Abstract
Helicobacter pylori eradication rate with conventional standard therapy is decreasing owing to antibiotic resistance, necessitating novel antibacterial strategies against H. pylori. We evaluated the efficacy of a gentamicin-intercalated smectite hybrid (S-GM)-based treatment and analyzed fecal microbiome composition in H. pylori-infected mice. To evaluate anti-H. pylori efficacy, mice were divided into eight groups, and H. pylori eradication was assessed by a Campylobacter-like organism (CLO) test and PCR assay of H. pylori in gastric mucosa. One week after H. pylori eradication, pro-inflammatory cytokine levels and atrophic changes in gastric mucosa were examined. Stool specimens were collected and analyzed for microbiome changes. The S-GM-based triple regimen decreased bacterial burden in vivo, compared with that in untreated mice or mice treated with other regimens. The therapeutic reactions in the CLO test from gastric mucosa were both 90% in the standard triple therapy and S-GM therapy group, respectively. Those of H. pylori PCR in mouse gastric mucosa were significantly lower in standard triple therapy and S-GM therapy groups than in the non-treatment group. Toxicity test results showed that S-GM therapy reduced IL-8 level and atrophic changes in gastric mucosa. Stool microbiome analysis revealed that compared with mice treated with the standard triple therapy, mice treated with the S-GM therapy showed microbiome diversity and abundant microorganisms at the phylum level. Our results suggested that S-GM is a promising and effective therapeutic agent against H. pylori infection.
1. Introduction
In 1983, Warren and Marchall described the Gram-negative, spiral-shaped microaerophilic bacterium Helicobacter pylori (H. pylori) that colonizes the human stomach. H. pylori triggers numerous pathologic alterations in the stomach, including peptic ulcer disease, primary gastritis, and gastric cancer [1,2]. H. pylori eradication cures gastritis and alters the complication or recurrence of gastrointestinal diseases [3]. The standard treatment for H. pylori infection is a triple therapy combining a proton pump inhibitor (PPI), clarithromycin, metronidazole, or amoxicillin [4]. This regimen, however, fails to eradicate infection in 10–40% of patients and sometimes causes side effects [4,5,6]. A major cause of this failure is the increase in multidrug-resistant H. pylori strains; hence, there is an alarming need to develop alternative antimicrobial agents with improved effectiveness.
Previously, we have confirmed that aminoglycosides have a low minimum inhibitory concentration for recently isolated H. pylori, including major drug-resistant strains [7]. However, aminoglycosides are polar, water-soluble compounds with very poor intestinal membrane permeability, resulting in low oral bioavailability [8,9]. Therefore, we used smectite clay, comprising tetrahedral sheets of SiO4 units and octahedral sheets of Al3+ ions [10], as a carrier of hydrophilic drugs to synthesize a gentamicin (GM)-intercalated smectite hybrid (S-GM) as a novel therapeutic agent. We previously identified that S-GM stably releases GM to the gastric wall, and an S-GM-based triple regimen decreases bacterial burden in vivo compared with that in untreated mice or mice treated with other regimens [11].
The human gut microbiota interacts with the host immune system and maintains metabolic homeostasis; thus, it is associated with obesity, inflammatory bowel disorder, allergic diseases, and neurological disorders [12,13]. Despite anatomical and compositional differences between human and mouse microbiota, some studies reported a concordance of microbiota shift in murine models and human diseases [14]. Therefore, we analyzed changes in fecal microbiota in an H. pylori-infected murine model to examine the toxicity of S-GM. Because S-GM is not absorbed in the gastrointestinal tract, it is impossible to evaluate its pharmacokinetics (PK) and pharmacodynamics (PD). Moreover, in contrast to previous studies [11], S-GM was administered less frequently in this study.
Here, we aimed to evaluate the effect of dosing interval on the daily administration of S-GM and to assess the safety of S-GM. Changes in inflammatory cytokine levels, atrophy of gastric mucosa, and fecal microbiota were analyzed after eradication of H. pylori with S-GM and compared with those after the standard triple therapy.
2. Results
2.1. CLO test and PCR Assay of H. pylori in Gastric Mucosa
The S-GM-based regimen decreased H. pylori bacterial burden in vivo, compared with that in the untreated mice or mice treated with other regimens. Table 1 shows the therapeutic effect of each regimen on H. pylori infection. Campylobacter-like organism (CLO) test results showed that the therapeutic reactions in gastric mucosa were 90%, 90%, 80%, 80%, 70%, and 10% in groups III, IV, V, VI, VII, and VIII, respectively (Table 1). The CLO scores of groups III and IV were the lowest among the H. pylori-infected groups and were significantly lower than of group II. The S-GM based therapy was not inferior to the standard triple therapy with amoxicillin and clarithromycin. Three or four doses per week also showed significant therapeutic results in the CLO test, although lower than that of daily administration.

Table 1.
Individual data of the CLO test and quantitative PCR of mouse gastric mucosa after treatment of H. pylori infection.
A polymerase chain reaction (PCR) assay was conducted to evaluate the therapeutic effects of S-GM in H. pylori-infected mice (Table 1). The amount of H. pylori deoxyribonucleic acid (DNA) in mouse gastric mucosa was significantly lower in groups III to VIII than in group II.
2.2. Pro-Inflammatory Cytokines and Atrophy of Gastric Mucosa
S-GM-based therapy reduced interleukin-8 (IL-8) and tumor necrosis factor-α (TNF-α) levels compared with the standard triple therapy (group III). The degree of atrophic changes in the gastric mucosa was analyzed in gastric tissue specimens; compared with the standard triple therapy (group III), S-GM based therapy (group IV) led to less atrophic changes in mouse stomach, but it showed no statistical significance (Table 2).

Table 2.
Plasma cytokine concentrations of IL-8 and TNF- α in each group.
