Synergy of Linezolid with Several Antimicrobial Agents against Linezolid-Methicillin-Resistant Staphylococcal Strains
Abstract
:1. Introduction
2. Results
2.1. Antimicrobial Susceptibility Testing
2.2. Checkerboard Results
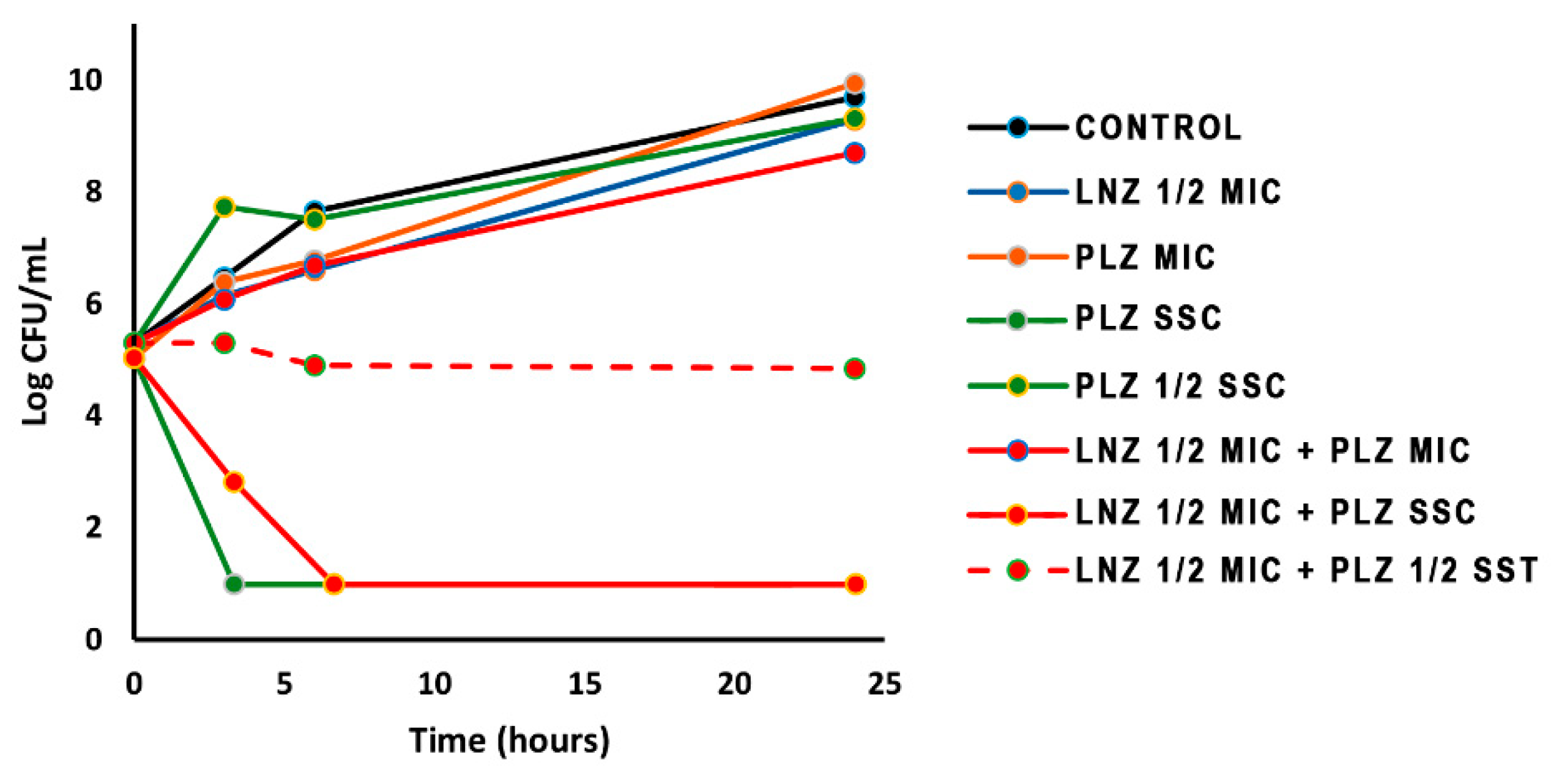
2.3. Time-Kill Curves
3. Discussion
4. Materials and Methods
4.1. Strains
4.2. Antimicrobial Agents
4.3. Susceptibility Testing
4.4. Checkerboard Technique
4.5. Time-Kill Assays
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Decousser, J.-W.; Desrochesa, M.; Bourgeois-Nicolaosa, N.; Potiera, J.; Jehl, F.; Linae, G.; Vincent Cattoir, V.; Vandeneshe, F.; Doucet-Populairea, F.; on behalf of the Microb. Study Group. Susceptibility trends including emergence of linezolid resistance among coagulase-negative staphylococci and meticillin-resistant Staphylococcus aureus from invasive infections. Int. J. Antimicrob. Agents 2015, 46, 622–630. [Google Scholar] [CrossRef] [PubMed]
- European Centre for Disease Prevention and Control. Surveillance of Antimicrobial Resistance in Europe—Annual Report of the European Antimicrobial Resistance Surveillance Network (EARS-Net) 2017; European Centre for Disease Prevention and Control: Stockholm, Sweden, 2018.
- Howden, B.P.; Davies, J.K.; Johnson, P.D.R.; Stinear, T.P.; Grayson, M.L. Reduced vancomycin vusceptibility in Staphylococcus aureus, including vancomycin-intermediate and heterogeneous vancomycin-intermediate strains: Resistance mechanisms, laboratory detection, and clinical implications. Clin. Microbiol. Rev. 2010, 23, 99–139. [Google Scholar] [CrossRef] [Green Version]
- Rae, N.; Jarchow-Macdonald, A.; Nathwani, P.; Marwick, C.A. MRSA: Treating people with infection. BMJ Clin. Evid. 2016, 2, 922. [Google Scholar]
- Mendes, R.E.; Hogan, P.A.; Streit, J.M.; Jones, R.N.; Flamm, R.K. Zyvox annual appraisal of potency and spectrum (ZAAPS) program: Report of linezolid activity over 9 years (2004–12). J. Antimicrob. Chemother. 2014, 69, 582–1588. [Google Scholar] [CrossRef] [PubMed]
- Diekema, D.I.; Jones, R.N. Oxazolidinone antibiotics. Lancet 2001, 358, 1975–1982. [Google Scholar] [CrossRef]
- Pagano, P.J.; Buchanan, L.V.; Dailey, C.F.; Haas, J.V.; Van Enk, R.A.; Gibson, J.K. Effects of linezolid on staphylococcal adherence versus time of treatment. Int. J. Antimicrob. Agents 2004, 23, 226–234. [Google Scholar] [CrossRef]
- Zurenko, G.E.; Yagi, B.H.; Schaadt, R.D.; Allison, J.W.; Kilburn, J.O.; Glickman, S.E.; Hutchinson, D.K.; Barbachyn, M.R.; Brickner, S.J. In vitro activities of U-100592 and U-100766, novel oxazolidinone antibacterial agents. Antimicrob. Agents Chemother. 1996, 40, 839–845. [Google Scholar] [CrossRef] [Green Version]
