X-ray Photoelectron Spectroscopy Analysis of Nitrogen-Doped TiO2 Films Prepared by Reactive-Ion-Beam Sputtering with Various NH3/O2 Gas Mixture Ratios
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Coated Surfaces
2.2. Investigation of Samples
3. Results and Discussion
3.1. XRD of N-Doped TiO2 Films
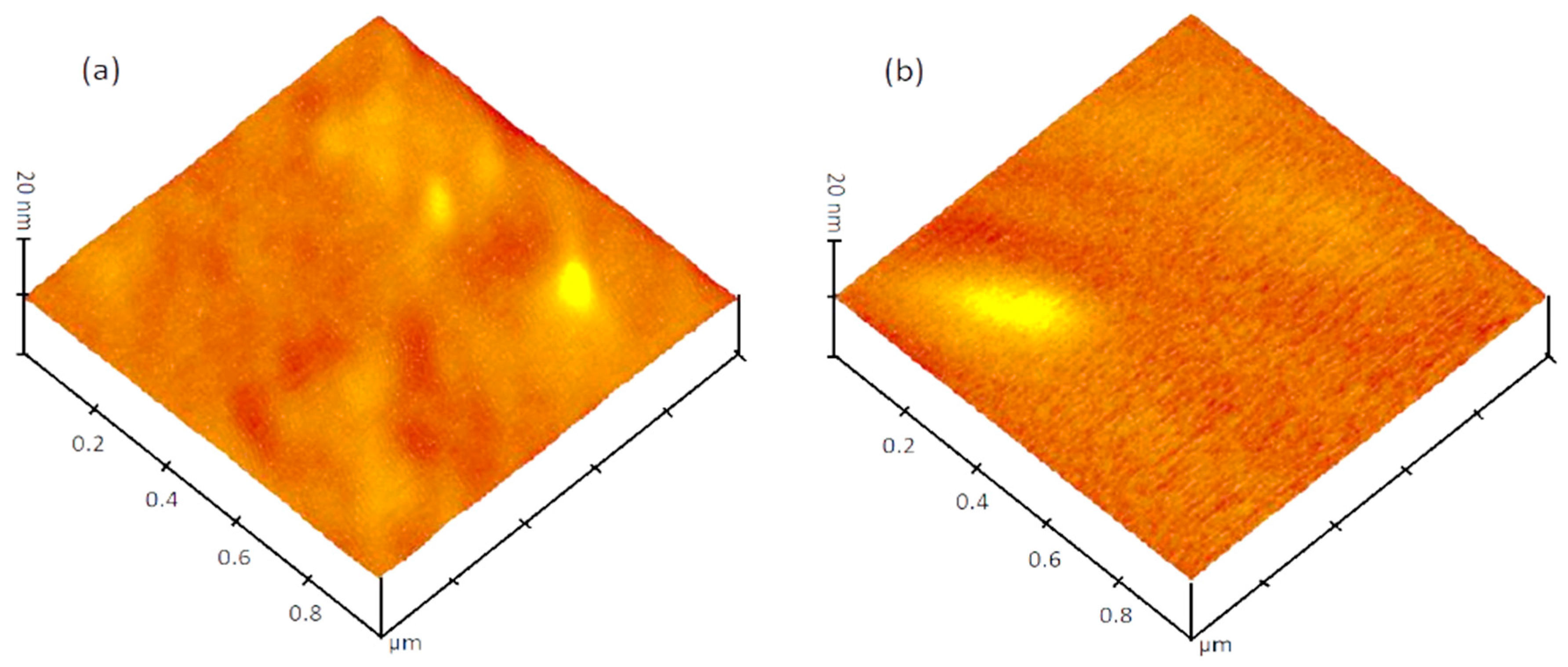
3.2. Surface Morphology of N-Doped TiO2 Films
3.3. FTIR Spectra of N-Doped TiO2 Films
3.4. X-ray Photoelectron Spectra of N-Doped TiO2 Films
3.4.1. O1s XPS Spectra
3.4.2. X-ray Photoelectron Spectroscopy of Ti
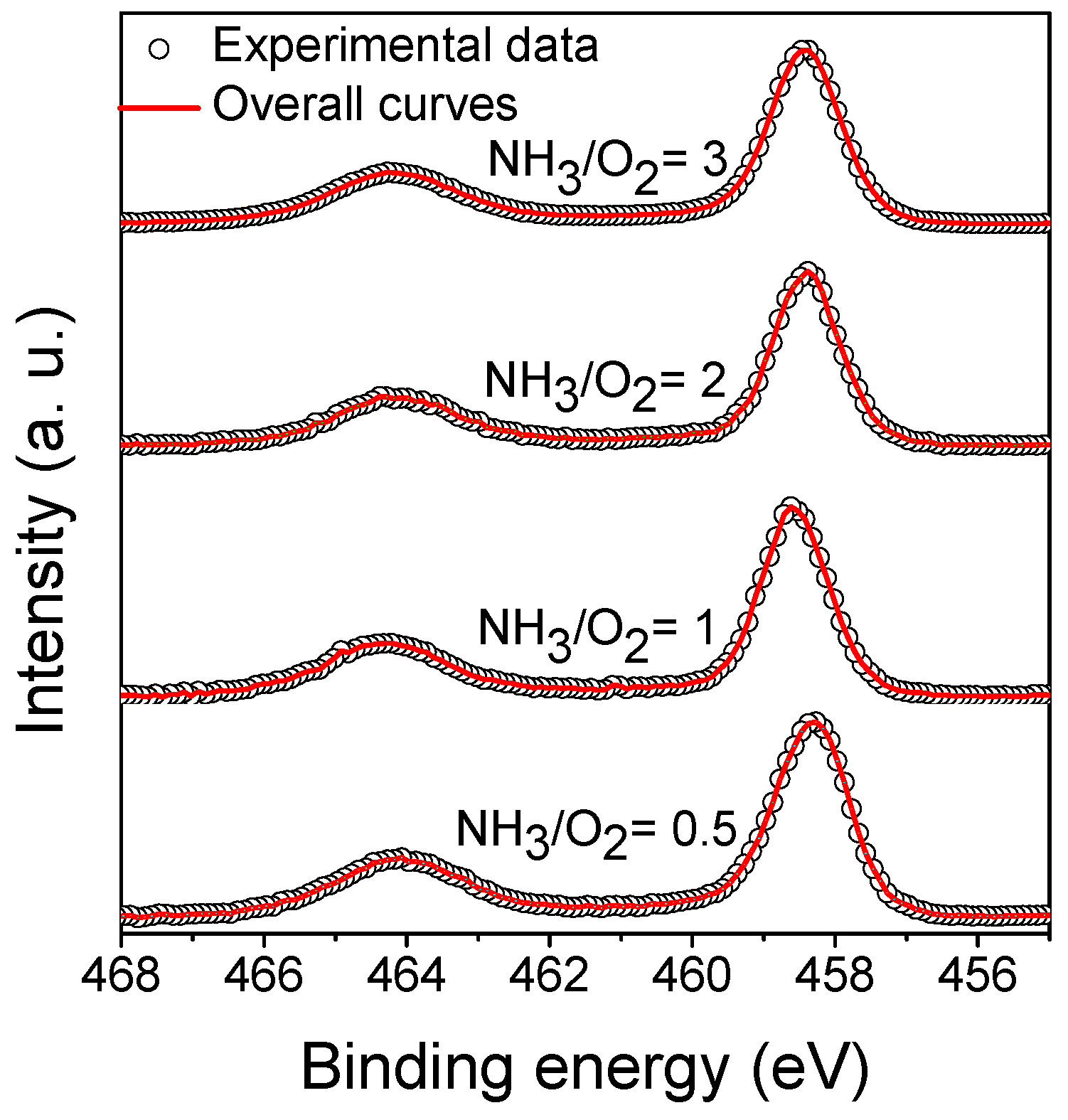
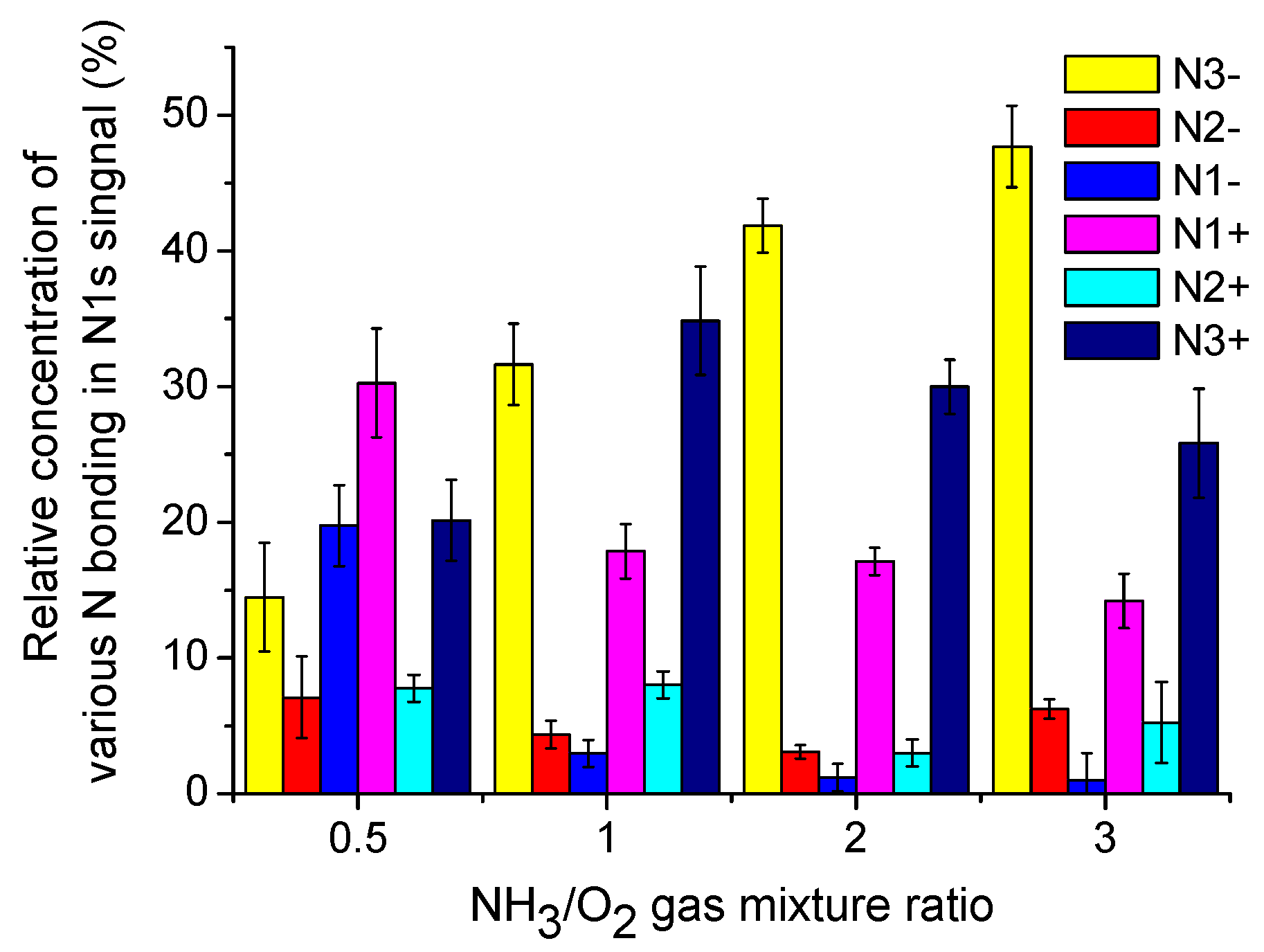
3.4.3. XPS of N
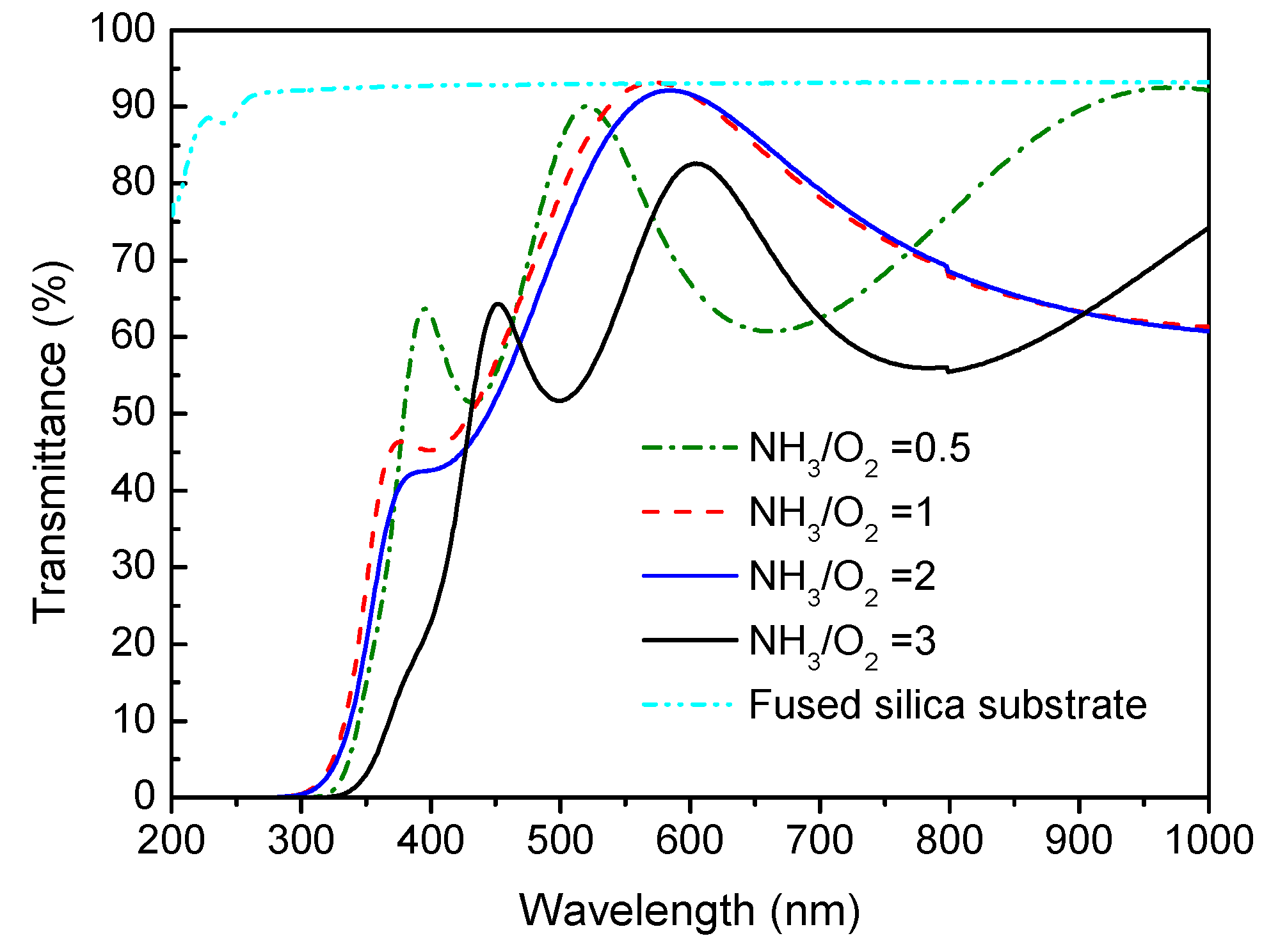
3.5. Optical UV-Vis Transmittance of Nitrogen-Doped TiO2 Films
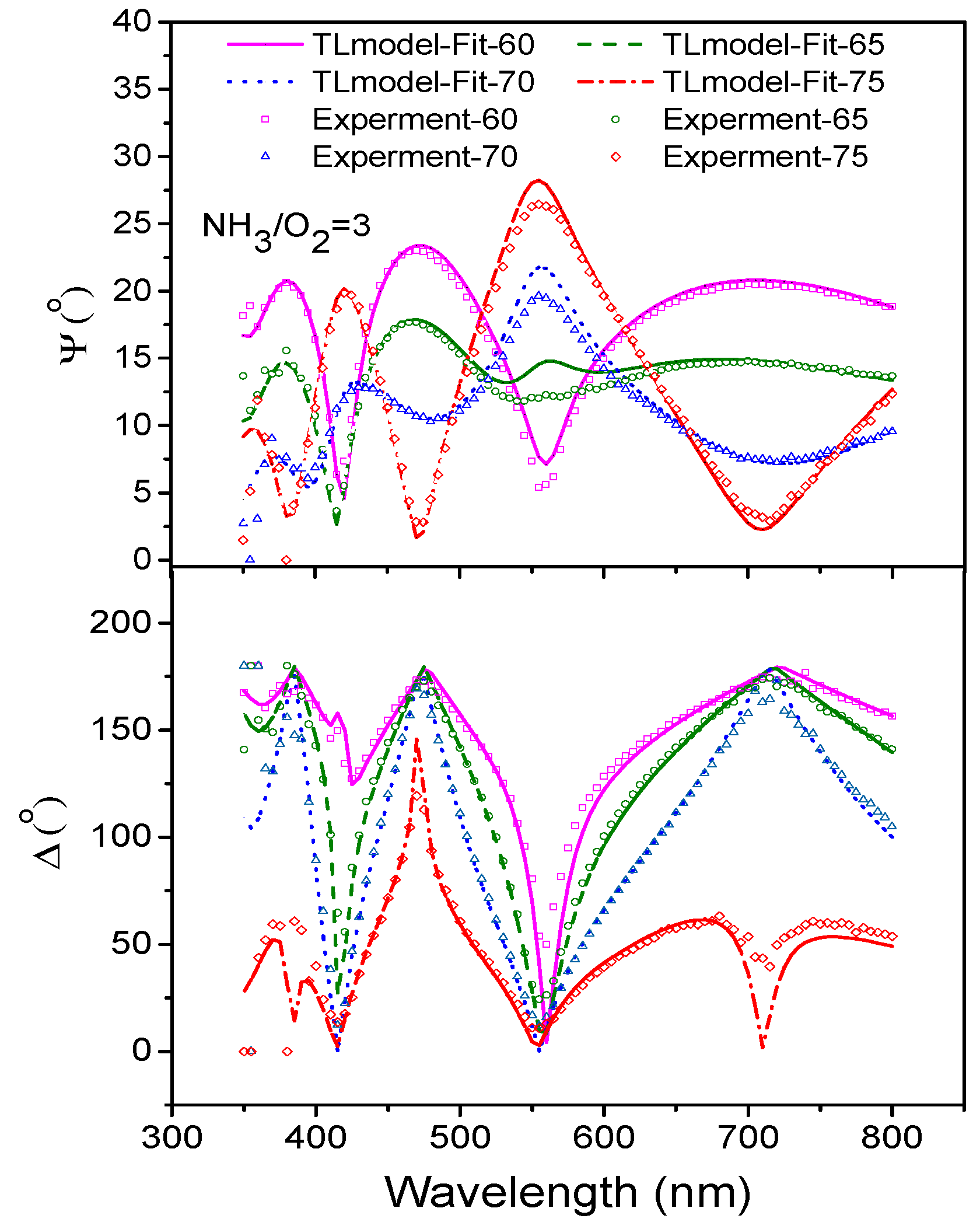
3.6. Ellipsometry of Nitrogen-Doped TiO2 Films
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Zaleska, A. Doped-TiO2: A review. Recent Pat. Eng. 2008, 2, 157–164. [Google Scholar] [CrossRef]
- Asahi, R.; Morikawa, T.; Ohwaki, T.; Aoki, K.; Taga, Y. Visible-light photocatalysis in nitrogen-doped titanium dioxide. Science 2001, 293, 269–271. [Google Scholar] [CrossRef] [PubMed]
- Irie, H.; Watanabe, Y.; Hashimoto, K. Nitrogen-concentration dependence on photocatalytic activity of TiO2−xNx powders. J. Phys. Chem. B 2003, 107, 5483–5486. [Google Scholar] [CrossRef]
- Zhao, Z.; Liu, Q. Mechanism of higher photocatalytic activity of anatase TiO2 doped with nitrogen under visible-light irradiation from density functional theory calculation. J. Phys. D Appl. Phys. 2007, 41, 025105. [Google Scholar] [CrossRef]
- Ihara, T.; Miyoshi, M.; Iriyama, Y.; Matsumoto, O.; Sugihara, S. Visible-light-active titanium oxide photocatalyst realized by an oxygen-deficient structure and by nitrogen doping. Appl. Catal. B Environ. 2003, 42, 403–409. [Google Scholar] [CrossRef]
