Electrodeposition of ZnO/Cu2O Heterojunctions on Ni-Mo-P Electroless Coating
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Electroless Plating of the Ceramic Surface
2.3. Electrodeposition of ZnO on Ni-Mo-P/Ceramic Substrate
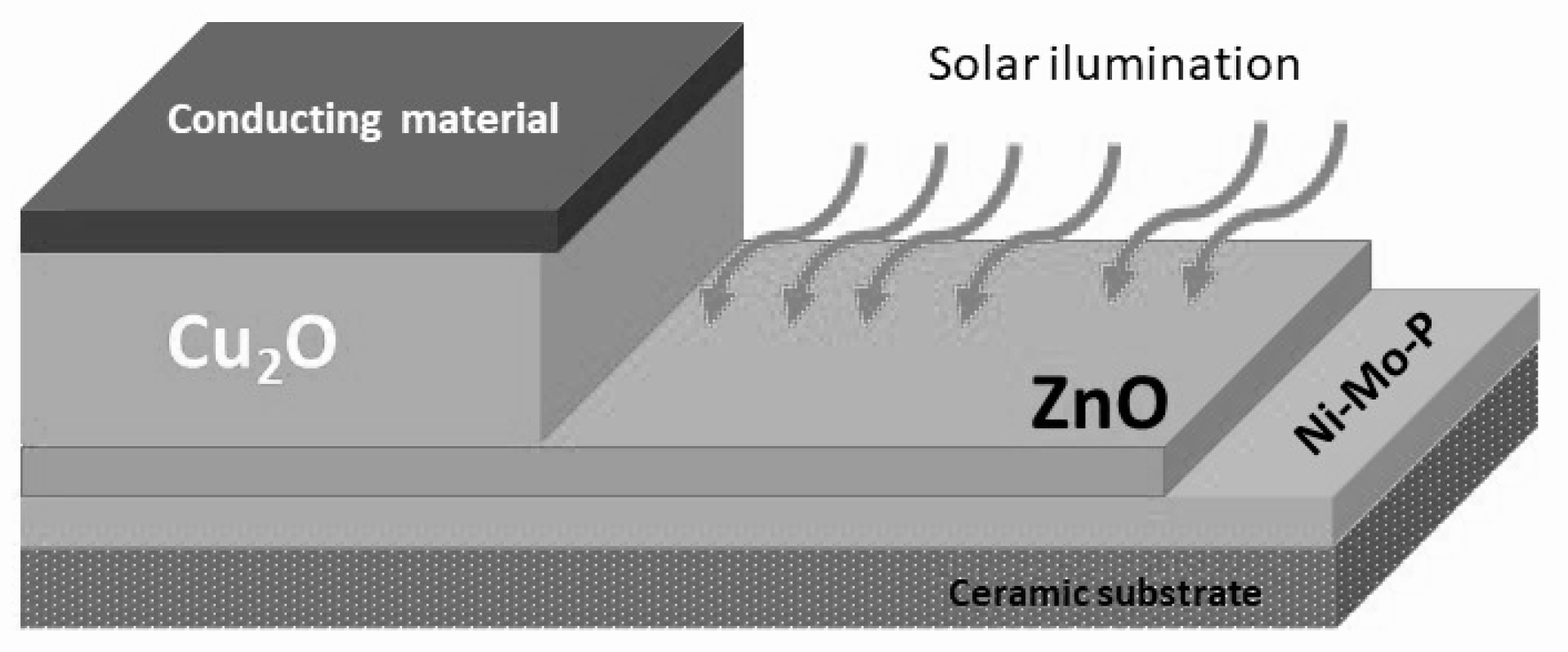
2.4. Fabrication of Cu2O/ZnO Heterojunction Solar Cells on Ni-Mo-P/Ceramic Substrate
2.5. Methods
3. Results and Discussion
3.1. Electrodeposition Study
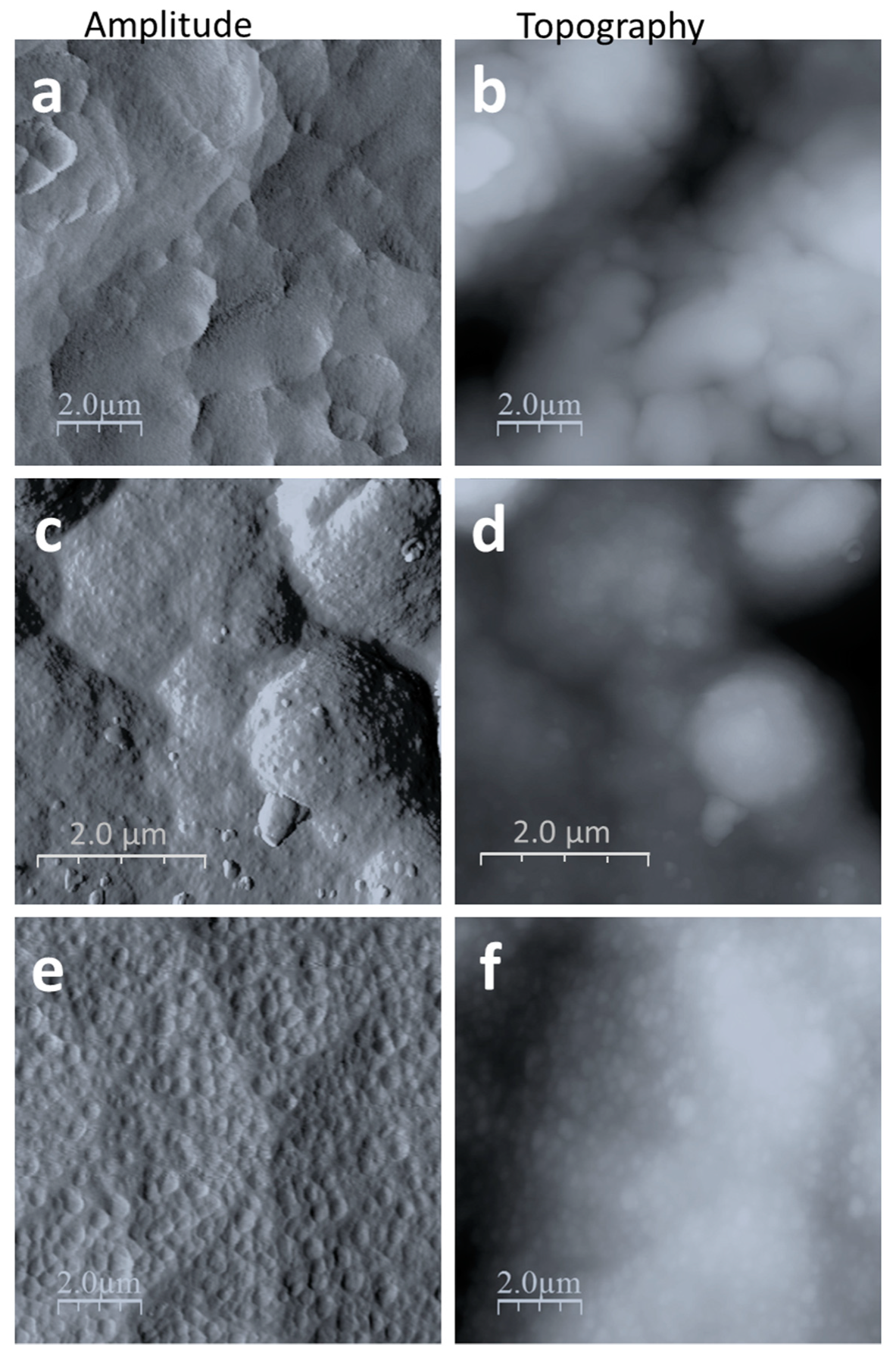
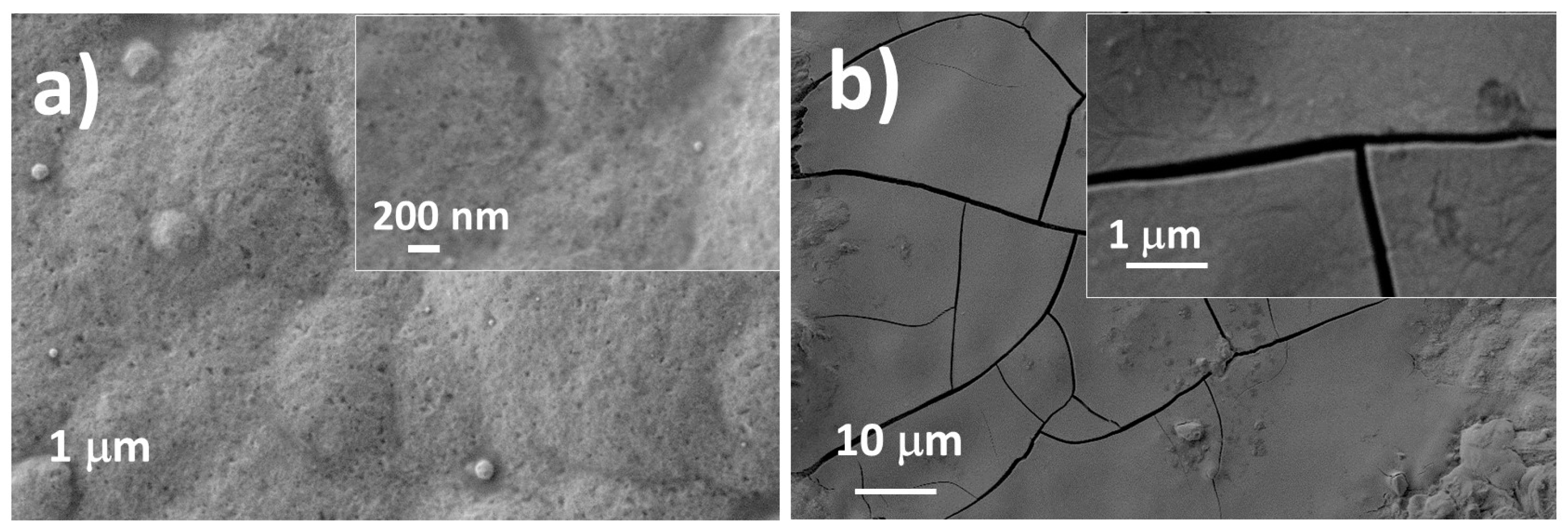
3.2. Morphology Analysis
3.3. Structure Analysis
3.4. Photoelectical Properties of Cu2O/ZnO Heterojunction Solar Cells Supported onto Ni-Mo-P-Coated Ceramic
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kishawy, H.A.; Hosseini, A. Machining Difficult-to-Cut Materials; Materials Forming, Machining and Tribology; Springer International Publishing: Cham, Switzerland, 2019; ISBN 978-3-319-95965-8. [Google Scholar]
- Leali, P.T.; Merolli, A. Fundamentals of Biomaterials. In Biomaterials in Hand Surgery; Springer Milan: Milano, Italy, 2009; pp. 1–11. ISBN 9788847011946. [Google Scholar]
- Ghosh, S.; Pal, K.S.; Dandapat, N.; Datta, S.; Basu, D. Interfacial properties of metallized alumina ceramics. Met. Mater. Int. 2012, 18, 625–630. [Google Scholar] [CrossRef]
- Ene Hourdequin, H.; Laudebat, L.; Locatelli, M.-L.; Valdez-Nava, Z.; Bidan, P. Metallized ceramic substrate with mesa structure for voltage ramp-up of power modules. Eur. Phys. J. Appl. Phys 2019, 87, 20903. [Google Scholar] [CrossRef]
- Mirone, G.; Sitta, A.; D’Arrigo, G.; Calabretta, M. Material Characterization and Warpage Modeling for Power Devices Active Metal Brazed Substrates. IEEE Trans. Device Mater. Reliab. 2019, 19, 537–542. [Google Scholar] [CrossRef]
- Sampaio, P.G.V.; Gonz�lez, M.O.A. Photovoltaic solar energy: Conceptual framework. Renew. Sustain. Energy Rev. 2017, 74, 590–601. [Google Scholar] [CrossRef]
- Agarwala, R.C.; Agarwala, V. Electroless alloy/composite coatings: A review. Sadhana 2003, 28, 475–493. [Google Scholar] [CrossRef]
