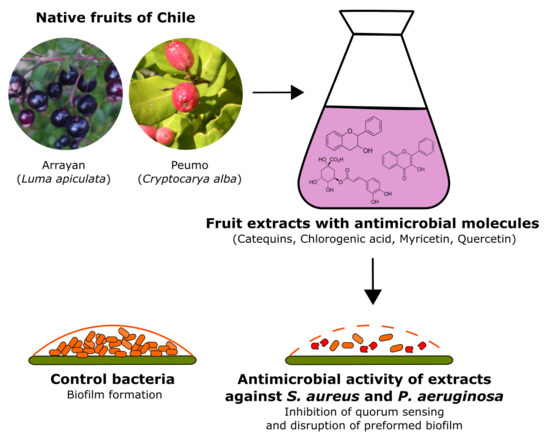
Antimicrobial Activity of Extracts of Two Native Fruits of Chile: Arrayan (Luma apiculata) and Peumo (Cryptocarya alba)
Abstract
:1. Introduction
2. Results
2.1. Inhibition of Multidrug-Resistant Bacterial Strains
2.2. Inhibition of Biofilm Formation
2.3. Disruption of Mature Biofilm
2.4. Inhibition of Quorum Sensing
2.5. High-Resolution Liquid Chromatography Coupled with Mass Spectrometry (U-HPLC/MS) of Arrayan and Peumo Extracts
3. Discussion
4. Materials and Methods
4.1. Plant Material
4.2. Samples Drying and Extracts Preparation
4.3. Inhibition of Multidrug-Resistant Bacterial Strains
4.4. Inhibition of Biofilm Formation
4.5. Disruption of Mature Biofilm
4.6. Inhibition of Quorum Sensing
4.7. Data Processing and Statistical Analysis
4.8. Ultrahigh-Pressure Liquid Chromatography-Mass Spectrometry (U-HPLC/MS) Analysis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Schmeda-Hirschmann, G.; Jiménez-Aspee, F.; Theoduloz, C.; Ladio, A. Patagonian berries as native food and medicine. J. Ethnopharmacol. 2019, 214, 111979. [Google Scholar] [CrossRef] [PubMed]
- Fuentes, L.; Figueroa, C.R.; Valdenegro, M.; Vinet, R. Patagonian Berries: Healthy Potential and the Path to Becoming Functional Foods. Foods 2019, 8, 289. [Google Scholar] [CrossRef] [Green Version]
- Hechenleitner, P.V.; Mf, G.; Thomas, P.; Echeverria, C.; Escobar, B.; Brownless, P.; Martínez, C. Las plantas amenazadas del centro-sur de chile. Distribucón, Conservacíon y Propagación; Universidad Austral de Chile y Real Jardın Botá nico de Edimburgo: Valdivia, Chile, 2005; p. 188. [Google Scholar]
- Simirgiotis, M.J.; Borquez, J.; Schmeda-Hirschmann, G. Antioxidant capacity, polyphenolic content and tandem hplc-dad-esi/ms profiling of phenolic compounds from the south american berries Luma apiculata and L. Chequen. Food Chem. 2013, 139, 289–299. [Google Scholar] [CrossRef] [PubMed]
- Jara-Seguel, P.; Carcamo-Fincheira, P.; Palma-Rojas, C.; von Brand, E. Karyotype morphology of Luma apiculata (dc.) burret (myrtaceae). Gayana Bot. 2013, 70, 395–397. [Google Scholar] [CrossRef] [Green Version]
- Fuentes, L.; Valdenegro, M.; Gomez, M.G.; Ayala-Raso, A.; Quiroga, E.; Martinez, J.P.; Vinet, R.; Caballero, E.; Figueroa, C.R. Characterization of fruit development and potential health benefits of arrayan (Luma apiculata), a native berry of south america. Food Chem. 2016, 196, 1239–1247. [Google Scholar] [CrossRef] [PubMed]
- Falkenberg, S.S.; Tarnow, I.; Guzman, A.; Molgaard, P.; Simonsen, H.T. Mapuche herbal medicine inhibits blood platelet aggregation. Evidence-Based Complement. Altern. 2012, 2012, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Bohm, L.; Arismendi, N.; Ciampi, L. Nematicidal activity of leaves of common shrub and tree species from southern chile against meloidogyne hapla. Cienc. Investig. Agrar. 2009, 36, 249–257. [Google Scholar] [CrossRef] [Green Version]