- Kusano, D.; Emori, M.; Sakama, H. Influence of electronic structure on visible light photocatalytic activity of nitrogen-doped TiO2. RSC Adv. 2017, 7, 1887–1898. [Google Scholar] [CrossRef] [Green Version]
- Chen, H.; Dawson, J.A. Nature of nitrogen-doped anatase TiO2 and the origin of its visible-light activity. J. Phys. Chem. C 2015, 119, 15890–15895. [Google Scholar] [CrossRef]
- Livraghi, S.; Chierotti, M.R.; Giamello, E.; Magnacca, G.; Paganini, M.C.; Cappelletti, G.; Bianchi, C.L. Nitrogen-doped titanium dioxide active in photocatalytic reactions with visible light: A multi-technique characterization of differently prepared materials. J. Phys. Chem. C 2008, 112, 17244–17252. [Google Scholar] [CrossRef]
- Sakthivel, S.; Kisch, H. Photocatalytic and photoelectrochemical properties of nitrogen-doped titanium dioxide. Chem. Phys. Chem. 2003, 4, 487–490. [Google Scholar] [CrossRef]
- Bouhadoun, S.; Guillard, C.; Sorgues, S.; Hérissan, A.; Colbeau-Justin, C.; Dapozze, F.; Habert, A.; Maurl, V.; Herlin-Boime, N. Laser synthesized TiO2-based nanoparticles and their efficiency in the photocatalytic degradation of linear carboxylic acids. Sci. Technol. Adv. Mater. 2017, 18, 805–815. [Google Scholar] [CrossRef] [Green Version]
- Youssef, L.; Leoga, A.J.K.; Roualdes, S.; Bassil, J.; Zakhour, M.; Rouessac, V.; Ayral, A.; Nakhl, M. Optimization of N-doped TiO2 multifunctional thin layers by low frequency PECVD process. J. Eur. Ceram. Soc. 2017, 37, 5289–5303. [Google Scholar] [CrossRef]
- Wang, J.; Tafen, D.N.; Lewis, J.P.; Hong, Z.; Manivannan, A.; Zhi, M.; Li, M.; Wu, N. Origin of photocatalytic activity of nitrogen-doped TiO2 nanobelts. J. Am. Chem. Soc. 2009, 131, 12290–12297. [Google Scholar] [CrossRef] [PubMed]
- Miyauchi, M.; Ikezawa, A.; Tobimatsu, H.; Irie, H.; Hashimoto, K. Zeta potential and photocatalytic activity of nitrogen doped TiO2 thin films. Phys. Chem. Chem. Phys. 2004, 6, 865–870. [Google Scholar] [CrossRef]