- Rosas-Laverde, N.M.; Pruna, A.; Cembrero, J.; Pascual, M.; Orozco-Messana, J. Optimizing Electroless Plating of Ni–Mo–P Coatings Toward Functional Ceramics. Acta Metall. Sin. Engl. Lett. 2020, 33, 1–9. [Google Scholar] [CrossRef]
- Badán, J.A.; Navarrete-Astorga, E.; Henríquez, R.; Martín, F.; Marotti, R.E.; Ramos-Barrado, J.R.; Dalchiele, E.A. Optical properties of silver nanoparticles deposited onto silicon substrates by different soft-solution processing techniques. Opt. Mater. (Amst) 2020, 100, 109651. [Google Scholar] [CrossRef]
- Ma, H.; Liu, Z.; Wu, L.; Wang, Y.; Wang, X. Study of a pre-treatment process for electroless copper plating on ceramics. Thin Solid Films 2011, 519, 7860–7863. [Google Scholar] [CrossRef]
- Zhang, B. Electroless Plating Baths of Metals, Binary Alloys, and Multicomponent Alloys. In Amorphous and Nano Alloys Electroless Depositions: Technology, Composition. Structure and Theory; Tian, S., Ed.; Elsevier: Amsterdam, The Netherlands, 2016; pp. 51–106. ISBN 9780128026854. [Google Scholar]
- Zhang, B. Impact Parameters and Deposition Rate. In Amorphous and Nano Alloys Electroless Depositions: Technology, Composition. Structure and Theory; Tian, S., Ed.; Elsevier: Amsterdam, The Netherlands, 2016; pp. 323–381. ISBN 9780128026854. [Google Scholar]
- Heidarzadeh, A.; Mousavian, R.T.; Khosroshahi, R.A.; Afkham, Y.A.; Pouraliakbar, H. Empirical model to predict mass gain of cobalt electroless deposition on ceramic particles using response surface methodology. Rare Met. 2017, 36, 209–219. [Google Scholar] [CrossRef]
- Zhou, R.; Chen, H.; Xu, C.; Hou, X.; Liu, G.; Liu, Y. Facile synthesis of electromagnetic Ni@glass fiber composites via electroless deposition method. J. Mater. Sci. Mater. Electron. 2015, 26, 3530–3537. [Google Scholar] [CrossRef]
- Tai, Y.; Chen, H.; Xu, C.; Liu, Y. Conductive glass fabrics@nickel composites prepared by a facile electroless deposition method. Mater. Lett. 2016, 171, 158–161. [Google Scholar] [CrossRef]