2.3. Changes in Fecal Microbiota
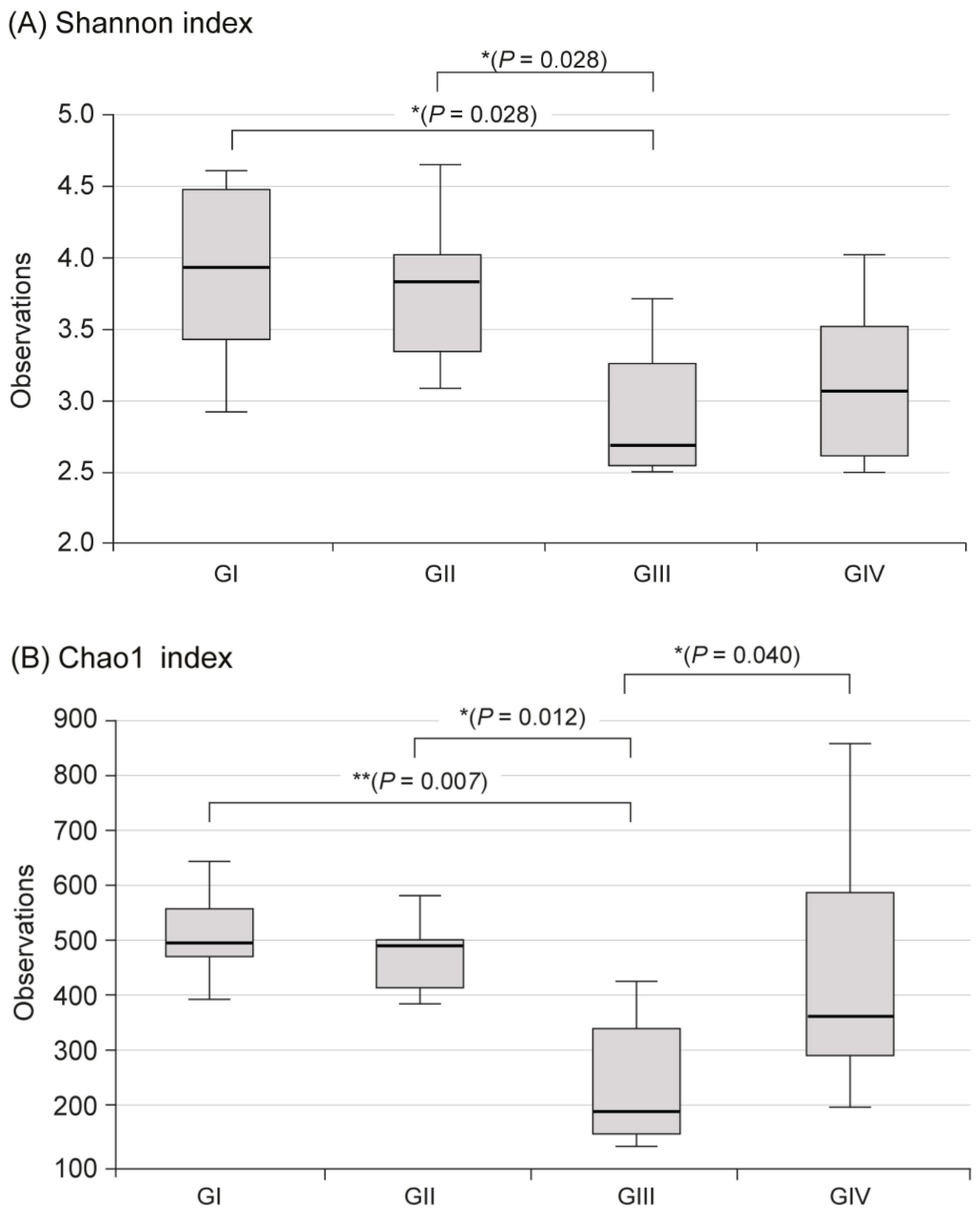
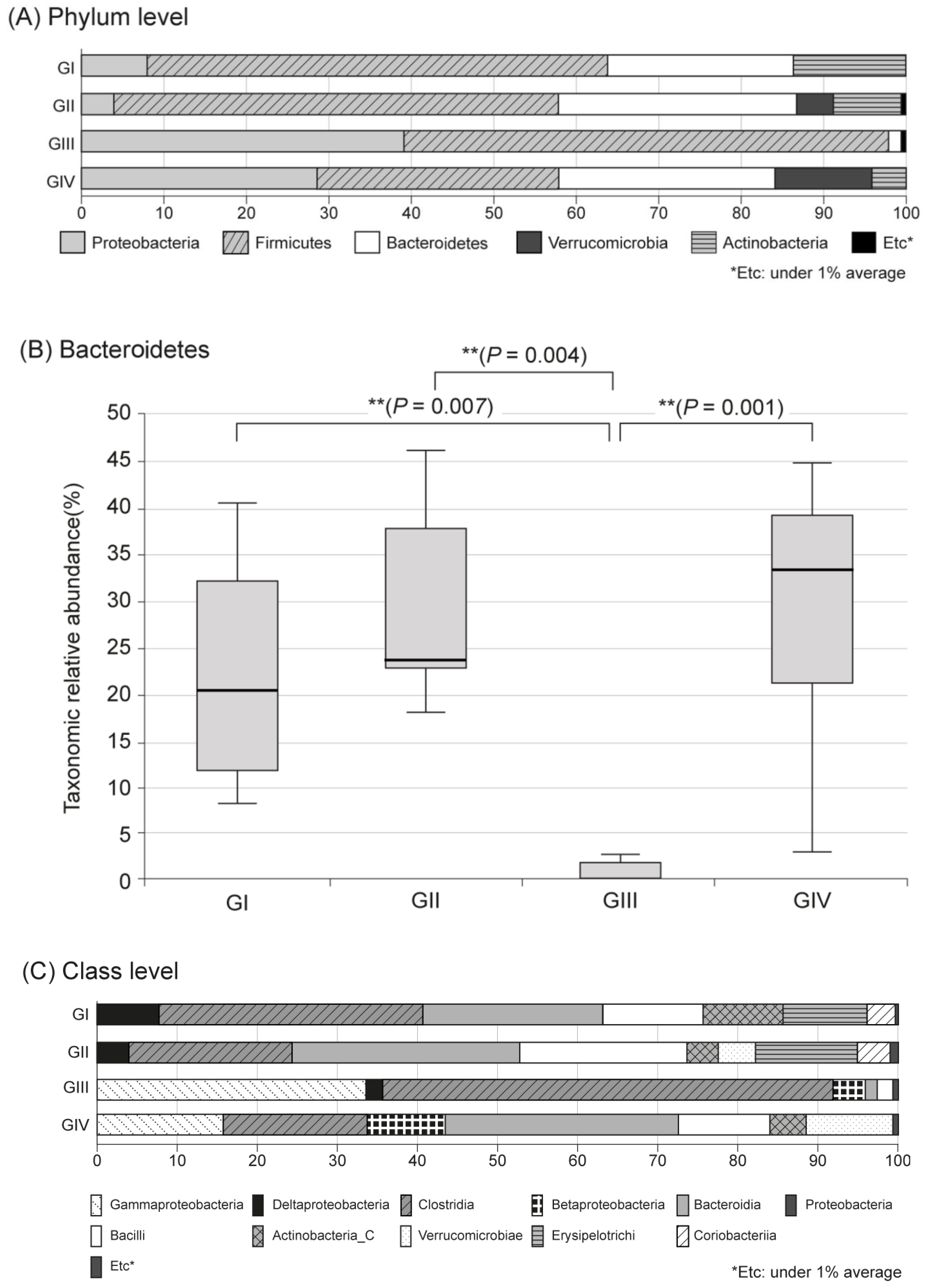
In the metagenomic analysis of S-GM, changes in the diversity and abundance of stool microbiome were identified (Table 2). Alpha diversity was the lowest in the standard triple therapy group (group III). The Shannon and Chao1 indexes were relatively preserved in the S-GM therapy group (group IV) compared with those in the standard triple therapy group (group III vs. group IV, Shannon index, 2.92 ± 0.53 vs. 3.13 ± 0.55; Chao 1, 245.71 ± 121.23 vs. 440.45 ± 213.56). The abundance in group III decreased significantly, while in group IV it was preserved with a similar trend to group II (Figure 1). Focusing on changes in each species, the composition of the stool microbial community was analyzed at the phylum and the class levels. In the phylum level, the amount of Bacteroidetes was significantly reduced, summation of two phyla Proteobacteria and Firmicutes took up above 95% in group III, and qualitative amounts of other phyla showed a decrease. While in group IV, total microbiota composition was evenly preserved, and taxonomic relative abundance presented as similar to the non-antibiotic treated groups (group II). Analogous trends were observed in the analysis of microbiomes with class level. In group III, diversity of class was prominent by only two types of Gammaproteobacteria and Clostridia, while group IV continued to have similar abundant diversity to group I and group II (Figure 2).
