Appl. Res. Nat. Prod. 2016, 9, 17–26. [Google Scholar]
- Jenssen, H. Anti herpes simplex virus activity of lactoferrin/lactoferricin—An example of antiviral activity of antimicrobial protein/peptide. Cell. Mol. Life Sci. 2005, 62, 3002–3013. [Google Scholar] [CrossRef]
- Kanyshkova, T.G.; Buneva, V.; Nevinsky, G.A. Lactoferrin and Its biological functions. Biochemistry 2001, 66, 1–7. [Google Scholar] [CrossRef]
- Sohrabi, S.M.; Niazi, A.; Chahardoli, M.; Hortamani, A.; Setoodeh, P. In silico investigation of lactoferrin protein characterizations for the prediction of anti-microbial properties. Mol. Biol. Res. Commun. 2014, 3, 85–100. [Google Scholar]
- Van Der Strate, B.W.; Beljaars, L.; Molema, G.; Harmsen, M.C.; Meijer, D. Antiviral activities of lactoferrin. Antivir. Res. 2001, 52, 225–239. [Google Scholar] [CrossRef]
- Adlerova, L.; Bartoskova, A.; Faldyna, M. Lactoferrin: A review. Vet. Med. 2008, 53, 457–468. [Google Scholar] [CrossRef] [Green Version]
- Redwan, E.M.; Uversky, V.N.; El-Fakharany, E.M.; Al-Mehdar, H. Potential lactoferrin activity against pathogenic viruses. Comptes Rendus Biol. 2014, 337, 581–595. [Google Scholar] [CrossRef] [PubMed]
- Sitohy, M.; Chobert, J.; Haertlé, T. Simplified short-time method for the esterification of milk proteins. Milchwissenschaft 2001, 56, 127–131. [Google Scholar]
- Wakabayashi, H.; Oda, H.; Yamauchi, K.; Abe, F. Lactoferrin for prevention of common viral infections. J. Infect. Chemother. 2014, 20, 666–671. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moreno-Expósito, L.; Illescas-Montes, R.; Melguizo-Rodríguez, L.; Ruiz, C.; Ramos-Torrecillas, J.; De Luna-Bertos, E. Multifunctional capacity and therapeutic potential of lactoferrin. Life Sci. 2018, 195, 61–64. [Google Scholar] [CrossRef]
- Sitohy, M.Z.; Desoky, E.-S.M.; Osman, A.; Rady, M.M. Pumpkin seed protein hydrolysate treatment alleviates salt stress effects on Phaseolus vulgaris by elevating antioxidant capacity and recovering ion homeostasis. Sci. Hortic. 2020, 271, 109495. [Google Scholar] [CrossRef]
- Abbas, E.; Osman, A.; Sitohy, M.Z. Biochemical control of Alternaria tenuissima infecting post-harvest fig fruit by chickpea vicilin. J. Sci. Food Agric. 2020, 100, 2889–2897. [Google Scholar] [CrossRef]
- Sitohy, M.; Chobert, J.-M.; Haertlé, T. Study of factors influencing protein esterification using?-lactoglobulin as a model. J. Food Biochem. 2000, 24, 381–398. [Google Scholar] [CrossRef]
- Bertrand-Harb, C.; Chobert, J.; Dufour, E.; Haertle, T. Esterification of food proteins: Characterization of the derivatives by a colorimetric method and by electrophoresis. Sci. Aliment. 1991, 11, 641–652. [Google Scholar]
- Elwan, E.; Aleem, E.E.A.; Fattouh, F.A.; Green, K.J.; Tran, L.T.; Karasev, A.V. Occurrence of Diverse Recombinant Strains of Potato virus Y Circulating in Potato Fields in Egypt. Plant Dis. 2017, 101, 1463–1469. [Google Scholar] [CrossRef] [Green Version]
- Clark, M.F.; Adams, A.N.; Graham, F.L.; Smiley, J.; Russell, W.C.; Nairn, R. Characteristics of the Microplate Method of Enzyme-Linked Immunosorbent Assay for the Detection of Plant Viruses. J. Gen. Virol. 1977, 34, 475–483. [Google Scholar] [CrossRef]