- Lee, C.-L.; Wan, C.-C.; Wang, Y.-Y. Pd Nanoparticles as a New Activator for Electroless Copper Deposition. J. Electrochem. Soc. 2003, 150, C125. [Google Scholar] [CrossRef]
- Lin, J.D.; Kuo, C.L. Effects of hydrogen plasma treatment on microstructure evolution and electrical conductivity of electroless Ni-P coatings on polyimide and glass substrates. Surf. Coatings Technol. 2012, 209, 80–89. [Google Scholar] [CrossRef]
- Kazemi, A.; Yang, S. Atomistic Study of the Effect of Magnesium Dopants on the Strength of Nanocrystalline Aluminum. JOM 2019, 71, 1209–1214. [Google Scholar] [CrossRef]
- Li, Q.; Xu, M.; Fan, H.; Wang, H.; Peng, B.; Long, C.; Zhai, Y. Dielectric properties investigation of Cu2O/ZnO heterojunction thin films by electrodeposition. Mater. Sci. Eng. B Solid-State Mater. Adv. Technol. 2013, 178, 496–501. [Google Scholar] [CrossRef]
- Li, J.; Li, H.; Xue, Y.; Fang, H.; Wang, W. Facile electrodeposition of environment-friendly Cu2O/ZnO heterojunction for robust photoelectrochemical biosensing. Sensors Actuators B Chem. 2014, 191, 619–624. [Google Scholar] [CrossRef]
- Bhattacharya, R.N.; Deb, S.K. A Low-Cost Approach to Fabrication of Multinary Compounds for Energy-Related Applications. Jpn. J. Appl. Phys. 2000, 39, 424. [Google Scholar] [CrossRef]
- Kang, D.; Lee, D.; Choi, K.-S. Electrochemical Synthesis of Highly Oriented, Transparent, and Pinhole-Free ZnO and Al-Doped ZnO Films and Their Use in Heterojunction Solar Cells. Langmuir 2016, 32, 10459–10466. [Google Scholar] [CrossRef]
- Shah, A.V.; Schade, H.; Vanecek, M.; Meier, J.; Vallat-Sauvain, E.; Wyrsch, N.; Kroll, U.; Droz, C.; Bailat, J. Thin-film silicon solar cell technology. Prog. Photovoltaics Res. Appl. 2004, 12, 113–142. [Google Scholar] [CrossRef]
- Rosa, G.; Bosio, A.; Menossi, D.; Romeo, N. How the Starting Precursor Influences the Properties of Polycrystalline CuInGaSe2 Thin Films Prepared by Sputtering and Selenization. Energies 2016, 9, 354. [Google Scholar] [CrossRef]