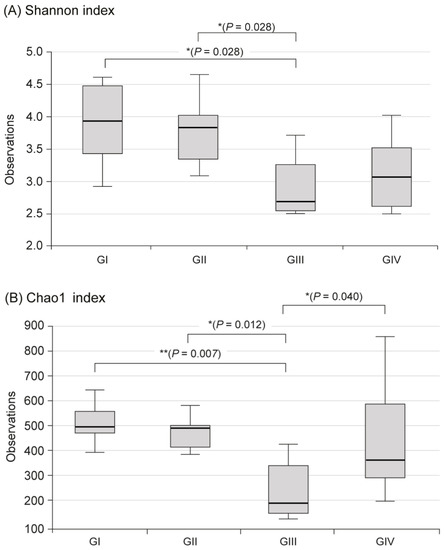
Figure 1.
Alpha diversity in fecal microbiome between four groups. (A) Shannon index, (B) Chao index, Group I, normal group consisting of uninfected mice; group II to IV, infected mice with H. pylori; group II, a negative control group, received distilled water as a vehicle; group III, a positive control group, treated with the standard triple therapy consisting of amoxicillin (14.25 mg/kg), clarithromycin (14.3 mg/kg), and omeprazole (138 mg/kg); group IV, treated with amoxicillin (14.25 mg/kg), gentamicin-intercalated smectite hybrid which emitted 8 mg/kg of gentamicin, and omeprazole (138 mg/kg). Shannon index, information about community composition and richness; Cho1 index, estimate diversity from abundance data.
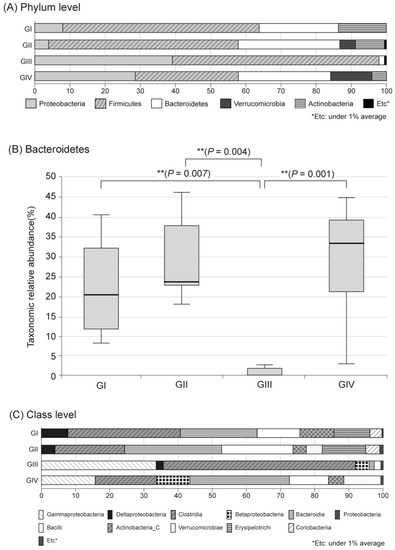
Figure 2.
Microbiota composition and relative abundance distributions in four groups. (A) Phylum level, (B) Bacteroidetes, (C) Class level, Group I, normal group consisting of uninfected mice; group II to IV, infected mice with H. pylori; group II, a negative control group, received distilled water as a vehicle; group III, a positive control group, treated with the standard triple therapy consisting of amoxicillin (14.25 mg/kg), clarithromycin (14.3 mg/kg), and omeprazole (138 mg/kg); group IV, treated with amoxicillin (14.25 mg/kg), gentamicin-intercalated smectite hybrid which emitted 8 mg/kg of gentamicin, and omeprazole (138 mg/kg).
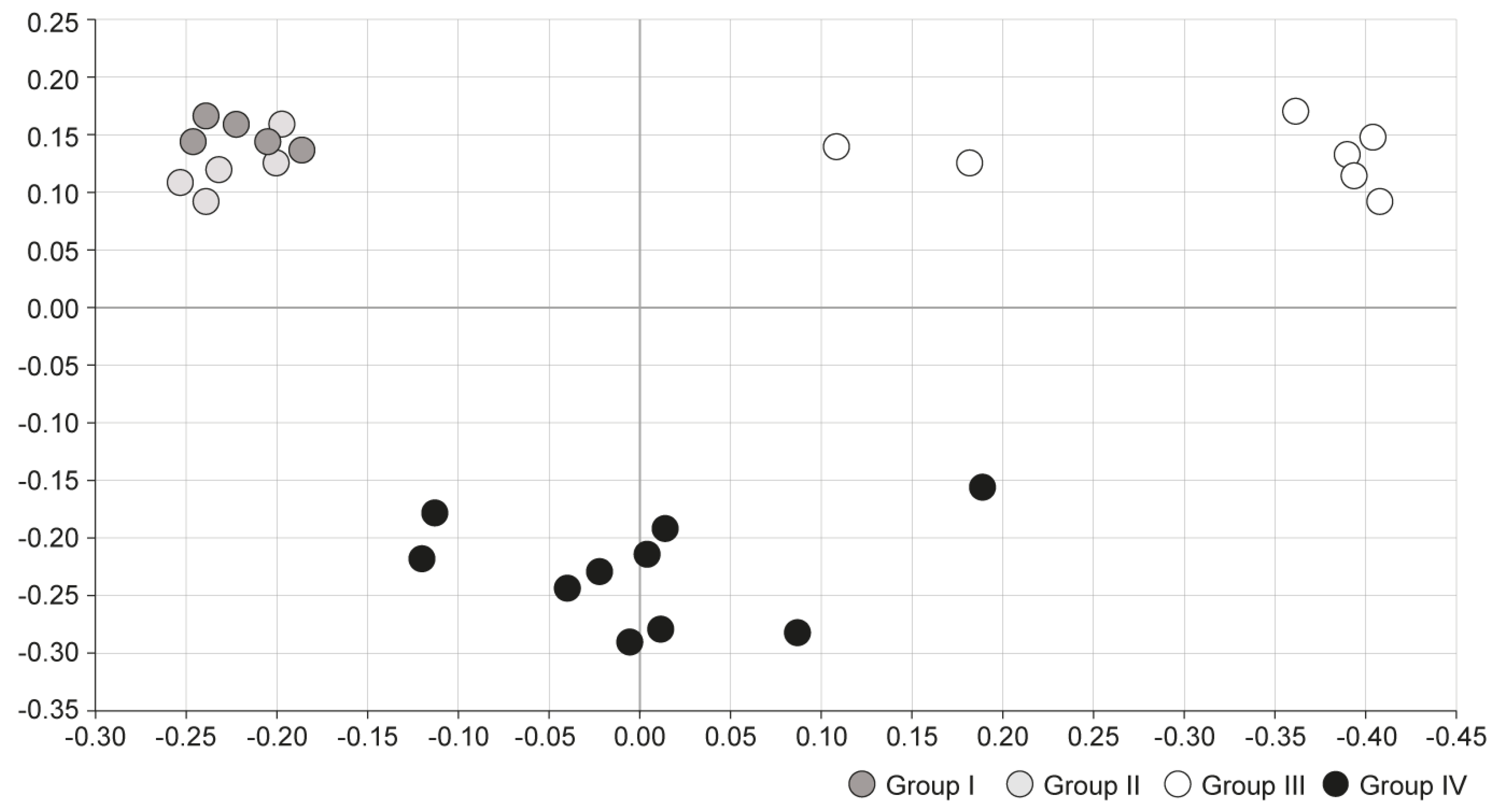
Principal coordinates analysis (PCoA) was conducted to compare microbial communities between the four groups. In PCoA analysis, points that are closer together represent microbial communities that are more similar in sequence composition. Group I and II showed similar trends, whereas group III showed a distinctly different trend of microbiome composition. Group IV, which was treated with S-GM therapy, showed moderate disposition (Figure 3).