- Kerr, J.A. A ready-reference book of chemical and physical data. In CRC Handbook of Chemistry and Physics, 1st ed.; Lide, D.R., Ed.; CRC Press: Florida, FL, USA, 2000. [Google Scholar]
- Lee, C.C.; Hsu, J.C.; Wong, D.H. The characteristics of some metallic oxides prepared in high vacuum by ion beam sputtering. Appl. Surf. Sci. 2001, 171, 151–156. [Google Scholar] [CrossRef]
- Jellison, G.E., Jr.; Modine, F.A. Parameterization of the optical functions of amorphous materials in the interband region. Appl. Phys. Lett. 1996, 69, 371–373. [Google Scholar] [CrossRef]
- Tauc, J.; Grigorovici, R.; Vancu, A. Optical properties and electronic structure of amorphous germanium. Phys. Status Solidi B 1966, 15, 627–637. [Google Scholar] [CrossRef]
- Forouhi, A.R.; Bloomer, I. Optical dispersion model relations for amorphous semiconductors and amorphous dielectrics. Phys. Rev. B 1986, 34, 7018–7026. [Google Scholar] [CrossRef]
- Woollam, J.A. Guide to Using WVASE 32; J. A. Woollam Co. Inc.: Nebraska, NE, USA, 2002. [Google Scholar]
- Selcuk, S.; Selloni, A. Facet-dependent trapping and dynamics of excess electrons at anatase TiO2 surfaces and aqueous interfaces. Nat. Mater. 2016, 15, 1107–1112. [Google Scholar] [CrossRef]
- Hsu, J.C.; Lee, C.C. Single- and dual-ion-beam sputter deposition of titanium oxide films. Appl. Opt. 1998, 37, 1171–1176. [Google Scholar] [CrossRef]
- Karunagaran, B.; Rajendra Kumar, R.T.; Mangalaraj, D.; Narayandass, S.K.; Mohan Rao, G. Influence of thermal annealing on the composition and structural parameters of DC magnetron sputtered titanium dioxide thin films. Cryst. Res. Technol. 2002, 37, 1285–1292. [Google Scholar] [CrossRef]
- Hsu, J.C.; Wang, P.W.; Lee, C.C. X-ray photoelectron spectroscopy study of thin TiO2 films cosputtered with Al. Appl. Opt. 2006, 45, 4303–4309. [Google Scholar] [CrossRef] [PubMed]