- Calvet, I.; Barrachina, E.; Martí, R.; Fraga, D.; Stoyanova Lyubenova, T.; Carda, J.B. Development of photovoltaic ceramic tile based on CZTSSe absorber. Mater. Lett. 2015, 161, 636–639. [Google Scholar] [CrossRef]
- Lin, S.; Li, X.X.; Pan, H.; Chen, H.; Li, X.X.; Li, Y.; Zhou, J. Numerical analysis of InxGa1-xN/SnS and AlxGa1-xN/SnS heterojunction solar cells. Energy Convers. Manag. 2016, 119, 361–367. [Google Scholar] [CrossRef]
- Cheng, K.; Li, Q.; Meng, J.; Han, X.; Wu, Y.; Wang, S.; Qian, L.; Du, Z. Interface engineering for efficient charge collection in Cu2O/ZnO heterojunction solar cells with ordered ZnO cavity-like nanopatterns. Sol. Energy Mater. Sol. Cells 2013, 116, 120–125. [Google Scholar] [CrossRef]
- Reinders, A.; Verlinden, P.; Van Sark, W.; Freundlich, A. (Eds.) Photovoltaic Solar Energy; John Wiley & Sons, Ltd.: Chichester, UK, 2016; ISBN 9781118927496. [Google Scholar]
- Rosas-Laverde, N.M.; Pruna, A.; Cembrero, J.; Orozco-Messana, J.; Manjón, F.J. Performance of graphene oxide-modified electrodeposited ZnO/Cu2O heterojunction solar cells. Boletín Soc. Española Cerámica Vidr. 2019, 58, 263–273. [Google Scholar] [CrossRef]
- Rosas-Laverde, N.M.; Pruna, A.; Busquets-Mataix, D.; Marí, B.; Cembrero, J.; Salas Vicente, F.; Orozco-Messana, J. Improving the properties of Cu2O/ZnO heterojunction for photovoltaic application by graphene oxide. Ceram. Int. 2018, 44, 23045–23051. [Google Scholar] [CrossRef]
- Wang, C.; Xu, J.; Shi, S.; Zhang, Y.; Gao, Y.; Liu, Z.; Zhang, X.; Li, L. Optimizing performance of Cu2O/ZnO nanorods heterojunction based self-powered photodetector with ZnO seed layer. J. Phys. Chem. Solids 2017, 103, 218–223. [Google Scholar] [CrossRef]
- Elfadill, N.G.; Hashim, M.R.; Chahrour, K.M.; Qaeed, M.A.; Bououdina, M. The influence of Cu2O crystal structure on the Cu2O/ZnO heterojunction photovoltaic performance. Superlattices Microstruct. 2015, 85, 908–917. [Google Scholar] [CrossRef]
- Bai, Z.; Zhang, Y. Self-powered UV-visible photodetectors based on ZnO/Cu2O nanowire/electrolyte heterojunctions. J. Alloys Compd. 2016, 675, 325–330. [Google Scholar] [CrossRef]