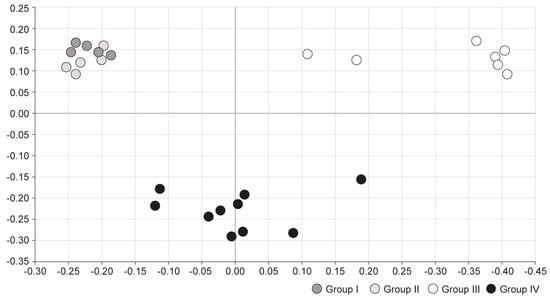
Figure 3.
Comparison of microbial communities using principal coordinate analysis. Group I, normal group consisting of uninfected mice; group II to IV, infected mice with H. pylori; group II, a negative control group, received distilled water as a vehicle; group III, a positive control group, treated with the standard triple therapy consisting of amoxicillin (14.25 mg/kg), clarithromycin (14.3 mg/kg), and omeprazole (138 mg/kg); group IV, treated with amoxicillin (14.25 mg/kg), gentamicin-intercalated smectite hybrid which emitted 8 mg/kg of gentamicin, and omeprazole (138 mg/kg). PCoA, principal coordinate analysis, is a statistical method to explore similarities in a data set.
3. Discussion
The existence of H. pylori in the human stomach has been known since as early as 60,000 years ago [15]; it has been isolated from the gastric antrum and cultivated in vitro [16]. Early eradication-based therapies regress H. pylori-associated diseases [3,4]. However, the eradication treatment efficacy has been compromised in many countries owing to the increasing resistance to antimicrobial agents [4,5,6,17,18]. Additionally, the recurrence of H. pylori remains a serious challenge worldwide, particularly in developing countries. The annual recurrence risk was 3.4% (95% CI, 3.1–3.7%) in high-income countries and 8.7% (95% CI, 8.8–9.6%) in low-income countries.
To improve the eradication efficacy, studies continue to evaluate novel treatment regimens, including quintuple therapies [19], high-dose dual therapies [20], and standard triple therapies with probiotics [21]. However, the evidence is insufficient, and the side and cost effects of such therapies should be considered.
In our previous work, we demonstrated the high anti-H. pylori efficacy of S-GM in reducing H. pylori load in mouse stomachs [11]. Here, we found no significant difference between daily administration and three- or four-time administration per week of S-GM. Therefore, we examined the possibility of administration three or four times a week for H. pylori eradication. However, the single-dose therapy (group VIII) showed a significantly reduced therapeutic effect. Further, in future studies, S-GM efficacy should be confirmed with reduced overall treatment durations, such as 3, 5, and 7 days of daily treatment, not three or four times per week.
However, we could still assess the efficacy of S-GM in eradicating H. pylori in this study. GM concentration in the S-GM hybrid was intercalated only up to 8 mg/kg. If GM concentration is increased, or if it is intercalated with another drug delivery system similar to smectite capable of delivering antibiotics to the stomach wall, we can achieve prolonged and improved drug release. The intercalation of GM high concentrations is difficult to consider owing to the systemic side effects associated with intravenous administration, but its effectiveness can be expected in targeted localized therapy, such as H. pylori eradication. Further research is needed to compare the therapeutic effect of S-GM with other drug delivery systems, such as alginate and a composite. With chitosan-treated beads, alginated-antibiotic hybrids may achieve pH-dependent retarded release of highly soluble drug [22].
In the present study, S-GM triple therapy reduced IL-8 level and atrophic changes in gastric mucosa. IL-8 promotes inflammation, and atrophy of gastric mucosa suggests post-inflammatory changes. Therefore, the reduction of the inflammatory response by S-GM suggests that the immune response and tissue injury of H. pylori can be suppressed. Further, stool microbiome analysis showed that microbiome diversity and microorganism abundance at the phylum level were preserved in the S-GM triple therapy group. This indicates that the composition of the microbiome in the standard therapy group consisting of amoxicillin and clarithromycin antibiotics (group III) was significantly reduced compared to the normal control (group I) and the H. pylori-infected but untreated mice (Group II). This suggests that the composition of the microbiome shows relatively small dysbiosis in the S-GM therapy group (group IV) than standard therapy (group I). This is considered an advantage of S-GM, which has little effect on the systemic adverse reaction and overall gut microbiota. It provides topical therapeutic benefits through localized effect, compared to a standard regimen with systemic effects by oral absorption. PK/PD analysis is required to examine the toxicity of S-GM; however, because S-GM is not absorbed systemically, PK/PD analysis is not feasible for S-GM. S-GM as a localized therapy showed a bactericidal effect against H. pylori attached to the gastric wall. Therefore, toxicity analysis of this treatment was focused on changes in the intestinal bacterial microbiome, and the results confirmed that the components of the microbiome were well preserved compared with those after the standard therapy.
In this study, amoxicillin- and clarithromycin-based standard therapies have been shown to lead to microbiome dysbiosis, which is associated with various metabolic diseases, gastrointestinal diseases, and even gastric cancer. H. pylori infection itself, as well as decreased microbial diversity and abundance, are correlated with gastric carcinoma [23,24]. Therefore, our results indicated that S-GM therapy could not only block gastric carcinogenesis but also reduce the incidence of diseases, such as inflammatory bowel and metabolic diseases, by minimalizing changes in gut microbiota with low toxicity in addition to sufficient efficacy compared with standard systemic therapy.
A previous H. pylori and microbiome study revealed a dramatic decrease in microbiome diversity immediately within one week after eradication, indicating that the bacterial community resembled and recovered the pre-antibiotic period only four years after a long-term follow-up. In humans, Actinobacteria was the most affected by antibiotics [25]. In our mouse model study, Actinobacteria reduction was also noticeable after the use of amoxicillin and clarithromycin (group III). Thus, to reduce prolonged dysbiosis and its consequences, it is necessary to eradicate H. pylori with minimal use of antibiotics, for which S-GM may be an effective strategy.
Therefore, to decrease antibiotic-related gut dysbiosis in patients and maintain microbiome components, targeted therapy for H. pylori attached to the gastric wall is needed instead of therapy with systemic antibiotics. Moreover, the use of a smectite applied to the stomach wall as a drug delivery system would be a significant turning point for H. pylori eradication.
There were, however, several limitations in this study. First, the study used animal models; thus, the actual clinical dysbiosis may differ in humans. Second, the stool microbiome analysis was not conducted for each individual mouse, and stool was extracted within the same treatment group. The mouse itself could share the same microbiome environment owing to co-housing in the same cage [26,27]. Therefore, for more accurate analysis, it is necessary to analyze the feces of each mouse subject and compare the individual eradication rate with a specific therapeutic regimen. Third, long-term follow-up after S-GM treatment is needed.