- Hsu, J.C.; Lee, C.C.; Chen, H.L.; Kuo, C.C.; Wang, P.W. Investigation of thin TiO2 films cosputtered with Si species. Appl. Surf. Sci. 2009, 255, 4852–4858. [Google Scholar] [CrossRef]
- Lee, C.C.; Wei, D.T.; Hsu, J.C.; Shen, C.H. Influence of oxygen on some oxide films prepared by ion beam sputter deposition. Thin Solid Films 1996, 290, 88–93. [Google Scholar] [CrossRef]
- Pore, V.J.; Okura, S.; Suemori, H. Process for Deposition of Titanium Oxynitride for Use in Integrated Circuit Fabrication. U.S. Patent No. 9,523,148, 20 December 2016. [Google Scholar]
- De Vietro, N.; Favia, P.; Fracassi, F.; d’Agostino, R. Acid/Base Gas/Liquid adsorption properties of carbon black modified in NH3/O2 RF glow discharges. Plasma Process. Polym. 2010, 7, 582–587. [Google Scholar] [CrossRef]
- Delegan, N.; Daghrir, R.; Drogui, P.; El Khakani, M.A. Bandgap tailoring of in-situ nitrogen-doped TiO2 sputtered films intended for electrophotocatalytic applications under solar light. J. Appl. Phys. 2014, 116, 153510. [Google Scholar] [CrossRef]
- Pai, P.G.; Chao, S.S.; Takagi, Y.; Lucovsky, G. Infrared spectroscopic study of SiOx films produced by plasma enhanced chemical vapor deposition. J. Vac. Sci. Technol. A 1986, 4, 689–694. [Google Scholar] [CrossRef] [Green Version]
- Shahzadi, P.; Muhammad, A.; Mehmood, F.; Chaudhry, M.Y. Synthesis of 3, 7-dimethyl-2, 6-octadienal acetals from citral extracted from lemon grass, cymbopogon citrates L. J. Antivir. Antiretrovir. 2014, 6, 028–031. [Google Scholar] [CrossRef] [Green Version]
- Abdullah, M.F.E.; Madzrol, N.; Sandin, R.W.P. Effects of biodiesel saturation degrees on NOx emission and FTIR spectroscopy. J. Appl. Sci. Proc. Eng. 2016, 3, 1. [Google Scholar] [CrossRef] [Green Version]