- Bai, Z.; Liu, J.; Liu, F.; Zhang, Y. Enhanced photoresponse performance of self-powered UV–visible photodetectors based on ZnO/Cu2O/electrolyte heterojunctions via graphene incorporation. J. Alloys Compd. 2017, 726, 803–809. [Google Scholar] [CrossRef]
- Tran, M.H.; Cho, J.Y.; Sinha, S.; Gang, M.G.; Heo, J. Cu2O/ZnO heterojunction thin-film solar cells: The effect of electrodeposition condition and thickness of Cu2O. Thin Solid Films 2018, 661, 132–136. [Google Scholar] [CrossRef]
- Messaoudi, O.; Makhlouf, H.; Souissi, A.; Ben assaker, I.; Amiri, G.; Bardaoui, A.; Oueslati, M.; Bechelany, M.; Chtourou, R. Synthesis and characterization of ZnO/Cu2O core–shell nanowires grown by two-step electrodeposition method. Appl. Surf. Sci. 2015, 343, 148–152. [Google Scholar] [CrossRef]
- Jiang, X.; Lin, Q.; Zhang, M.; He, G.; Sun, Z. Microstructure, optical properties, and catalytic performance of Cu2O-modified ZnO nanorods prepared by electrodeposition. Nanoscale Res. Lett. 2015, 2–7. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.W.; Perng, D.C.; Fang, J.F. Nano-structured Cu2O solar cells fabricated on sparse ZnO nanorods. Sol. Energy Mater. Sol. Cells 2011, 95, 2471–2477. [Google Scholar] [CrossRef]
- Panigrahi, S.; Nunes, D.; Calmeiro, T.; Kardarian, K.; Martins, R.; Fortunato, E. Oxide-Based Solar Cell: Impact of Layer Thicknesses on the Device Performance. ACS Comb. Sci. 2017, 19, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Jeong, S.; Aydil, E.S. Heteroepitaxial growth of Cu2O thin film on ZnO by metal organic chemical vapor deposition. J. Cryst. Growth 2009, 311, 4188–4192. [Google Scholar] [CrossRef]
- Kathalingam, A.; Vikraman, D.; Kim, H.S.; Park, H.J. Facile fabrication of n-ZnO nanorods/p-Cu2O heterojunction and its photodiode property. Opt. Mater. (Amst.) 2017, 66, 122–130. [Google Scholar] [CrossRef]