- Pacheco, P.; Sierra, J.; Schmedahirschmann, G.; Potter, C.W.; Jones, B.M.; Moshref, M. Antiviral activity of chilean medicinal plant-extracts. Phytother. Res. 1993, 7, 415–418. [Google Scholar] [CrossRef]
- Giordano, A.; Fuentes-Barros, G.; Castro-Saavedra, S.; Gonzalez-Cooper, A.; Suarez-Rozas, C.; Salas-Norambuena, J.; Acevedo-Fuentes, W.; Leyton, F.; Tirapegui, C.; Echeverria, J.; et al. Variation of secondary metabolites in the aerial biomass of cryptocarya alba. Nat. Prod. Commun. 2019, 14, 11. [Google Scholar] [CrossRef]
- Bravo, J.; Carbonell, V.; Sepulveda, B.; Delporte, C.; Valdovinos, C.E.; Martin-Hernandez, R.; Higes, M. Antifungal activity of the essential oil obtained from cryptocarya alba against infection in honey bees by nosema ceranae. J. Invertebr. Pathol. 2017, 149, 141–147. [Google Scholar] [CrossRef]
- Pinto, J.J.; Silva, G.; Figueroa, I.; Tapia, M.; Urbina, A.; Rodriguez, J.C.; Lagunes, A. Insecticidal activity of powder and essential oil of cryptocarya alba (molina) looser against sitophilus zeamais motschulsky. Chil. J. Agric. Res. 2016, 76, 48–54. [Google Scholar] [CrossRef] [Green Version]
- Di Cosmo, D.; Santander, R.; Urzua, A.; Palacios, S.M.; Rossi, Y. Insecticidal effect of cryptocarya alba essential oil on the housefly, musca domestica L. Bol. Latinoam. Caribe Plantas Med. 2015, 14, 113–117. [Google Scholar]
- Carmona, E.R.; Reyes-Diaz, M.; Parodi, J.; Inostroza-Blancheteau, C. Antimutagenic evaluation of traditional medicinal plants from south america peumus boldus and cryptocarya alba using drosophila melanogaster. J. Toxicol. Environ. Health A 2017, 80, 208–217. [Google Scholar] [CrossRef] [PubMed]
- Simirgiotis, M.J. Antioxidant capacity and hplc-dad-ms profiling of chilean peumo (Cryptocarya alba) fruits and comparison with german peumo (Crataegus monogyna) from southern chile. Molecules 2013, 18, 2061–2080. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schmeda-Hirschmann, G.; Razmilic, I.; Gutierrez, M.I.; Loyola, J.I. Proximate composition and biological activity of food plants gathered by chilean amerindians. Econ. Bot. 1999, 53, 177–187. [Google Scholar] [CrossRef]
- Schmeda-Hirschmann, G.; Astudillo, L.; Bastida, J.; Codina, C.; De Arias, A.R.; Ferreira, M.E.; Inchaustti, A.; Yaluff, G. Cryptofolione derivatives from cryptocarya alba fruits. J. Pharm. Pharmacol. 2001, 53, 563–567. [Google Scholar] [CrossRef]
- Bansal, S.; Choudhary, S.; Sharma, M.; Kumar, S.; Lohan, S.; Bhardwaj, V.; Syan, N.; Jyoti, S. Tea: A native source of antimicrobial agents. Food Res. Int. 2013, 53, 568–584. [Google Scholar] [CrossRef]