- Gu, B.; Kelesidis, T.; Tsiodras, S.; Hindler, J.; Humphries, R.M. The emerging problem of linezolid-resistant Staphylococcus. J. Antimicrob. Chemother. 2013, 68, 4–11. [Google Scholar] [CrossRef] [Green Version]
- Liu, C.; Bayer, A.; Cosgrove, S.E.; Daum, R.S.; Fridkin, S.C.; Gorwitz, R.J.; Kaplan, S.L.; Karchmer, A.V.; Levine, D.P.; Murray, B.A.; et al. Clinical practice guidelines by the Infectious Diseases Society of America for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children. Clin. Infect. Dis. 2011, 52, 18–55. [Google Scholar] [CrossRef] [Green Version]
- Eliopoulos, G.M.; Eliopoulos, T. Clinical antibiotic combinations: Should they be tested? Microbiol. Rev. 1988, 1, 139–156. [Google Scholar] [CrossRef]
- Chen, H.; Li, L.; Liu, Y.; Wu, M.; Xu, S.; Zhang, G.; Qi, C.; Du, Y.; Wang, M.; Li, J.; et al. In vitro activity and post-antibiotic effects of linezolid in combination with fosfomycin against clinical isolates of Staphylococcus aureus. Infect. Drug Resist. 2018, 11, 2107–2115. [Google Scholar] [CrossRef] [Green Version]
- Lee, Y.-C.; Chen, P.-Y.; Wang, J.-T.; Chan, S.-D. A study on combination of daptomycin with selected antimicrobial agents: In vitro synergistic effect of MIC value of 1mg/L against MRSA strains. BMC Pharmacol. Toxicol. 2019, 20, 25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sánchez, M.; de la Torre, M.A.; Morales, G.; Pelaez, B.; Tolón, M.J.; Domingo, S.; Candel, J.F.; Andrade, R.; Arribi, A.; Garcıia, N.; et al. Clinical outbreak of linezolid-resistant Staphylococcus aureus in an intensive care unit. JAMA 2010, 303, 2260–2264. [Google Scholar] [CrossRef] [Green Version]
- Cataneli, V.; Calixto, L.; Pinheiro-Hubinger, L.; Oliveira, A.; Benini, K.; Ribeiro, M.L. Coagulase-negative staphylococci: A 20-year study on the antimicrobial resistance profile of blood culture isolates from a teaching hospital. Braz. J. Infect. Dis. 2020, 24, 160–169. [Google Scholar] [CrossRef]
- Pfaller, M.A.; Mendes, R.E.; Streit, J.M.; Hogan, P.A.; Flamm, R.K. Five-year summary of in vitro activity and resistance mechanisms of linezolid against clinically important Gram positive cocci in the United States from the LEADER Surveillance Program (2011 to 2015). Antimicrob. Agents Chemother. 2018, 61, e00609–e00617. [Google Scholar] [CrossRef] [Green Version]
- Morales, G.; Picazo, J.J.; Baos, E.; Candel, F.J.; Arribi, A.; Peláez, B.; Andrade, R.; de la Torre, A.A.; Ferreres, J.; Sánchez-García, M. Resistance to linezolid is mediated by the cfr gene in the first report of an outbreak of linezolid-resistant Staphylococcus aureus. Clin. Infect. Dis. 2010, 50, 821–825. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marshall, S.H.; Donskey, C.J.; Hutton-Thomas, R.; Salata, R.A.; Rice, L.B. Gene dosage and linezolid resistance in Enterococcus faecium and Enterococcus faecalis. Antimicrob. Agents Chemother. 