Formation of Chitosan/Sodium Phytate/Nano-Fe3O4 Magnetic Coatings on Wood Surfaces via Layer-by-Layer Self-Assembly
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Cationic and Anionic Polyelectrolyte Solutions
2.3. Preparation of Positively-Charged Fe3O4 Nanoparticles Suspension
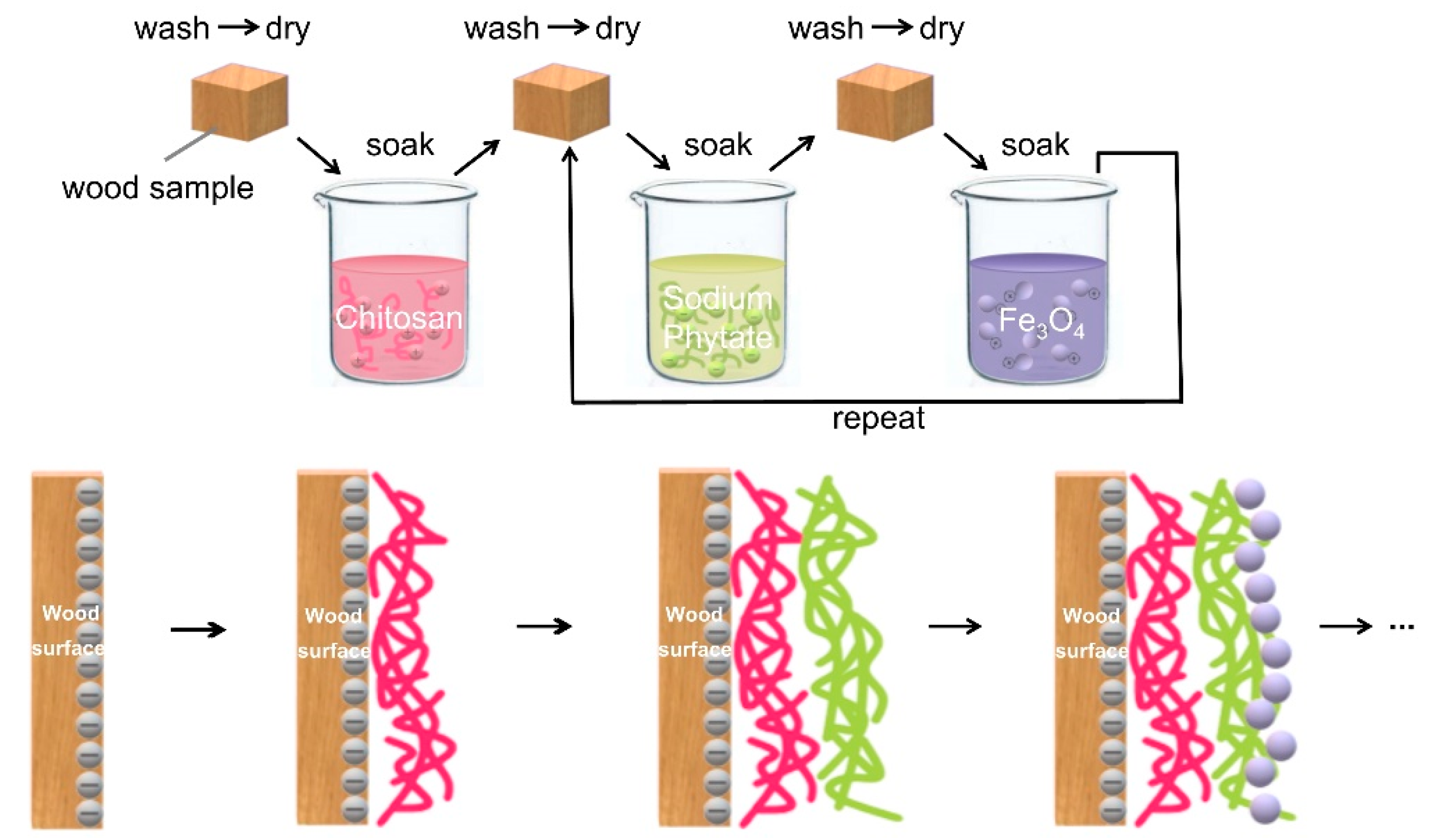
2.4. Formation of Magnetic Coatings on Wood Surfaces by Layer-by-Layer Self-Assembly
2.5. Characterization and Analysis
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Oka, H.; Narita, K.; Osada, H.; Seki, K. Experimental results on indoor electromagnetic wave absorber using magnetic wood. J. Appl. Phys. 2002, 91, 7008–7010. [Google Scholar] [CrossRef]
- Oka, H.; Tanaka, K.; Osada, H.; Kubota, K. Study of electromagnetic wave absorption characteristics and component parameters of laminated-type magnetic wood with stainless steel and ferrite powder for use as building materials. J. Appl. Phys. 2009, 105, 07E701. [Google Scholar] [CrossRef]
- Oka, H.; Terui, M.; Osada, H.; Sekino, N. Electromagnetic wave absorption characteristics adjustment method of recycled powder-type magnetic wood for use as a building material. IEEE Trans. Magn. 2012, 48, 3498–3500. [Google Scholar] [CrossRef]
- Gan, W.T.; Liu, Y.; Gao, L.K.; Zhan, X.X.; Li, J. Magnetic property, thermal stability, UV-resistance, and moisture absorption behavior of magnetic wood composites. Polym. Compos. 2015, 38, 1646–1654. [Google Scholar] [CrossRef]
- Gan, W.T.; Gao, L.K.; Sun, Q.F.; Jin, C.D.; Lu, Y.; Li, J. Multifunctional wood materials with magnetic, superhydrophobicand anti-ultraviolet properties. Appl. Surf. Sci. 2015, 332, 565–572. [Google Scholar] [CrossRef]
- Gan, W.T.; Xiao, S.L.; Gao, L.K.; Gao, R.N.; Li, J.; Zhan, X.X. Luminescent and transparent wood composites fabricated by poly(methyl methacrylate) and γ-Fe2O3@YVO4:Eu3+ nanoparticle impregnation. ACS Sustain. Chem. Eng. 2017, 5, 3855–3862. [Google Scholar] [CrossRef]
- Oka, H.; Hojo, A.; Osada, H.; Namizaki, Y.; Taniuchi, H. Manufacturing methods and magnetic characteristics of magnetic wood. J. Magn. Magn. Mater. 2004, 272–276, 2332–2334. [Google Scholar] [CrossRef]
- Oka, H.; Kataoka, Y.; Osada, H.; Aruga, Y.; Izumida, F. Experimental study on electromagnetic wave absorbing control of coating-type magnetic wood using a grooving process. J. Magn. Magn. Mater. 2007, 310, 1028–1029. [Google Scholar] [CrossRef]
- Oka, H.; Uchidata, S.; Sekino, N.; Namizaki, Y.; Taniuchi, H. Electromagnetic wave absorption characteristics of half carbonized powder-type magnetic wood. IEEE Trans. Magn. 2011, 47, 3078–3080. [Google Scholar] [CrossRef]