- Shen, X.; Sun, X.; Xie, Q.; Liu, H.; Zhao, Y.; Pan, Y.; Hwang, C.-A.; Wu, V.C.H. Antimicrobial effect of blueberry (Vaccinium corymbosum L.) extracts against the growth of listeria monocytogenes and salmonella enteritidis. Food Control. 2014, 35, 159–165. [Google Scholar] [CrossRef]
- Sáez, F.; Narváez, G.F.; Morales, M.; Bello-Toledo, H.; Balbontin, C.; Figueroa, C. Physiochemical and antibacterial characterization of fruits of citronella mucronata (cardiopteridaceae), pitavia punctata (rutaceae) and beilschmiedia berteroana (lauraceae), three endemic and threatened chilean trees. Fruits 2017, 72, 87–96. [Google Scholar] [CrossRef]
- Cabrera, G.; Wilkens, M.; Giordano, A.; Bernardo, Y.; Delgado, N. Chemical composition and antibacterial activity of red murta (Ugni molinae Turcz.) seeds: An undervalued chilean resource. J. Food Meas. Charact. 2020, 14, 1810–1821. [Google Scholar] [CrossRef]
- Mason, T.L. Inactivation of red beet beta glucan synthase by native and oxidized phenolic compounds. Phytochemistry 1987, 26, 2197–2202. [Google Scholar] [CrossRef]
- Araya-Contreras, T.; Veas, R.; Escobar, C.A.; Machuca, P.; Bittner, M. Antibacterial effect of Luma apiculata (dc.) burret extracts in clinically important bacteria. Int. J. Microbiol. 2019, 2019, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Wikimedia Commons. Available online: https://commons.wikimedia.org/wiki/Main_Page (accessed on 22 July 2020).
- Miller, M.B.; Bassler, B.L. Quorum sensing in bacteria. Annu. Rev. Microbiol. 2001, 55, 165–199. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Timmermann, B.N.; Valcic, S.; Liu, Y.L.; Montenegro, G. Flavonols from cryptocarya alba. Z. Naturforsch. C 1995, 50, 898–899. [Google Scholar] [CrossRef]
- Castro-Saavedra, S.; Fuentes-Barros, G.; Tirapegui, C.; Acevedo-Fuentes, W.; Cassels, B.K.; Barriga, A.; Vilches-Herrera, M. Phytochemical analysis of alkaloids from the chilean endemic tree Cryptocarya alba. J. Chil. Chem. Soc. 2016, 61, 3076–3080. [Google Scholar] [CrossRef] [Green Version]
- Cushnie, T.P.; Lamb, A.J. Antimicrobial activity of flavonoids. Int. J. Antimicrob. Agents 2005, 26, 343–356. [Google Scholar] [CrossRef]
- Sato, Y.; Suzaki, S.; Nishikawa, T.; Kihara, M.; Shibata, H.; Higuti, T. Phytochemical flavones isolated from scutellaria barbata and antibacterial activity against methicillin-resistant Staphylococcus aureus. J. Ethnopharmacol. 2000, 72, 483–488. [Google Scholar] [CrossRef]
- Rauha, J.P.; Remes, S.; Heinonen, M.; Hopia, A.; Kahkonen, M.; Kujala, T.; Pihlaja, K.; Vuorela, H.; Vuorela, P. Antimicrobial effects of finnish plant extracts containing flavonoids and other phenolic compounds. Int. J. Food Microbiol. 2000, 56, 3–12. [Google Scholar] [CrossRef]