- Amores, J.G.; Escribano, V.S.; Ramis, G.; Busca, G. An FT-IR study of ammonia adsorption and oxidation over anatase-supported metal oxides. Appl. Catal. B Environ. 1997, 13, 45–58. [Google Scholar] [CrossRef]
- Hadjiivanov, K. FTIR study of CO and NH3 co-adsorption on TiO2 (rutile). Appl. Surf. Sci. 1998, 135, 331–338. [Google Scholar] [CrossRef]
- Wu, Y.T.; Yu, Y.H.; Nguyen, V.H.; Wu, J.C. In-situ FTIR spectroscopic study of the mechanism of photocatalytic reduction of NO with methane over Pt/TiO2 photocatalysts. Res. Chem. Intermed. 2015, 41, 2153–2164. [Google Scholar] [CrossRef]
- Wagner, C.; Riggs, W.; Davis, L.; Moulder, J.; Muilenberg, G. Handbook of X-Ray Photoelectron Spectroscopy; Perkin-Elmer Inc.: Massachusetts, MA, USA, 1979. [Google Scholar]
- Yu, Y.P.; Xing, X.J.; Xu, L.M.; Wu, S.X.; Li, S.W. N-derived signals in the X-ray photoelectron spectra of N-doped anatase TiO2. J. Appl. Phys. 2009, 105, 123535. [Google Scholar] [CrossRef]
- Zajac, A.T.; Radecka, M.; Zakrzewska, K.; Brudnik, A.; Kusior, E.; Bourgeois, S.; Marco de Lucas, M.C.; Imhoff, L. Structural and electrical properties of magnetron sputtered Ti(ON) thin films: The case of TiN doped in situ with oxygen. J. Power Sources 2009, 194, 93–103. [Google Scholar] [CrossRef]
- Lahoz, R.; Espinós, J.P.; Fuente, G.F.D.; Elipe, A.R.G. “in situ” XPS studies of laser induced surface cleaning and nitridation of Ti. Surf. Coat. Tech. 2008, 202, 1486–1492. [Google Scholar] [CrossRef]
- Panda, A.B.; Laha, P.; Harish, K.; Sarkar, B.; Chaure, S.V.; Sayyad, W.A.; Jadhav, V.S.; Kulkarni, G.R.; Sasmal, D.; Barhai, P.K.; et al. Study of bactericidal efficiency of magnetron sputtered TiO2 films deposited at varying oxygen partial pressure. Surf. Coat. Tech. 2010, 205.5, 1611–1617. [Google Scholar] [CrossRef]
- Jia, T.; Fu, F.; Yu, D.; Cao, J.; Sun, G. Facile synthesis and characterization of N-doped TiO2/C nanocomposites with enhanced visible-light photocatalytic performance. Appl. Surf. Sci. 2018, 430, 438–447. [Google Scholar] [CrossRef]