2002, 46, 3334–3336. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Campanille, F.; Mongelli, G.; Bongiorno, D.; Adembri, C.; Ballardini, M.; Falcone, M.; Menichetti, F. Worrisome trend of new multiple mechanisms of linezolid resistance in staphylococcal clones diffused in Italy. J. Clin. Microbiol. 2013, 51, 1256–1259. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smith, T.T.; Tamma, P.D.; Do, T.D.; Dzintars, K.E.; Zhao, Y.; Cosgrove, S.E.; Avdic, E. Prolonged linezolid use is associated with the development of linezolid-resistant Enterococcus faecium. Diagn. Microbiol. Infect. Dis. 2018, 91, 161–163. [Google Scholar] [CrossRef]
- Baos, E.; Candel, F.J.; Merino, P.; Pena, I.; Picazo, J.J. Characterization and monitoring of linezolid-resistant clinical isolates of Staphylococcus epidermidis in an intensive care unit 4 years after an outbreak of infection by cfr-mediated linezolid-resistant Staphylococcus aureus. Diagn. Microbiol. Infect. Dis. 2013, 76, 325–329. [Google Scholar] [CrossRef]
- Otto, M. Staphylococcus epidermidis—The “accidental” pathogen. Nat. Rev. Microbiol. 2009, 7, 555–567. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shaeer, K.M.; Zmarlicka, M.T.; Chahine, E.B.; Piccicacco, N.; Cho, C.J. Plazomicin: A next-generation aminoglycoside. Pharmacotherapy 2019, 39, 77–93. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liakopoulos, A.; Damani, A.; Kanellopoulou, M.; Schoina, S.; Papafragas, E.; Marangos, M.; Fligou, F.; Zakythinos, E.; Makris, D.; Protonotariou, E.; et al. Dissemination of two international linezolid-resistant Staphylococcus epidermidis clones in Greek hospitals. J. Antimicrob. Chemother. 2010, 65, 1070–1077. [Google Scholar] [CrossRef]
- Sauquillo, J.M.; Colomo, E.; Gil, A.; Ortiz, R.; Cantón, R.; Gobernado, M. In vitro activity of linezolid in combination with doxycycline, fosfomycin, levofloxacin, rifampicin and vancomycin against methicillin-susceptible Staphylococcus aureus. Rev. Esp. Quimioterap. 2006, 19, 252–257. [Google Scholar]
- Grohs, P.; Kitzis, M.-D.; Gutmann, L. In vitro bactericidal activities of linezolid in combination with vancomycin, gentamicin, ciprofloxacin, fusidic acid, and rifampin against Staphylococcus aureus. Antimicrob. Agents Chemother. 2003, 47, 418–420. [Google Scholar] [CrossRef] [Green Version]
- Kuli, B.; Barbeyrac, B.; Dauchy, F.A.; Dutronc, H.; Bébéar, C.; Mégraud, F.; Dupon, M. In vitro activities of daptomycin, tigecycline, linezolid and eight other antibiotics, alone and in combination, against 41 Staphylococcus spp. clinical isolates from bone and joint infections. Int. J. Antimicrob. Agents 2009, 33, 487–495. [Google Scholar] [CrossRef]