- Fiamegos, Y.C.; Kastritis, P.L.; Exarchou, V.; Han, H.; Bonvin, A.M.J.J.; Vervoort, J.; Lewis, K.; Hamblin, M.R.; Tegos, G.P. Antimicrobial and efflux pump inhibitory activity of caffeoylquinic acids from artemisia absinthium against gram-positive pathogenic bacteria. PLoS ONE 2011, 6, e18127. [Google Scholar] [CrossRef] [Green Version]
- Santiago, C.; Pang, E.L.; Lim, K.H.; Loh, H.S.; Ting, K.N. Inhibition of penicillin-binding protein 2a (pbp2a) in methicillin resistant staphylococcus aureus (mrsa) by combination of ampicillin and a bioactive fraction from duabanga grandiflora. BMC Complement. Altern. Med. 2015, 15, 178. [Google Scholar] [CrossRef] [Green Version]
- Rani, N.; Vijayakumar, S.; PTV, L.; Arunachalam, A. Allosteric site-mediated active site inhibition of pbp2a using quercetin 3-o-rutinoside and its combination. J. Biomol. Struct. Dyn. 2016, 34, 1778–1796. [Google Scholar] [CrossRef]
- Rani, N.; Vijayakumar, S.; Thanga Velan, L.P.; Arunachalam, A. Quercetin 3-o-rutinoside mediated inhibition of pbp2a: Computational and experimental evidence to its anti-mrsa activity. Mol. Biosyst. 2014, 10, 3229–3237. [Google Scholar] [CrossRef] [PubMed]
- Karaman, İ.; Sahin, F.; Güllüce, M.; Ogutcu, H.; Sengül, M.; Adiguzel, A. Antimicrobial activity of aqueous and methanol extracts of juniperus L. J. Ethnopharmacol. 2003, 85, 231–235. [Google Scholar] [CrossRef]
- Viktorova, J.; Dobiasova, S.; Rehorova, K.; Biedermann, D.; Kanova, K.; Seborova, K.; Vaclavikova, R.; Valentova, K.; Ruml, T.; Kren, V.; et al. Antioxidant, anti-inflammatory, and multidrug resistance modulation activity of silychristin derivatives. Antioxidants 2019, 8, 303. [Google Scholar] [CrossRef] [PubMed] [Green Version]




| Sample | IC50 (mg/mL) | |||
|---|---|---|---|---|
| Extract/Compound | S. aureus (ATCC 25923) | S. aureus (NEM 449) | P. aeruginosa (ATCC 27853) | P. aeruginosa (NEM 986) |
| sensitive strain | resistant strain * | sensitive strain | resistant strain ** | |
| Peumo | 0.533 ± 0.018 c | 0.557 ± 0.034 c | >1 | 0.778 ± 0.004 b |
| Arrayan | 0.354 ± 0.007 b | 0.385 ± 0.013 b | >1 | >1 |
| Antibiotics | 0.0046 ± 0.0002 a | 0.040 ± 0.002 a | 0.0002 ± 0.000 | >0.150 a |
| Sample | Fold | |
|---|---|---|
| Extract | S. aureus (NEM 449) | P. aeruginosa (NEM 986) |
| resistant strain | resistant strain | |
| Peumo | 0.388 ± 0.0002 | 0.547 ± 0.0003 |
| Arrayán | 0.865 ± 0.0006 | 0.485 ± 0.0007 |
| Sample | IC50 (mg/mL) | |
|---|---|---|
| Extract | S. aureus (ATCC 25923) | P. aeruginosa (ATCC 27853) |
| Peumo | 0.473 ± 0.028 b | 0.346 ± 0.013 b |
| Arrayan | 0.229 ± 0.017 a | 0.288 ± 0.021 a |
| Sample | IC50 (mg/mL) | |