- Cheung, S.H.; Nachimuthu, P.; Joly, A.G.; Engelhard, M.H.; Bowman, M.K.; Chambers, S.A. N incorporation and electronic structure in N-doped TiO2 (110) rutile. Surf. Sci. 2007, 601, 1754. [Google Scholar] [CrossRef]
- Gartner, M.; Osiceanu, P.; Anastasescu, M.; Stoica, T.; Stoica, T.F.; Trapalis, C.; Todorova, N.; Lagoyannis, A. Investigation on the nitrogen doping of multilayered, porous TiO2 thin films. Thin Solid Films 2008, 516, 8184–8189. [Google Scholar] [CrossRef]
- Yamada, K.; Yamane, H.; Matsushima, S.; Nakamura, H.; Sonoda, T.; Miura, S.; Kumada, K. Photocatalytic activity of TiO2 thin films doped with nitrogen using a cathodic magnetron plasma treatment. Thin Solid Films 2008, 516, 7560–7564. [Google Scholar] [CrossRef]
- Balaceanu, M.; Braic, V.; Braic, M.; Kiss, A.; Zoita, C.N.; Vladescu, A.; Drob, P.; Vasilescu, C.; Dudu, D.; Muresanu, O. Structural, mechanical and corrosion properties of TiOxNy/ZrOxNy multilayer coatings. Surf. Coat. Technol. 2008, 202, 2384–2388. [Google Scholar] [CrossRef]
- Glaser, A.; Surnev, S.; Netzer, F.P.; Fateh, N.; Fontalvo, G.A.; Mitterer, C. Oxidation of vanadium nitride and titanium nitride coatings. Surf. Sci. 2007, 601, 1153–1159. [Google Scholar] [CrossRef]
- Jeyachandran, Y.L.; Venkatachalam, S.; Karunagaran, B.; Narayandass, S.K.; Mangalaraj, D.; Bao, C.Y.; Zhang, C.L. Bacterial adhesion studies on titanium, titanium nitride and modified hydroxyapatite thin films. Mater. Sci. Eng. C 2007, 27, 35–41. [Google Scholar] [CrossRef]
- Zhang, M.; Lin, G.; Dong, C.; Kim, K.H. Mechanical and optical properties of composite TiOxNy films prepared by pulsed bias arc ion plating. Curr. Appl. Phys. 2009, 9, S174–S178. [Google Scholar] [CrossRef]