Nevertheless, this is the first study to verify the gut dysbiosis of the S-GM as an alternative therapy for H. pylori eradication to overcome the increasing antibiotic resistance to other regimens. Moreover, localized H. pylori eradication will make a novel paradigm shift in H. pylori treatment.
4. Materials and Methods
4.1. Intercalation of GM
GM (2 mg/mL) solution was prepared using the gentamicin sulfate of USP grade produced by BIO BASIC INC (Toronto, Canada). Ca-smectite was prepared by purifying the bentonite found in the area of Gampo, Korea. To generate a GM-intercalated smectite hybrid, GM solution was mixed with Ca-smectite to a concentration of 250 mL/g, and the mixture was stirred vigorously for 24 h. Next, the hybrid solution was dialyzed with 5 L of distilled water for ~8 h at 50 °C, and the dialysis was repeated three to four times until sulfate ions could not be detected by PbCl2. A hybrid powder was finally obtained by frieze-drying the dialyzed hybrid solution for 2 to 3 days. The amount of GM released from the hybrid was determined by batch-release test using 25 mL of pH 1.2 solution for 100 mg of the hybrid powder. The total amount of GM released within 1 h was determined to be ~5.0 mg per 100 mg of the hybrid.
4.2. Animal Preparation
The Institutional Animal Care and Use Committee at Daegu-Gyeongbuk Medical Innovation Foundation (DGMIF), Daegu, Korea, approved the animal procedures. Four-week-old male C57BL/6 mice were purchased from Japan SLC, Inc., Shizuoka, Japan. The mice were 5 weeks of age and weighed 18–20 g at the start of the experiment. The animal experiments were reviewed and approved by the Institutional Animal Care and Use Committee of the DGMIF (Reg. No. DGMIF-19031801-00).
4.3. Anti-H. pylori Efficacy In Vivo
4.3.1. H. pylori Strains and Culture Conditions
H. pylori SS1 was used in this study. The bacteria were maintained and grown on Brucella agar (Merck, Germany) supplemented with 10% fetal bovine serum (Gibco, USA), and incubated under microaerobic conditions (5% O2, 10% CO2, and 85% N2) at 37 °C for 72 h.
4.3.2. Inoculation of Experimental Animals
For in vivo assessment of anti-H. pylori effect, 80 mice were allowed to acclimatize for 1 week before the initiation of the experiment. After the acclimatization period, the animals were fasted for 12 h, and 70 of them were intragastrically infected with 0.5 mL of 2.0 × 109 CFU/mL H. pylori suspension by oral gavage every 48 h, and this was repeated three times in 1 week.
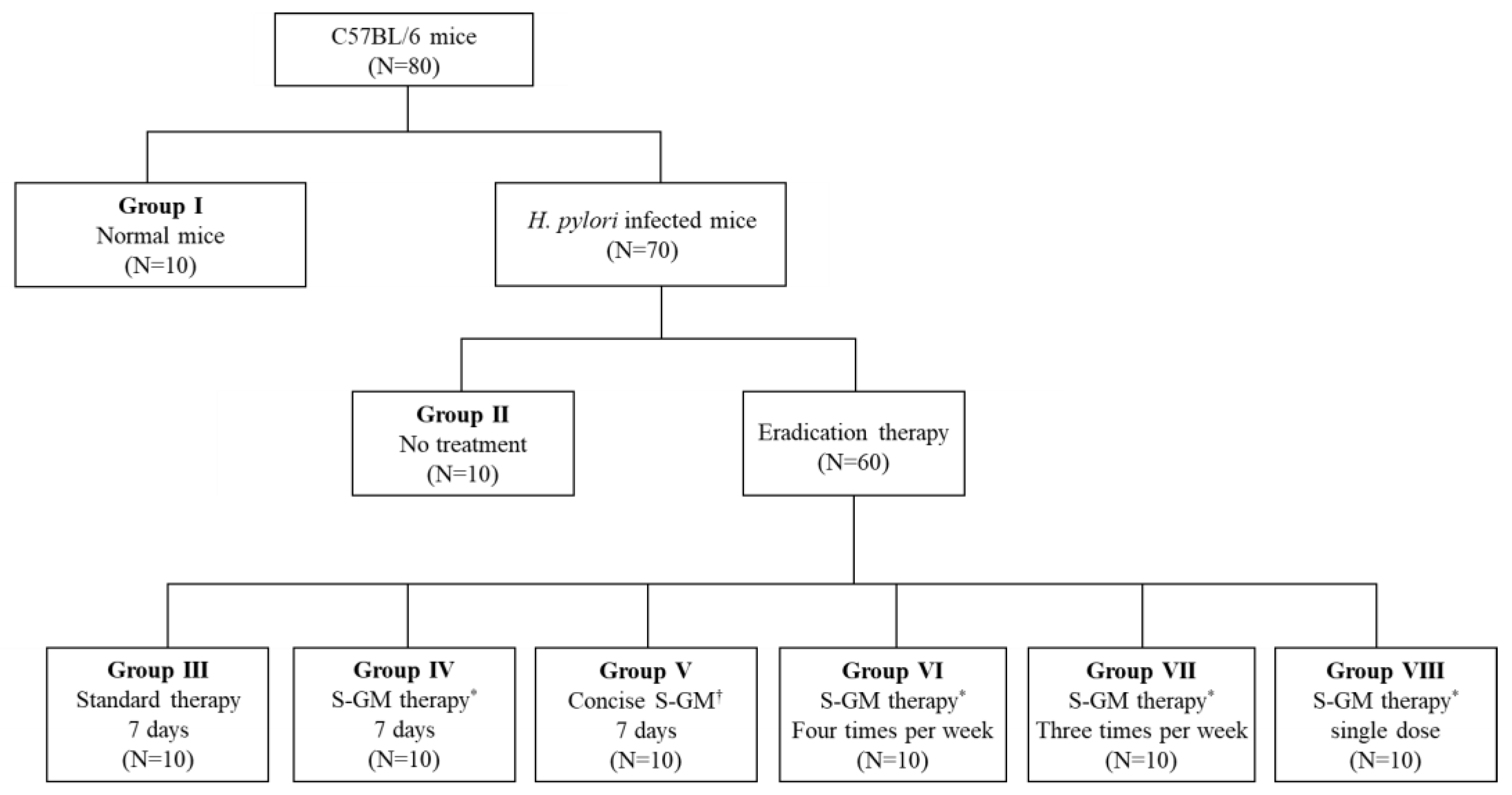
4.3.3. Distribution of Animals
A total of 80 mice were used for analysis, and 70 H. pylori-infected mice were distributed into seven groups and allowed to rest for 1 week after the last inoculation. Group I was a normal group consisting of uninfected mice. Group II, a negative control group, received distilled water as a vehicle. Group III, a positive control group, was treated with the standard triple therapy consisting of amoxicillin (14.25 mg/kg), clarithromycin (14.3 mg/kg), and a PPI (omeprazole 138 mg/kg). Group IV was treated with amoxicillin (14.25 mg/kg), S-GM (which emitted 8 mg/kg of GM), and a PPI (138 mg/kg). Group V was treated with S-GM (which emitted 8 mg/kg of GM) and a PPI (138 mg/kg). Groups V to VIII were treated with the same regimen as that of Group IV, but with different administration intervals of four times per week, three times per week, and a single dose per week, respectively. In Groups I to IV, the treatments were orally administered to mice once a day for 7 consecutive days (Figure 4). The H. pylori immunoglobulin G (IgG) level was checked with an enzyme-linked immunosorbent assay kit (Cusabio Biotech Co., USA) before the treatment period to confirm the serological status of H. pylori-infected mice.