- La Plante, K.; Rybak, M.J. Impact of high-inoculum Staphylococcus aureus on the activities of nafcillin, vancomycin, linezolid, and daptomycin, alone and in combination with gentamicin, in an in vitro pharmacodynamic model. Antimicrob. Agents Chemother. 2004, 48, 4665–4672. [Google Scholar] [CrossRef] [Green Version]
- Ono, D.; Yamaguchi, T.; Hamada, M.; Sonoda, S.; Sato, A.; Aoki, A.; Kajiwara, C.; Kimura, S.; Fujisaki, M.; Tojo, H.; et al. Analysis of synergy between beta-lactams and anti-methicillinresistant Staphylococcus aureus agents from the standpoint of strain characteristics and binding action. J. Infect. Chemother. 2019, 25, 273–280. [Google Scholar] [CrossRef]
- Singh, S.R.; Bacon, A.E.; Young, D.C.; Couch, K.A. in vitro 24-hour time-kill studies of vancomycin and linezolid in combination versus methicillin-resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 2009, 53, 4495–4497. [Google Scholar] [CrossRef] [Green Version]
- Lopez, M.C.; Rios, E.; Rodriguez-Avial, I.; Simaluiza, R.J.; Picazo, J.P.; Culebras, E. In-vitro activity of several antimicrobial agents against methicillin-resistant Staphylococcus aureus (MRSA) isolates expressing aminoglycoside-modifying enzymes: Potency of plazomicin alone and in combination with other agents. Int. J. Antimicrob. Agents 2017, 50, 191–196. [Google Scholar] [CrossRef]
- Lin, G.; Ednie, L.M.; Appelbaum, P.C. Antistaphylococcal activity of ACHN-490 tested alone and in combination with other agents by time-kill assay. Antimicrob. Agents Chemother. 2010, 54, 2258–2261. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grif, K.; Dierich, M.P.; Pfaller, K.; Miglioli, P.A.; Allerberger, F. In vitro activity of fosfomycin in combination with various antistaphylococcal substances. J. Antimicrob. Chemother. 2001, 48, 2009–2017. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jacqueline, C.; Caillon, J.; Grossi, O.; Le Mabecque, V.; Miegeville, A.F.; Bugnon, D.; Batard, E.; Potel, P. In vitro and in vivo assessment of linezolid combined with ertapenem: A highly synergistic combination against methicillin-resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 2006, 50, 2547–2549. [Google Scholar] [CrossRef] [Green Version]
- Pachón-Ibáñez, M.E.; Ribes, S.; Domínguez, M.A.; Fernández, R.; Tubau, F.; Ariza, J.; Gudiol, F.; Cabellos, C. Efficacy of fosfomycin and its combination with linezolid, vancomycin and imipenem in an experimental peritonitis model caused by a Staphylococcus aureus strain with reduced susceptibility to vancomycin. Eur. J. Clin. Microbiol. Infect. Dis. 2011, 30, 89–95. [Google Scholar] [CrossRef]