- Merk, V.; Chanana, M.; Gierlinger, N.; Hirt, A.M.; Burgert, I. Hybrid wood materials with magnetic anisotropy dictated by the hierarchical cell structure. ACS Appl. Mater. Interfaces 2014, 6, 9760–9767. [Google Scholar] [CrossRef]
- Segmehl, J.S.; Laromaine, A.; Keplinger, T.; Masnou, A.M.; Roig, A. Magnetic wood by in situ synthesis of iron oxide nanoparticles via a microwave-assisted route. J. Mater. Chem. C 2018, 6, 3395–3402. [Google Scholar] [CrossRef] [Green Version]
- Gan, W.T.; Gao, L.K.; Zhan, X.X.; Li, J. Hydrothermal synthesis of magnetic wood composites and improved wood properties by precipitation with CoFe2O4/hydroxyapatite. RSC Adv. 2015, 5, 45919–45927. [Google Scholar] [CrossRef]
- Qian, T.M.; Dang, B.K.; Chen, Y.P.; Jin, C.D.; Qian, J.; Sun, Q.F. Fabrication of magnetic phase change n-eicosane@Fe3O4/SiO2 microcapsules on wood surface via sol-gel method. J. Alloys Compd. 2019, 772, 871–876. [Google Scholar] [CrossRef]
- Agarwal, M.; Lvov, Y.; Varahramyan, K. Conductive wood microfibres for smart paper through layer-by-layer nanocoating. Nanotechnology 2006, 17, 5319–5325. [Google Scholar] [CrossRef]
- Zhao, Z.Y.; Sun, S.J.; Wu, D.; Zhang, M.; Huang, C.X.; Umemura, K.; Yong, Q. Synthesis and characterization of sucrose and ammonium dihydrogen phosphate (SADP) adhesive for plywood. Polymers 2019, 11, 1909. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koklukaya, O.; Carosio, F.; Grunlan, J.C.; Wågberg, L. Flame-retardant paper from wood fibers functionalized via layer by-layer assembly. ACS Appl. Mater. Interfaces 2015, 7, 23750–23759. [Google Scholar] [CrossRef]
- Renneckar, S.; Zhou, Y. Nanoscale coatings on wood: Polyelectrolyte adsorption and layer-by-layer assembled film formation. ACS Appl. Mater. Interfaces 2009, 1, 559–566. [Google Scholar] [CrossRef]
- Rao, X.; Liu, Y.Z.; Fu, Y.C.; Liu, Y.X.; Yu, H.P. Formation and properties of polyelectrolytes/TiO2 composite coating on wood surfaces through layer-by-layer assembly method. Holzforschung 2016, 70, 361–367. [Google Scholar] [CrossRef]
- Decher, G.; Hong, J.D. Buildup of ultrathin multilayer films by a self-assembly process, 1 consecutive adsorption of anionic and cationic bipolar amphiphiles on charged surfaces. Macromol. Symp. 1991, 46, 321–327. [Google Scholar] [CrossRef]
- Schoeler, B.; Guruswamy, K.A.; Caruso, F. Investigation of the influence of polyelectrolyte charge density on the growth of multilayer thin films prepared by the layer-by-layer technique. Macromolecules 2002, 35, 889–897. [Google Scholar] [CrossRef]