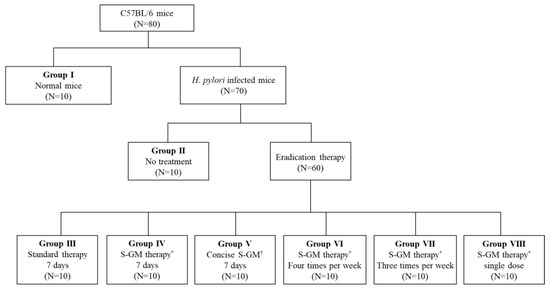
Figure 4.
Flow chart for study design. * S-GM therapy, amoxicillin (14.25 mg/kg), gentamicin-intercalated smectite hybrid (S-GM) which emitted 8 mg/kg of gentamicin and omeprazole (138 mg/kg); † Concise S-GM therapy, gentamicin-intercalated smectite hybrid (S-GM) which emitted 8 mg/kg of gentamicin and omeprazole (138 mg/kg).
4.3.4. CLO Test and PCR Assay of H. pylori in Gastric Mucosa
At 12 h after the last administration, mice were euthanized, and their stomachs were removed from their abdominal cavities. Samples of gastric mucosa from the pyloric region were assayed with CLO kits (Asan Pharmaceutical Co., Seoul, Korea) and incubated at 37 °C for 12 h to examine urease activity. The reaction score was graded from 0 to 3 with 0 = no color change, 1 = bright red, 2 = light purple, and 3 = dark red. The CLO test, also called the rapid urease test, is a rapid diagnostic test for the diagnosis of H. pylori based on the ability of urease secretion, and the color change of dark red means positive for H. pylori.
H. pylori DNA was prepared using the bead beater-phenol extraction method [28]. A bacterial suspension was placed in a 2.0-mL screw-cap microcentrifuge tube filled with glass beads (Biospec Products, Bartlesville, OK, USA) and 200 μL of phenol:chloroform:isoamyl alcohol solution (50:49:1). After an initial denaturation/activation step (95 °C for 5 min), DNA (50 ng) was amplified in a 20-μL volume for 35 cycles of denaturation (94 °C for 60 s), annealing (62 °C for 60 s), and extension (72 °C for 90 s) using the following primers: H. pylori-specific ureA and ureC, sense, 5′-TGATGCTCCACTACGCTGGA-3′, and antisense, 5′-GGGTATGCACGGTTACGAGT-3′ (expected product 265 bp); [29] and GAPDH, sense, 5′-TGGGGTGATGCTGGTGCTG-AG-3′, and antisense, 5′-GGTTTCTCCAGGCGGCATGTC-3′ (expected product 497 bp) [30]. The PCR products were analyzed by electrophoresis in 1.5% agarose gels.
4.3.5. Pro-Inflammatory Cytokines and Atrophy of Gastric Mucosa
Plasma was obtained on day 21 through the insertion of a heparinized microhematocrit tube into the ophthalmic venous plexus of mice. Plasma IL-8 and TNF-α levels were measured using mouse ELISA kits (R&D System, Minneapolis, MN, USA).
For histopathologic analysis, the stomach was fixed in 10% neutralized buffered formalin, and embedded in paraffin. Sections (4-μm thick) were then stained with hematoxylin and eosin. The glandular mucosae of the corpus and antrum were examined histologically. Atrophic changes, as defined by atrophy of glandular cells and hyperplasia of mucus cells, were determined in a blinded fashion and scored based on the percentage of altered gastric mucosa [31]: 0 = no mucosal alterations; 1 = less than 5%; 2 = 10% to 25%; 3 = 25% to 50%; 4 = 50% to 75%.
4.4. Fecal Microbiota
4.4.1. DNA Extraction from Fecal Materials
After examination of IgG level post-treatment, the mice were sacrificed. Their feces were collected from each group and frozen at −80 °C until processed. From the fecal materials of each mouse group, DNA was extracted using the FastDNA® SPIN Kit (MP Biomedicals, Solon, OH, USA). The samples were lysed with FastPrep® Instruments and centrifuged, and DNA was isolated from the supernatant using the procedure of silica-based GENECLEAN® and SPIN filters (MP Biomedicals, Solon, OH, USA) [32].
4.4.2. PCR Amplification and 16S rRNA Gene Sequencing
Using the extracted metagenomic DNA as a template, PCR was performed for amplification of the V3–V4 regions of the bacterial 16S rRNA gene using the primers 341F (5′-TCGTCGGCAGCGTC-AGATGTGTATAAGAGACAG-CCTACGGGNGGCWGCAG-3′; the underlined sequence indicates the target region primer) and 805R (5′-GTCTCGTGGGCTCGG-AGATGTGTATAAGAGACAG-ACTACHVGGGTATCTAATCC-3′). Next, secondary amplification for attachment of the Illumina NexTera barcode was performed using the following primers (X indicates the barcode region): i5 forward primer, 5′-AATGATACGGCGACCACCGAGATCTACAC-XXXXXXXX-TCGTCGGCAGCGTC-3′; and i7 reverse primer, 5′-CAAGCAGAAGACGGCATACGAGAT-XXXXXXXX-AGTCTCGTGGGCTCGG-3′. The PCR products were identified via 1% agarose gel electrophoresis and visualized in a Gel Doc system (BioRad, Hercules, CA, USA).
After purification of the amplified products using Clean PCR (CleanNA, Waddinxveen, The Netherlands), qualified products were assessed on a Bioanalyzer 2100 (Agilent, Palo Alto, CA, USA). The libraries were prepared for analysis, and gene sequencing was performed using an Illumina MiSeq Sequencing System (Illumina, San Diego, CA, USA) according to the manufacturer’s instructions.
4.4.3. Bioinformatics for Microbiota Analysis
The EzBioCloud 16S rRNA database (https://www.ezbiocloud.net) operated by ChunLab (ChunLab, Inc., Seoul, Korea) was used as a bioinformatics cloud platform for accurate pairwise and taxonomic assignments. Chimeric reads were filtered on reads with <97% similarity based on the UCHIME algorithm [33], and operational taxonomic units (OTU)s with single and un-clustered reads are omitted from further analysis. Alpha diversity, which measures the diversity and abundance of bacterial species, was analyzed by the Shannon and Chao1 index. As for diversity, it is necessary to take into account not only qualitative amounts of species but also the abundance of species. Shannon index measures the number of different species per sample defined as abundance. In addition, the Chao 1 index qualitatively measures alpha diversity, giving more weight to rare species [34]. The Wilcoxon rank-sum test was used to examine differences in the number of OTUs. Beta diversity shows a compositional difference between microbial communities, and it was measured using the unweighted UniFrac method. The results were presented by PCoA, it was visualized as a two-dimensional scatter plot to reveal the microbial compositional differences between samples [35].