- Ribes, S.; Pachón-Ibáñez, M.E.; Domínguez, M.A.; Fernández, R.; Tubau, F.; Ariza, J.; Gudiol, F.; Cabellos, C. In vitro and in vivo activities of linezolid alone and combined with vancomycin and imipenem against Staphylococcus aureus with reduced susceptibility to glycopeptides. Eur. J. Clin. Microbiol. Infect. Dis. 2010, 29, 1361–1367. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu-Hong, Y.; Falagas, M.E.; Dong, W.; Karageorgopoulos, D.E.; De-Fen, L.; Rui, W. In vitro activity of fosfomycin in combination with linezolid against clinical isolates of methicillin-resistant Staphylococcus aureus. J. Antibiot. 2014, 67, 369–371. [Google Scholar] [CrossRef]
- Campanile, F.; Bongiorno, D.; Mongelli, G.; Zanghì, G.; Stefani, S. Bactericidal activity of ceftobiprole combined with different antibiotics against selected Gram-positive isolates. Diagn. Microbiol. Infect. Dis. 2019, 93, 77–81. [Google Scholar] [CrossRef]
- Soriano, A.; Jurado, A.; Marco, F.; Almela, M.; Ortega, M.; Mensa, J. Actividad in vitro de linezolid, moxifloxacino, levofloxacino, clindamicina y rifampicina, solos o en combinación, frente a Staphylococcus aureus y Staphylococcus epidermidis. Rev. Esp. Quimioterap. 2005, 18, 168–172. [Google Scholar]
- Wadhwa, R.R.; Cascella, M. Steady State Concentration; StatPearls Publishing LLC: Treasure Island, FL, USA, 2020. [Google Scholar]
- Foweraker, J.L.; Laughton, C.R.; Brown, D.F.; Bilton, D. Comparison of methods to test antibiotic combinations against heterogeneous populations of multiresistant Pseudomonas aeruginosa from patients with acute infective exacerbations in cystic fibrosis. Antimicrob. Agents Chemother. 2009, 53, 4809–4815. [Google Scholar] [CrossRef] [Green Version]
- El Haj, C.; Murillo, O.; Ribera, A.; Lloberas, N.; Gomez-Junyent, J.; Tubau, F.; Fontova, P.; Cabellos, C.; Ariza, J. Evaluation of linezolid or trimethoprim/sulfamethoxazole in combination with rifampicin as alternative oral treatments based on an in vitro pharmacodynamic model of staphylococcal biofilm. Int. J. Antimicrob. Agents 2018, 51, 854–861. [Google Scholar] [CrossRef]
- EUCAST. The European Committee on Antimicrobial Susceptibility Testing. Routine and Extended Internal Quality Control for MIC Determination and Disk Diffusion as Recommended by EUCAST; Version 10.0; EUCAST: Vaxjo, Sweden, 2020. [Google Scholar]
- CLSI. M07-A9: Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria that Grow Aerobically; Approved Standard—Ninth Edition; Clinical and Laboratory Standards Institute: Wayne, NJ, USA, 2012. [Google Scholar]
- FDA. Antibacterial Susceptibility Test Interpretive Criteria. Available online: https://www.fda.gov/drugs/development-resources/antibacterial-susceptibility-test-interpretive-criteria (accessed on 4 June 2020).