- Forster, A.C.; Mclnnes, J.L.; Skingle, D.C.; Symons, R.H. Non-radioactive hybridization probes prepared by the chemical labelling of DNA and RNA with a novel reagent, photobiotin. Nucleic Acids Res. 1985, 13, 745–761. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Derrick, K.S. Assay for Viruses and Mycoplasmas Using Serologically Specific Electron Microscopy. Phytopathology 1976, 66, 815. [Google Scholar] [CrossRef] [Green Version]
- Duncan, D.B. Multiple Range and Multiple F Tests. Biometrics 1955, 11, 1–42. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds: MLF (methylated lactoferrin) and BLM (methylated β-lactoglobulin) are available from the authors. |




| Parameters | NC * | PC ** | BL | BLM | LF | MLF |
|---|---|---|---|---|---|---|
| No. of shoots | 3.10 ± 0.14 a | 4.4 ± 0.14 d | 3.4 ± 0.14 b | 3.8 ± 0.14 c | 3.15 ± 0.21a | 3.8 ± 0.14 c |
| Plant height (cm) | 58 ± 0.07 d | 52.55 ± 0.78 a | 54.8 ± 1.13 c | 54.95 ± 0.64 c | 53.75 ± 1.06 b | 53 ± 0.57 b |
| No of tubers/plant | 6.05 ± 0.07 a | 6.265 ± 0.09 b | 6.515 ± 0.16 c | 6.985 ± 0.12 e | 6.2 ± 0.14 b | 6.79 ± 0.08 d |
| Fresh weight (g) | 382 ± 2.83 b | 348.5 ± 2.12 a | 356 ± 4.24 c | 456.5 ± 3.54 c | 356.5 ± 2.12 c | 441.5 ± 2.12 d |
| Dry weight (g) | 60.5 ± 0.78 b | 56.75 ± 1.06 a | 67.365 ± 1.9 c | 79.4 ± 1.98 d | 68.45 ± 2.05 c | 83.7 ± 0.99 e |
| Tubers/plant (g) | 396.50 ± 2.1b | 346.5 ± 3.54 a | 433 ± 1.41 c | 516 ± 1.41 e | 431.5 ± 2.12 c | 494 ± 2.83 d |
| Total yield (ton/feddan) | 8.27 ± 0.09 b | 6.6 ± 0.14 a | 8.53 ± 0.18 c | 9.79 ± 0.13 de | 8.155 ± 0.08 b | 9.36 ± 0.08 d |
| % Increase/NC | - | - | 3 | 18 | −1 | 13 |
| % Increase/PC | - | - | 29 | 48 | 23 | 42 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sitohy, M.; Taha, S.; Osman, A.; Abdel-Hamid, M.; Hamed, A.; Abdelbacki, A. Antiviral Action of Native and Methylated Lactoferrin and β-Lactoglobulin against Potato Virus Y (PVY) Infected into Potato Plants Grown in an Open Field. Antibiotics 2020, 9, 430. https://doi.org/10.3390/antibiotics9070430
Sitohy M, Taha S, Osman A, Abdel-Hamid M, Hamed A, Abdelbacki A. Antiviral Action of Native and Methylated Lactoferrin and β-Lactoglobulin against Potato Virus Y (PVY) Infected into Potato Plants Grown in an Open Field. Antibiotics. 2020; 9(7):430. https://doi.org/10.3390/antibiotics9070430
Chicago/Turabian StyleSitohy, Mahmoud, Soad Taha, Ali Osman, Mahmoud Abdel-Hamid, Ali Hamed, and Ashraf Abdelbacki. 2020. "Antiviral Action of Native and Methylated Lactoferrin and β-Lactoglobulin against Potato Virus Y (PVY) Infected into Potato Plants Grown in an Open Field" Antibiotics 9, no. 7: 430. https://doi.org/10.3390/antibiotics9070430
APA StyleSitohy, M., Taha, S., Osman, A., Abdel-Hamid, M., Hamed, A., & Abdelbacki, A. (2020). Antiviral Action of Native and Methylated Lactoferrin and β-Lactoglobulin against Potato Virus Y (PVY) Infected into Potato Plants Grown in an Open Field. Antibiotics, 9(7), 430. https://doi.org/10.3390/antibiotics9070430