- Braic, M.; Balaceanu, M.; Vladescu, A.; Kiss, A.; Braic, V.; Epurescu, G.; Dinescu, G.; Moldovan, A.; Birjega, R.; Dinescu, M. Preparation and characterization of titanium oxy-nitride thin films. Appl. Surf. Sci. 2007, 253, 8210–8214. [Google Scholar] [CrossRef]
- Chan, M.H.; Lu, F.H. Preparation of titanium oxynitride thin films by reactive sputtering using air/Ar mixtures. Surf. Coat. Technol. 2008, 203, 614–618. [Google Scholar] [CrossRef]
- Peng, F.; Cai, L.; Huang, L.; Yu, H.; Wang, H. Preparation of nitrogen-doped titanium dioxide with visible-light photocatalytic activity using a facile hydrothermal method. J. Phys. Chem. Solids 2008, 69, 1657–1664. [Google Scholar] [CrossRef]
- Yang, T.S.; Yang, M.C.; Shiu, C.B.; Chang, W.K.; Wong, M.S. Effect of N2 ion flux on the photocatalysis of nitrogen-doped titanium oxide films by electron-beam evaporation. Appl. Surf. Sci. 2006, 252, 3729–3736. [Google Scholar] [CrossRef]
- Mohamed, S.H.; Kappertz, O.; Niemeier, T.; Drese, R.; Wakkad, M.M.; Wuttig, M. Effect of heat treatment on structural, optical and mechanical properties of sputtered TiOxNy films. Thin Solid Films 2004, 468, 48–56. [Google Scholar] [CrossRef]
- Nakano, Y.; Morikawa, T.; Ohwaki, T.; Taga, Y. Deep-level optical spectroscopy investigation of N-doped TiO2 films. Appl. Phys. Lett. 2005, 86, 132104. [Google Scholar] [CrossRef]






| Oxidation States | N3+ | N2+ | N1+ | N1− | N2− | N3− | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TiON-0.5 | BE | 401.6 | 400.5 | 399.5 | 397.9 | 396.9 | 395.9 | ||||||
| Δ * | – | 1.0 | 1.1 | – | 1.1 | 1.0 | – | ||||||
| FWHM | 0.60 | 0.6 | 0.7 | 0.7 | 0.7 | 0.7 | |||||||
| TiON-1 | BE | 401.7 | 400.6 | 399.6 | 397.8 | 396.9 | 395.9 | ||||||
| Δ | – | 1.1 | 1.0 | – | 0.9 | 1.0 | – | ||||||
| FWHM | 0.6 | 0.6 | 0.7 | 0.5 | 0.6 | 0.5 | |||||||
| TiON-2 | BE | 401.7 | 400.6 | 399.6 | 397.8 | 396.9 | 395.9 | ||||||
| Δ | – | 1.1 | 1.0 | – | 0.9 | 1.0 | – | ||||||