- CLSI. M26-A: Methods for Determining Bactericidal Activity of Antimicrobial Agents; Approved Guideline; Clinical Laboratory Standard Institute: Wayne, NJ, USA, 1998. [Google Scholar]
- Scheetz, M.H.; Qi, C.; Warren, J.R.; Postelnick, M.J.; Zembower, T.; Obias, A.; Noskin, G.A. In vitro activities of various antimicrobials alone and in combination with tigecycline against carbapenem-intermediate or -resistant Acinetobacter baumannii. Antimicrob. Agents Chemother. 2007, 51, 1621–1626. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Álvarez-Lerma, F.; Olaeche, P.; Grau, S.; Marín, M.; Domínguez, A.; Martínez-Lanao, J.; Soy, D.; Alos, M.; Calvo, M.V.; Sadaba, B.; et al. Recomendaciones para la monitorización de antibióticos en pacientes críticos ingresados en UCI. Enferm. Infecc. Microbiol. Clin. 2008, 26, 230–239. [Google Scholar] [CrossRef]
- Tängdén, R.A.; Hickman, P.; Forsberg, P.; Lagerbäck, C.; Giske, C.; Carsa, O. Evaluation of double- and triple-antibiotic combinations for VIM and NDM-producing Klebsiella pneumoniae by in vitro time-kill experiments. Antimicrob. Agents Chemother. 2014, 58, 1757–1762. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Sample Availability: Samples of the compounds …… are available from the authors. |
| Bacterial Species | Isolate Number | MIC (mg/L) (Breakpoint) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| LNZ (4) | AMK (8) | GM (1) | PLZ (2) | FOSF (32) | IMP * | MER * | DOR * | ||
| S. aureus | HCSC-Sa3 | 32 | 4 | 64 | 0.25 | 128 | 128 | 128 | 64 |
| S. aureus | HCSC-Sa8 | 32 | 4 | 64 | 0.25 | 256 | 128 | 128 | 64 |
| S. aureus | HCSC-Sa11 | 32 | 4 | 64 | 0.25 | 128 | 128 | 128 | 64 |
| S. aureus | HCSC-Sa13 | 32 | 4 | 64 | 0.25 | 512 | 128 | 128 | 64 |
| S. aureus | HCSC-Sa14 | 32 | 4 | 64 | 0.25 | 128 | 128 | 128 | 64 |
| S. epidermidis | HCSC-Se12 | 256 | 8 | 64 | 0.25 | 2 | 4 | 16 | 4 |
| S. epidermidis | HCSC-Se31 | 16 | 128 | 128 | 0.25 | 1 | 4 | 16 | 4 |
| S. epidermidis | HCSC-Se39 | 16 | 128 | 128 | 0.25 | 2 | 2 | 8 | 2 |
| S. epidermidis | HCSC-Se45 | 64 | 64 | 128 | 0.125 | 2 | 8 | 16 | 8 |
| S. epidermidis | HCSC-Se47 | 16 | 128 | 128 | 0.25 | 2 | 8 | 16 | 8 |
| Isolate Number | FICImin (Interpretation) Minimum MICs (mg/L) at Synergistic Point | ||||||
|---|---|---|---|---|---|---|---|