4.5. Statistical Analysis
Data are presented as means ± standard error, and the non-parametric Mann–Whitney test was used to compare groups. Multiple differences between groups were evaluated using one-way analysis of variance (ANOVA) multiple comparison test. The 95% confidential interval (CI) of the detection rate was obtained using the MINITAB statistical software (Minitab, Inc., State College, PA, USA). If two values were not overlapped between its 95% CI, the difference was considered significant. A p-value of <0.05 was considered statistically significant. Results were analyzed using the Statistics Package for Social Science (SPSS 15.0 for Windows; SPSS Inc., Chicago, IL, USA).
5. Conclusions
The eradication rate of H. pylori showed a decreasing trend due to antibiotic resistance, especially clarithromycin. Therefore, we made a smectite hybrid as a drug delivery system using an aminoglycoside antibiotic, gentamicin, and applied it to the mouse stomach wall to confirm the localized therapeutic effect and set the different treatment duration to verify the effect. As a result, it was confirmed that the therapeutic efficacy of S-GM was not inferior to the existing standard triple therapy, based on amoxicillin and clarithromycin, and preserved the diversity of gut microbiome composition. Therefore, an S-GM treatment is expected to be a new alternative regimen to H. pylori infection.
Author Contributions
S.J.J., K.H.L., J.-H.K., and Y.G.S. conceived and designed the research; S.Y.P. performed the experiments; J.-H.K. and Y.G.S. analyzed the data; S.J.J. and K.H.L. wrote the draft; Y.G.S. was involved in the review and editing of the final manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Basic Research Project (Study No. GP2017-020) of the Korea Institute of Geoscience and Mineral Resources (KIGAM), funded by the Ministry of Science, ICT, and Future Planning of Korea. This work was also supported by the Seyoung Association (SYH) research grant from the Department of Internal Medicine, Gangnam Severance Hospital (2017-GNS-001).
Acknowledgments
We thank Myoung Ju Choi, Kwang Hoon Lee, and Jae Min Kim for helpful research support and discussions.
Conflicts of Interest
All authors report no potential conflicts of interest.
Abbreviations
| ANOVA | Analysis of variance |
| CLO | Campylobacter-like organism test |
| DNA | Deoxyribonucleic acid |
| ELISA | Enzyme-linked immunosorbent assay |
| GM | Gentamicin |
| H. pylori | Helicobacter pylori |
| IgG | Immunoglobulin G |
| IL-6 | Interleukin-6 |
| OUT | Operational taxonomic units |
| PCoA | Principal coordinates analysis |
| PCR | Polymerase chain reaction |
| PD | Pharmacodynamics |
| PK | Pharmacokinetics |
| PPI | Proton pump inhibitor |
| RNA | Ribonucleic acid |
| S-GM | Gentamicin-intercalated smectite hybrid |
| TNF-α | Tumor necrosis factor-α |
References
- Coussens, L.M.; Werb, Z. Inflammation and cancer. Nature 2002, 420, 860–867. [Google Scholar] [CrossRef] [PubMed]
- Kusters, J.G.; van Vliet, A.H.; Kuipers, E.J. Pathogenesis of Helicobacter pylori infection. Clin. Microbiol. Rev. 2006, 19, 449–490. [Google Scholar] [CrossRef] [PubMed]
- Malfertheiner, P.; Megraud, F.; O’Morain, C.A.; Gisbert, J.P.; Kuipers, E.J.; Axon, A.T.; Bazzoli, F.; Gasbarrini, A.; Atherton, J.; Graham, D.Y.; et al. Management of Helicobacter pylori infection-the maastricht v/florence consensus report. Gut 2017, 66, 6–30. [Google Scholar] [CrossRef] [PubMed]
- O’Morain, N.R.; Dore, M.P.; O’Connor, A.J.; Gisbert, J.P.; O’Morain, C.A. Treatment of h elicobacter pylori infection in 2018. Helicobacter 2018, 23, e12519. [Google Scholar]
- Kaakoush, N.O.; Asencio, C.; Mégraud, F.; Mendz, G.L. A redox basis for metronidazole resistance in Helicobacter pylori. Antimicrob. Agents Chemother. 2009, 53, 1884–1891. [Google Scholar] [CrossRef]
- Song, Z.; Zhang, J.; He, L.; Chen, M.; Hou, X.; Li, Z.; Zhou, L. Prospective multi-region study on primary antibiotic resistance of Helicobacter pylori strains isolated from chinese patients. Dig. Liver Dis. 2014, 46, 1077–1081. [Google Scholar] [CrossRef]
- Lee, K.H.; Park, S.Y.; Jeong, S.J.; Jung, D.H.; Kim, J.-H.; Jeong, S.H.; Kang, I.-M.; Song, Y.G. Can aminoglycosides be used as a new treatment for Helicobacter pylori? In Vitro activity of recently isolated Helicobacter pylori. Infect. Chemother. 2019, 51, 10–20. [Google Scholar] [CrossRef]
- Cox, C.E. Gentamicin. Med. Clin. N. Am. 1970, 54, 1305–1315. [Google Scholar] [CrossRef]
- Recchia, J.; Lurantos, M.H.; Amsden, J.A.; Storey, J.; Kensil, C.R. A semisynthetic quillaja saponin as a drug delivery agent for aminoglycoside antibiotics. Pharm. Res. 1995, 12, 1917–1923. [Google Scholar] [CrossRef]
- Thomas, F.; Michot, L.J.; Vantelon, D.; Montarges, E.; Prelot, B.; Cruchaudet, M.; Delon, J.-F. Layer charge and electrophoretic mobility of smectites. Colloids Surf. A Physicochem. Eng. Asp. 1999, 159, 351–358. [Google Scholar] [CrossRef]
- Jeong, S.J.; Kim, J.; Jung, D.H.; Lee, K.H.; Park, S.Y.; Kang, I.; Song, Y.G. Gentamicin-intercalated smectite as a new therapeutic option for Helicobacter pylori eradication. J. Antimicrob. Chemother. 2018, 73, 1324–1329. [Google Scholar]
- Nicholson, J.K.; Holmes, E.; Kinross, J.; Burcelin, R.; Gibson, G.; Jia, W.; Pettersson, S. Host-gut microbiota metabolic interactions. Science 2012, 336, 1262–1267. [Google Scholar] [CrossRef]