- Bieker, P.; Schönhoff, M. Linear and exponential growth regimes of multilayers of weak polyelectrolytes in dependence on pH. Macromolecules 2010, 43, 5052–5059. [Google Scholar] [CrossRef]
- Li, Q.; Du, Y.Z.; Yuan, H.; Zhang, X.G.; Miao, J.; Cui, F.D.; Hu, F.Q. Synthesis of lamivudine stearate and antiviral activity of stearic acid-g-chitosan oligosaccharide polymeric micelles delivery system. Eur. J. Pharm. Sci. 2010, 41, 498–507. [Google Scholar] [CrossRef] [PubMed]
- Sadeghi-Kiakhani, M.; Arami, M.; Gharanjig, K. Preparation of chitosan-ethyl acrylate as a biopolymer adsorbent for basic dyes removal from colored solutions. J. Environ. Chem. Eng. 2013, 1, 406–415. [Google Scholar] [CrossRef]
- Kai, C.; Catchmark, J.M. Improved eco-friendly barrier materials based on crystalline nanocellulose/chitosan/carboxymethyl cellulose polyelectrolyte complexes. Food Hydrocoll. 2018, 80, 195–205. [Google Scholar]
- Shi, H.Y.; Xue, L.X.; Gao, A.L.; Fu, Y.Y.; Zhou, Q.B.; Zhu, L.J. Fouling-resistant and adhesion-resistant surface modification of dual layer PVDF hollow fiber membrane by dopamine and quaternary polyethyleneimine. J. Membr. Sci. 2016, 498, 39–47. [Google Scholar] [CrossRef]
- Chen, M.J.; Shen, H.; Li, X.; Liu, H.F. Facile synthesis of oil-soluble Fe3O4 nanoparticles based on a phase transfer mechanism. Appl. Surf. Sci. 2014, 307, 306–310. [Google Scholar] [CrossRef]
- Gan, W.T.; Gao, L.K.; Xiao, S.L.; Gao, R.N.; Li, J.; Zhan, X.X. Magnetic wood as an effective induction heating material: Magnetocaloric effect and thermal insulation. Adv. Mater. Interfaces 2017, 4, 1700777. [Google Scholar] [CrossRef]





| Layer Number | Chitosan Concentration | Sodium Phytate Concentration | Fe3O4 Nanoparticles Concentration | Soaking Time |
|---|---|---|---|---|
| 1 bilayer | 1% | 1% | 1% | 90 min |
| 5 bilayers | 1% | 1% | 1% | 90 min |
| 10 bilayers | 1% | 1% | 1% | 90 min |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, T.; Fu, Y. Formation of Chitosan/Sodium Phytate/Nano-Fe3O4 Magnetic Coatings on Wood Surfaces via Layer-by-Layer Self-Assembly. Coatings 2020, 10, 51. https://doi.org/10.3390/coatings10010051
Tang T, Fu Y. Formation of Chitosan/Sodium Phytate/Nano-Fe3O4 Magnetic Coatings on Wood Surfaces via Layer-by-Layer Self-Assembly. Coatings. 2020; 10(1):51. https://doi.org/10.3390/coatings10010051
Chicago/Turabian StyleTang, Tingli, and Yanchun Fu. 2020. "Formation of Chitosan/Sodium Phytate/Nano-Fe3O4 Magnetic Coatings on Wood Surfaces via Layer-by-Layer Self-Assembly" Coatings 10, no. 1: 51. https://doi.org/10.3390/coatings10010051