| LNZ-AMK | LNZ-GM | LNZ-PLZ | LNZ-FOSF | LNZ-IMP | LNZ-MER | LNZ-DOR | |
| HCSC-Sa3 | 1 (I) | 0.53 (PS) | 1 (I) | 0.257 (S) L(0.25)/F(64) * | 0.625 (PS) | 0.56 (PS) | 0.625 (PS) |
| HCSC-Sa8 | 1 (I) | 0.75 (PS) | 0.75 (PS) | 0.75 (PS) | 0.375 (S) L(4)/I(32) | 0.75 (PS) | 1 (I) |
| HCSC-Sa11 | 1 (I) | 0.625 (PS) | 0.25 (S) L(4)/P(0.25) * | 0.75 (PS) | 0.5 (S) L(8)/I(32) | 0.75 (PS) | 0.625 (PS) |
| HCSC-Sa13 | 1 (I) | 0.75 (PS) | 0.14 (S) L(0.5)/P(0.25) * | 1 (I) | 0.375 (S) L(8)/I(32) | 0.75 (PS) | 0.75 (PS) |
| HCSC-Sa14 | 1 (I) | 0.625 (PS) | 0.26 (S) L(0.5)/P(0.125) * | 0.28 (S) L(1)/F(128) | 0.5 (S) L(8)/I(32) | 0.75 (PS) | 0.375 (S) L(4)/D(32) |
| HCSC-Se12 | 0.5 (S) | 0.625 (PS) | 0.75 (PS) | 0.625 (PS) | 0.5 (S) L(0.25)/I(2) * | 0.75 (PS) | 0.5 (S) L(128)/D(1) |
| HCSC-Se31 | 0.75 (PS) | 0.625 (PS) | 0.53 (PS) | 1 (I) | 0.375 (S) L(4)/I(2) * | 0.375 (S) L(8)/M(4) * | 0.25 (S) L(4)/D(2)* |
| HCSC-Se39 | 0.5 (S) L(0.125)/A(128) | 1 (I) | 0.625 (PS) | 0.5 (S) L(0.125)/F(2) * | 0.5 (S) L(2)/I(0.25) * | 0.625(PS) | 0.375 (S) L(8)/D(1) * |
| HCSC-Se45 | 0.09 (S) L(32)/A(8) | 0.09 (S) L(32)/G(16) | 0.078 (S) L(32)/P(0.0156) | 0.187 (S) L(32)/F(0.5) | 0,187 (S) L(4)/I(2) * | 0.078 (S) L(32)/M(0.25) | 0.0625 (S) L(16)/D(0.25) |
| HCSC-Se47 | 0.75 (PS) | 0.375 (S) L(8)/G(64) | 0.75 (PS) | 0.5 (S) L(8)/F(2) * | 0.315 (S) L(4)/I(2) * | 0.375 (S) L(8)/M(4) * | 0.3125 (S) L(8)/D(0.5) * |
| Antibiotic | S. aureus | S. epidermidis | ||||||
|---|---|---|---|---|---|---|---|---|
| HCSC-Sa3 | HCSC-Sa8 | HCSC-Sa13 | HCSC-Sa14 | HCSC-Se31 | HCSC-Se39 | HCSC-Se45 | HCSC-Se47 | |
| Control | 8.848 | 8.938 | 9.362 | 9.728 | 9.168 | 10.476 | 9.028 | 9.070 |
| LNZ 1/2MIC | 6.255 | 6.350 | 7.301 | 8.653 | 5.903 | 8.585 | 7.977 | 7.790 |
| AMK SSC | 0.845 | 1.778 | 5.204 | 1.000 | 8.398 | 4.000 | 8.176 | 9.301 |
| GM SSC | 0.845 | 1.778 | 3.568 | 9.356 | 2.477 | 9.019 | 5.316 | 0.845 |
| PLZ SSC | 0.845 | 1.000 | 0.845 | 0.845 | 0.845 | 0.845 | 0.845 | 1.477 |
| FOSF SSC | 5.829 | 6.973 | 7.970 | 7.954 | 8.664 | 9.015 | 8.889 | 9.591 |
| IMP SSC | 3.000 | 8.985 | 8.602 | ND | 7.966 | ND | 6.217 | 9.423 |
| MER SSC | 8.817 | 9.000 | 9.267 | 8.778 | 8.905 | 9.025 | 9.313 | 9.146 |
| LNZ+AMK | 4.921 | 2.332 | 0.845 | 2.455 | 7.628 | 5.000 | 3.279 * | 4.916 |
| LNZ+GM | 2.146 | 0.845 | 3.125 | 5.190 | 2.362 | 6.699 | 0.845 * | 2.875 |