- Fujimoto, K.; Oku, T.; Akiyama, T.; Suzuki, A. Fabrication and characterization of copper oxide-zinc oxide solar cells prepared by electrodeposition. J. Phys. Conf. Ser. 2013, 433, 012024. [Google Scholar] [CrossRef]
- Lahmar, H.; Setifi, F.; Azizi, A.; Schmerber, G.; Dinia, A. On the electrochemical synthesis and characterization of p-Cu2O/n-ZnO heterojunction. J. Alloys Compd. 2017, 718, 36–45. [Google Scholar] [CrossRef]
- Abdelfatah, M.; Ismail, W.; El-Shaer, A. Low cost inorganic white light emitting diode based on submicron ZnO rod arrays and electrodeposited Cu2O thin film. Mater. Sci. Semicond. Process. 2018, 81, 44–47. [Google Scholar] [CrossRef]
- Septina, W.; Ikeda, S.; Khan, M.A.; Hirai, T.; Harada, T.; Matsumura, M.; Peter, L.M. Potentiostatic electrodeposition of cuprous oxide thin films for photovoltaic applications. Electrochim. Acta 2011, 56, 4882–4888. [Google Scholar] [CrossRef]
- Han, G.; Zhang, S.; Boix, P.P.; Wong, L.H.; Sun, L.; Lien, S.-Y. Towards high efficiency thin film solar cells. Prog. Mater. Sci. 2017, 87, 246–291. [Google Scholar] [CrossRef]
- Barreau, N.; Duche, D.; Ruiz, C.M.; Escoubas, L.; Simon, J.-J.; Le Rouzo, J.; Bermudez, V. Innovative approaches in thin-film photovoltaic cells. In Optical Thin Films and Coatings; Elsevier: Amsterdam, The Netherlands, 2018; pp. 595–632. [Google Scholar]
- Hamelmann, F.U. Transparent Conductive Oxides in Thin Film Photovoltaics. J. Phys. Conf. Ser. 2014, 559, 012016. [Google Scholar] [CrossRef]
- Wu, S.; Yin, Z.; He, Q.; Huang, X.; Zhou, X.; Zhang, H. Electrochemical deposition of semiconductor oxides on reduced graphene oxide-based flexible, transparent, and conductive electrodes. J. Phys. Chem. C 2010, 114, 11816–11821. [Google Scholar] [CrossRef]
- Arefpour, M.; Almasi Kashi, M.; Bagheli, M. High Chemical and Thermal Stability of Ag Nanowire-Based Transparent Conductive Electrodes Induced by Electroless Ag Nanoparticle Decoration. Phys. status solidi 2020, 217, 1900957. [Google Scholar] [CrossRef]