| FWHM | 0.6 | 0.6 | 0.6 | 0.6 | 0.5 | 0.5 | |||||||
| TiON-3 | BE | 401.7 | 400.7 | 399.6 | – | 397.0 | 396.0 | ||||||
| Δ | – | 1.0 | 1.1 | – | – | 0.9 | – | ||||||
| FWHM | 0.6 | 0.6 | 0.6 | – | 0.5 | 0.5 | |||||||
| Average of BE | 401.7 | 400.6 | 399.6 | 397.8 | 396.9 | 395.9 | |||||||
| Oxidation States | Binding Energy (eV) | Bonding | Reference |
|---|---|---|---|
| N3− | 396 | N–Ti, N–Ti–O | [2,36,41] |
| N2− | 395.4, 395.7, 396, 396.3, 396.6, 396.9, 397.2, 397.5 | Ti–N–Ti–O, Ti–N–Ti–N, O–Ti–N (N3− substitute O2−) | [36,37,38,39,40,41,42,43,44,45,46,47,48,49,50] |
| N1−, N1+ | 398.7, 398.2, 399, 399.1 | N–O, N–Ti–O, N≡O (in TiOxNy) | [37,39,41,42,46,47,50] |
| N2+, N3+ | 399.8, 400, 400.2, 401.8, 402 | Ti–O–N–O | [41,43,44,47] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hsu, J.-C.; Lin, Y.-H.; Wang, P.W. X-ray Photoelectron Spectroscopy Analysis of Nitrogen-Doped TiO2 Films Prepared by Reactive-Ion-Beam Sputtering with Various NH3/O2 Gas Mixture Ratios. Coatings 2020, 10, 47. https://doi.org/10.3390/coatings10010047
Hsu J-C, Lin Y-H, Wang PW. X-ray Photoelectron Spectroscopy Analysis of Nitrogen-Doped TiO2 Films Prepared by Reactive-Ion-Beam Sputtering with Various NH3/O2 Gas Mixture Ratios. Coatings. 2020; 10(1):47. https://doi.org/10.3390/coatings10010047
Chicago/Turabian StyleHsu, Jin-Cherng, Yung-Hsin Lin, and Paul W. Wang. 2020. "X-ray Photoelectron Spectroscopy Analysis of Nitrogen-Doped TiO2 Films Prepared by Reactive-Ion-Beam Sputtering with Various NH3/O2 Gas Mixture Ratios" Coatings 10, no. 1: 47. https://doi.org/10.3390/coatings10010047
APA StyleHsu, J.-C., Lin, Y.-H., & Wang, P. W. (2020). X-ray Photoelectron Spectroscopy Analysis of Nitrogen-Doped TiO2 Films Prepared by Reactive-Ion-Beam Sputtering with Various NH3/O2 Gas Mixture Ratios. Coatings, 10(1), 47. https://doi.org/10.3390/coatings10010047