| LNZ+PLZ | 1.000 | 0.845 | 3.622 | 0.845 | 0.845 | 0.845 | 0.845 | 4.695 |
| LNZ+FOSF | 2.727 * | 3.845 | 7.423 | 8.000 | 5.450 | 0.845 * | 3.028 * | 7.618 |
| LNZ+IMP | 3.204 | 5.243 | 5.04 | ND | 3.527 | ND | 2.000 * | 3.994 * |
| LNZ+MER | 5.139 | 5.394 | 7.22 | 4.903 | 4.773 | 5.041 | 2.903 * | 2.934 * |
| Isolate | Clinical Sample | Linezolid Resistance Mechanism |
|---|---|---|
| Staphylococcus aureus [17] | ||
| HCSC-Sa3 | Bronchial aspirate | cfr + ∆Ser 145/His146Tyr (L3) |
| HCSC-Sa8 | Blood | cfr + ∆Ser 145/His146Tyr (L3) |
| HCSC-Sa11 | Bronchial aspirate | cfr + ∆Ser 145/His146Tyr (L3) |
| HCSC-Sa13 | Bronchial aspirate | cfr + ∆Ser 145/His146Tyr (L3) |
| HCSC-Sa14 | Catheter tip | cfr + ∆Ser 145/His146Tyr (L3) |
| Staphylococcus epidermidis [21] | ||
| HCSC-Se12 | Catheter tip | cfr + Gly2576Thr (rRNA—5 copies) |
| HCSC-Se31 | Blood | Gly152Ser (L3) |
| HCSC-Se39 | Catheter tip | cfr |
| HCSC-Se45 | Catheter tip | Gly152Ser (L3) + Asn158Ser (L4) |
| HCSC-Se47 | Catheter tip | Gly2576Thr (rRNA—1 copy) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Valderrama, M.-J.; Alfaro, M.; Rodríguez-Avial, I.; Baos, E.; Rodríguez-Avial, C.; Culebras, E. Synergy of Linezolid with Several Antimicrobial Agents against Linezolid-Methicillin-Resistant Staphylococcal Strains. Antibiotics 2020, 9, 496. https://doi.org/10.3390/antibiotics9080496
Valderrama M-J, Alfaro M, Rodríguez-Avial I, Baos E, Rodríguez-Avial C, Culebras E. Synergy of Linezolid with Several Antimicrobial Agents against Linezolid-Methicillin-Resistant Staphylococcal Strains. Antibiotics. 2020; 9(8):496. https://doi.org/10.3390/antibiotics9080496
Chicago/Turabian StyleValderrama, María-José, María Alfaro, Icíar Rodríguez-Avial, Elvira Baos, Carmen Rodríguez-Avial, and Esther Culebras. 2020. "Synergy of Linezolid with Several Antimicrobial Agents against Linezolid-Methicillin-Resistant Staphylococcal Strains" Antibiotics 9, no. 8: 496. https://doi.org/10.3390/antibiotics9080496
APA StyleValderrama, M.-J., Alfaro, M., Rodríguez-Avial, I., Baos, E., Rodríguez-Avial, C., & Culebras, E. (2020). Synergy of Linezolid with Several Antimicrobial Agents against Linezolid-Methicillin-Resistant Staphylococcal Strains. Antibiotics, 9(8), 496. https://doi.org/10.3390/antibiotics9080496