- Jia, G.; Plentz, J.; Dellith, A.; Schmidt, C.; Dellith, J.; Schmidl, G.; Andrä, G. Biomimic Vein-Like Transparent Conducting Electrodes with Low Sheet Resistance and Metal Consumption. Nano-Micro Lett. 2020, 12, 1–13. [Google Scholar] [CrossRef]
- Rosas-Laverde, N.M.; Pruna, A.I.; Busquets-Mataix, D. Graphene oxide-polypyrrole coating for functional ceramics. Nanomaterials 2020, 10, 1188. [Google Scholar] [CrossRef]
- Fetohi, A.E.; Abdel Hameed, R.M.; El-Khatib, K.M. Ni-P and Ni-Mo-P modified aluminium alloy 6061 as bipolar plate material for proton exchange membrane fuel cells. J. Power Sources 2013, 240, 589–597. [Google Scholar] [CrossRef]
- Nikoobakht, A.; Aghaei, J.; Massrur, H.R.; Hemmati, R. Decentralised hybrid robust/stochastic expansion planning in coordinated transmission and active distribution networks for hosting large-scale wind energy. IET Gener. Transm. Distrib. 2020, 14, 797–807. [Google Scholar] [CrossRef]
- Cembrero-Coca, P.; Cembrero, J.; Busquets-Mataix, D.; Pérez-Puig, M.A.; Marí, B.; Pruna, A. Factorial electrochemical design for tailoring of morphological and optical properties of Cu2O. Mater. Sci. Technol. (UK) 2017, 33, 2102–2109. [Google Scholar] [CrossRef]
- Yang, M.; Zhu, L.; Li, Y.; Cao, L.; Guo, Y. Asymmetric interface band alignments of Cu2O/ZnO and ZnO/Cu2O heterojunctions. J. Alloys Compd. 2013, 578, 143–147. [Google Scholar] [CrossRef]
- Pławecki, M.; Rówiński, E. Mieszczak Zinc oxide/cuprous(I) oxide-based solar cells prepared by electrodeposition. Acta Phys. Pol. A 2016, 130, 1144–1146. [Google Scholar] [CrossRef]
- Rosas-Laverde, N.M.; Pruna, A.; Busquets-Mataix, D.; Pullini, D. Graphene oxide-assisted morphology and structure of electrodeposited ZnO nanostructures. Materials (Basel) 2020, 13, 365. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, F.F.; Proenca, M.P.; Araújo, J.P.; Ventura, J. Electrodeposition of ZnO thin films on conducting flexible substrates. J. Mater. Sci. 2016, 51, 5589–5597. [Google Scholar] [CrossRef]