|---|---|---|
| Extract | S. aureus (ATCC 25923) | P. aeruginosa (ATCC 27853) |
| Peumo | No activity | 0.586 ± 0.042 a |
| Arrayan | No activity | 0.559 ± 0.040 a |
| Sample | IC50 (µg/mL) | |||
|---|---|---|---|---|
| Extract | AI-1 strain BAA 1118 (G−) | AI-2 strain BAA 1119 (G+, G−) | ||
| viability | communication | viability | communication | |
| Peumo | 165.7 ± 21.8 a | 25.6 ± 0.3 a | 147.4 ± 2.3 a | 96.2 ± 7.4 b |
| Arrayan | 447.7 ± 71.6 b | 127.2 ± 5.9 b | 111.5 ± 15.3 a | 39.9 ± 3.9 a |
| RT (min) | [M+X]+ (m/z) | [M−X]− (m/z) | [M] (m/z) | Fragments | MF | Tentative Compound |
|---|---|---|---|---|---|---|
| 4.02 | - | 466.0311 [M-H]− | 467 | 169.0149, 211.0259, 271.0476 | C26H10O9 | Unidentified |
| - | 933.0699 [2M-H]− | |||||
| 4.35 | - | 466.0311 [M-H]− | 467 | 156.1025, 184.0957 | C26H10O9 | Unidentified |
| 935.0790 [2M+H]+ | 933.0699 [2M-H]− | |||||
| 5.05 | 307.0813 [M+H]+ | 305.0685 [M-H]− | 306 | 125.0247, 137.0248, 151.0406, 167.0356, 179.0358, 219.0675, 221.0467 | C15H14O7 | Epigallocatechin Gallocatechin |
| - | 611.1443 [2M-H]− | |||||
| 5.29 | 465.1029 [M+H]+ | 463.0915 [M-H]− | 464 | −301.0375, 337.0589 | C21H20O12 | Quercetin 3-glucosideMyricetin Hyperoside Isoquercetrin |
| 5.63 | 479.1188 [M+H]+ | 477.1071 [M-H]− | 478 | −315.0533 | C22H22O12 | Isohamnetin-3-O-β-d-galactoside |
| 449.1083 [M+H]+ | 447.0964 [M-H]− | 448 | C21H20O11 | Quercitrin Isoorientin Luteolin 7-O-glucoside | ||
| 5.92 | 493.1346 [M+H]+ | 491.1230 [M-H]− | 492 | −169.0150, 305.0678, 331.0481 | C23H24O12 | Unidentified |
| 6.88 | 481.0974 [M+H]+ | 479.0858 [M-H]− | 480 | +245.0453, 263.0559, 273.0403, 319.0449 | C21H20O13 | Myricetin 3-O-galactoside |
| 7.14 | 465.1027 [M+H]+ | 463.0912 [M-H]− | 464 | C21H20O12 | myricitin Quercetin 3-glucoside Quercetin 3-O-β-d-allopyranoside Isoquercitrin Hyperoside | |
| 7.99 | 599.2699 [M+H]+ | 597.2589 [M-H]− | 598 | −271.0476, 313.0585, 485.1695 | C29H42O13 | Unidentified |
| 621.2517 [M+Na]+ | 1195.5236 [2M-H]− | |||||
| 9.14 | 387.1803 [M+H]+ | - | 386 | 289.1053 | C22H27O6 | Unidentified |
| 409.1621 [M+Na]+ | - | |||||
| 9.45 | 225.1121 [M+H]+ | 223.0987 [M-H]− | 224 | −179.1085 +139.0395, 155.0343, 207.1024 | C12H16O4 | Unidentified |
| 9.51 | 503.3371 [M+H]+ | 501.3255 [M-H]− | 502 | +139.0402, 155.0352, 165.0561, 207.1035 | C30H46O6 | Guavenoic acid Guavalanostenoic acid |
| 525.3193 [M+Na]+ | 1003.6580 [2M-H]− | |||||
| 9.63 | 415.2113 [M+H]+ | 414 | +303.1211 | C24H30O6 | Unidentified | |
| 437.1931 [M+Na]+ | ||||||
| 851.3975 [2M+Na]+ |
| RT (min) | [M+X]+ (m/z) | [M−X]− (m/z) | [M] (m/z) | Fragments | MF | Tentative Compound |
|---|---|---|---|---|---|---|