- Lozupone, C.A.; Stombaugh, J.I.; Gordon, J.I.; Jansson, J.K.; Knight, R. Diversity, stability and resilience of the human gut microbiota. Nature 2012, 489, 220–230. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.L.; Vieira Silva, S.; Liston, A.; Raes, J. How informative is the mouse for human gut microbiota research? Dis. Models Mech. 2015, 8, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Moodley, Y.; Linz, B.; Bond, R.P.; Nieuwoudt, M.; Soodyall, H.; Schlebusch, C.M.; Bernhöft, S.; Hale, J.; Suerbaum, S.; Mugisha, L.; et al. Age of the association between Helicobacter pylori and man. PLoS Pathog. 2012, 8, e1002693. [Google Scholar] [CrossRef] [PubMed]
- Warren, J.R.; Marshall, B. Unidentified curved bacilli on gastric epithelium in active chronic gastritis. Lancet 1983, 1, 1273–1275. [Google Scholar] [PubMed]
- Miftahussurur, M.; Syam, A.F.; Nusi, I.A.; Makmun, D.; Waskito, L.A.; Zein, L.H.; Akil, F.; Uwan, W.B.; Simanjuntak, D.; Wibawa, I.D.N.; et al. Surveillance of Helicobacter pylori antibiotic susceptibility in indonesia: Different resistance types among regions and with novel genetic mutations. PLoS ONE 2016, 11, e0166199. [Google Scholar] [CrossRef]
- Ontsira Ngoyi, E.N.; Atipo Ibara, B.I.; Moyen, R.; Ahoui Apendi, P.C.; Ibara, J.R.; Obengui, O.; Ossibi Ibara, R.B.; Nguimbi, E.; Niama, R.F.; Ouamba, J.M.; et al. Molecular detection of Helicobacter pylori and its antimicrobial resistance in brazzaville, congo. Helicobacter 2015, 20, 316–320. [Google Scholar] [CrossRef]
- De Bortoli, N.; Leonardi, G.; Ciancia, E.; Merlo, A.; Bellini, M.; Costa, F.; Mumolo, M.G.; Ricchiuti, A.; Cristiani, F.; Santi, S.; et al. Helicobacter pylori eradication: A randomized prospective study of triple therapy versus triple therapy plus lactoferrin and probiotics. Am. J. Gastroenterol. 2007, 102, 951–956. [Google Scholar] [CrossRef]
- Yang, J.; Lin, C.; Wang, H.; Chen, J.; Kao, J.Y.; Shun, C.; Lu, C.; Lin, B.; Shieh, M.; Chang, M.; et al. High-dose dual therapy is superior to standard first-line or rescue therapy for Helicobacter pylori infection. Clin. Gastroenterol. Hepatol. 2015, 13, 895–905. [Google Scholar] [CrossRef]
- Oh, B.; Kim, J.S.; Koh, S.; Kim, B.G.; Lee, K.L.; Chun, J. The effect of probiotics on gut microbiota during the Helicobacter pylori eradication: Randomized controlled trial. Helicobacter 2016, 21, 165–174. [Google Scholar] [CrossRef] [PubMed]
- Tønnesen, H.H.; Karlsen, J. Alginate in drug delivery systems. Drug Dev. Ind. Pharm. 2002, 28, 621–630. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, R.M.; Pereira Marques, J.; Pinto Ribeiro, I.; Costa, J.L.; Carneiro, F.; Machado, J.C.; Figueiredo, C. Gastric microbial community profiling reveals a dysbiotic cancer-associated microbiota. Gut 2018, 67, 226–236. [Google Scholar] [CrossRef]
- Jo, H.J.; Kim, N.; Park, J.H.; Nam, R.H.; Seok, Y.; Kim, Y.; Kim, J.M.; Lee, D.H.; Jung, H.C. Analysis of gastric microbiota by pyrosequencing: Minor role of bacteria other than Helicobacter pylori in the gastric carcinogenesis. Helicobacter 2016, 21, 364–374. [Google Scholar] [CrossRef]
- Jakobsson, H.E.; Jernberg, C.; Andersson, A.F.; Sjölund Karlsson, M.; Jansson, J.K.; Engstrand, L. Short-term antibiotic treatment has differing long-term impacts on the human throat and gut microbiome. PLoS ONE 2010, 5, e9836. [Google Scholar] [CrossRef] [PubMed]
- Shi, Z.; Zou, J.; Zhang, Z.; Zhao, X.; Noriega, J.; Zhang, B.; Zhao, C.; Ingle, H.; Bittinger, K.; Mattei, L.M.; et al. Segmented filamentous bacteria prevent and cure rotavirus infection. Cell 2019, 179, 644–658. [Google Scholar] [CrossRef]
- Robertson, S.J.; Lemire, P.; Maughan, H.; Goethel, A.; Turpin, W.; Bedrani, L.; Guttman, D.S.; Croitoru, K.; Girardin, S.E.; Philpott, D.J. Comparison of co-housing and littermate methods for microbiota standardization in mouse models. Cell Rep. 2019, 27, 1910–1919. [Google Scholar] [CrossRef]
- Kim, B.-J.; Lee, S.-H.; Lyu, M.-A.; Kim, S.-J.; Bai, G.-H.; Kim, S.-J.; Chae, G.-T.; Kim, E.-C.; Cha, C.-Y.; Kook, Y.-H. Identification of mycobacterial species by comparative sequence analysis of the rna polymerase gene (rpob). J. Clin. Microbiol. 1999, 37, 1714–1720. [Google Scholar] [CrossRef]
- Kim, Y.B.; Kim, S.T.; Lee, S.W.; Jeen, Y.T.; Chun, H.J.; Lee, H.S.; Song, C.W.; Um, S.H.; Choi, J.H.; Kim, C.D. The influence of number of gastroscopic biopsy specimens on follow-up campylobacter-like organism (clo) test. Korean J. Gastroenterol. 2000, 35, 422–428. [Google Scholar]
- Kundu, P.; Mukhopadhyay, A.K.; Patra, R.; Banerjee, A.; Berg, D.E.; Swarnakar, S. Cag pathogenicity island-independent up-regulation of matrix metalloproteinases-9 and -2 secretion and expression in mice by Helicobacter pylori infection. J. Biol. Chem. 2006, 281, 34651–34662. [Google Scholar] [CrossRef]
- Fox, J.G.; Beck, P.; Dangler, C.A.; Whary, M.T.; Wang, T.C.; Shi, H.N.; Nagler Anderson, C. Concurrent enteric helminth infection modulates inflammation and gastric immune responses and reduces helicobacter-induced gastric atrophy. Nat. Med. 2000, 6, 536–542. [Google Scholar] [CrossRef]
- Layton, A.; McKay, L.; Williams, D.; Garrett, V.; Gentry, R.; Sayler, G. Development of bacteroides 16s rrna gene taqman-based real-time pcr assays for estimation of total, human, and bovine fecal pollution in water. Appl. Environ. Microbiol. 2006, 72, 4214–4224. [Google Scholar] [CrossRef] [PubMed]
- Edgar, R.C.; Haas, B.J.; Clemente, J.C.; Quince, C.; Knight, R. Uchime improves sensitivity and speed of chimera detection. Bioinformatics 2011, 27, 2194–2200. [Google Scholar] [CrossRef] [PubMed]
- Willis, A.D. Rarefaction, alpha diversity, and statistics. Front. Microbiol. 2019, 10, 2407. [Google Scholar] [CrossRef]
- Lin, J. Divergence measures based on the shannon entropy. IEEE Trans. Inf. Theory 1991, 37, 145–151. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).