| 2.37 | - | 341.1032 [M-H]− 683.2142 [2M-H]− | 342 | −89.0227, 101.0226, 119.0330, 143.0329, 161.0433, 179.0537 | C19H18O6 | Unidentified |
| 5.23 | 579.1509 [M+H]+ | 577.1362 [M-H]− 599.1181 [M-Na]− | 578 | −289.0743, 407.0809, 425.0913, 451.1071 | C30H26O12 | Procyanidin B1 Procyanidin B2 |
| 6.11 | 355.1033 [M+H]+ | 353.0875 [M-H]− 707.1821 [2M-H]− | 354 | C16H18O9 | Chlorogenic acid 4-Caffeoylquinic acid | |
| 6.24 | - | 289.0718 [M-H]− 579.1511 [2M-H]− | 290 | −179.0357, 205.0516. 245.0811 | C15H14O6 | Catechin, Epicatechin |
| 6.38 | 470.1667 [M+H]+ 492.1486 [M+Na]+ | 468.1505 [M-H]− 937.3083 [2M-H]− | 469 | C21H27O11N | Unidentified | |
| 6.46 | 355.1030 [M+H]+ 731.1821 [2M+Na]+ | 353.0876 [M-H]− 707.1880 [2M-H]− | 354 | −191.0568 | C16H18O9 | Analogue of chlorogenic acid 4-Caffeoylquinic acid |
| 7.29 | 465.1034 [M+H]+ | 463.0881 [M-H]− | 464 | 301.0370 | C21H20O12 | Quercetin 3-glucoside Quercetin 3-O-β-d-allopyranoside Isoquercitrin Hyperoside |
| 7.58 | 449.1084 | 447.0930 | 448 | 301.0371 | C21H20O11 | Quercetin 3-O-α-d-rhamnopyranoside Quercitrin Kaempferol 3-O-galactoside Quercetin 3-O-β-d-rhamnoside Astragalin Orientin |
| 7.94 | 463.1241 [M+H]+ | 461.1087 [M-H]− 923.2253 [2M-H]− | 462 | C22H22O11 | Isorhamnetin-3-O-rhamnoside Luteolin 7-O-glucuronide | |
| 8.34 | - | 359.1504 | 360 | 313.1465, 327.1466, 341.1624 | C20H24O6 | (+)-Lariciresinol 4-O-Methylcedrusin |
| 8.52 | - | 343.1766 | 344 | 165.0564, 255.1717, 297.1727 | C17H28O7 | Unidentified |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Viktorová, J.; Kumar, R.; Řehořová, K.; Hoang, L.; Ruml, T.; Figueroa, C.R.; Valdenegro, M.; Fuentes, L. Antimicrobial Activity of Extracts of Two Native Fruits of Chile: Arrayan (Luma apiculata) and Peumo (Cryptocarya alba). Antibiotics 2020, 9, 444. https://doi.org/10.3390/antibiotics9080444
Viktorová J, Kumar R, Řehořová K, Hoang L, Ruml T, Figueroa CR, Valdenegro M, Fuentes L. Antimicrobial Activity of Extracts of Two Native Fruits of Chile: Arrayan (Luma apiculata) and Peumo (Cryptocarya alba). Antibiotics. 2020; 9(8):444. https://doi.org/10.3390/antibiotics9080444
Chicago/Turabian StyleViktorová, Jitka, Rohitesh Kumar, Kateřina Řehořová, Lan Hoang, Tomas Ruml, Carlos R. Figueroa, Monika Valdenegro, and Lida Fuentes. 2020. "Antimicrobial Activity of Extracts of Two Native Fruits of Chile: Arrayan (Luma apiculata) and Peumo (Cryptocarya alba)" Antibiotics 9, no. 8: 444. https://doi.org/10.3390/antibiotics9080444
APA StyleViktorová, J., Kumar, R., Řehořová, K., Hoang, L., Ruml, T., Figueroa, C. R., Valdenegro, M., & Fuentes, L. (2020). Antimicrobial Activity of Extracts of Two Native Fruits of Chile: Arrayan (Luma apiculata) and Peumo (Cryptocarya alba). Antibiotics, 9(8), 444. https://doi.org/10.3390/antibiotics9080444