- Marimuthu, T.; Anandhan, N.; Thangamuthu, R. Electrochemical synthesis of one-dimensional ZnO nanostructures on ZnO seed layer for DSSC applications. Appl. Surf. Sci. 2018, 428, 385–394. [Google Scholar] [CrossRef]
- Cui, X.; Hutt, D.A.; Conway, P.P. Evolution of microstructure and electrical conductivity of electroless copper deposits on a glass substrate. Thin Solid Films 2012, 520, 6095–6099. [Google Scholar] [CrossRef]
- Sridhar, N.; Udaya Bhat, K. Effect of Deposition Time on the Morphological Features and Corrosion Resistance of Electroless Ni-High P Coatings on Aluminium. J. Mater. 2013, 2013, 985763. [Google Scholar] [CrossRef]
- Liu, B.H.; Liao, F.Y.; Chen, J.H. Design, fabrication, and characterization of electroless Ni-P alloy films for micro heating devices. Thin Solid Films 2013, 537, 263–268. [Google Scholar] [CrossRef]
- Pruna, A.; Shao, Q.; Kamruzzaman, M.; Li, Y.Y.; Zapien, J.A.; Pullini, D.; Busquets Mataix, D.; Ruotolo, A. Effect of ZnO core electrodeposition conditions on electrochemical and photocatalytic properties of polypyrrole-graphene oxide shelled nanoarrays. Appl. Surf. Sci. 2017, 392, 801–809. [Google Scholar] [CrossRef]
- Prepelita, P.; Medianu, R.; Sbarcea, B.; Garoi, F.; Filipescu, M. The influence of using different substrates on the structural and optical characteristics of ZnO thin films. Appl. Surf. Sci. 2010, 256, 1807–1811. [Google Scholar] [CrossRef]
- Mukhopadhyay, A.; Barman, T.K. Effect of heat treatment on tribological behavior of electroless Ni-B coating at elevated temperatures. Surf. Rev. Lett. 2018, 25, 1850014. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, W. Influence of heat treatment for coating of nickel plating on hollow glass beads. Phys. Procedia 2013, 50, 219–224. [Google Scholar] [CrossRef][Green Version]
- Makkar, P.; Agarwala, R.C.; Agarwala, V. Chemical synthesis of TiO2 nanoparticles and their inclusion in Ni-P electroless coatings. Ceram. Int. 2013, 39, 9003–9008. [Google Scholar] [CrossRef]
- Ke, N.H.; Trinh, L.T.T.; Phung, P.K.; Loan, P.T.K.; Tuan, D.A.; Truong, N.H.; Tran, C.V.; Hung, L.V.T. Changing the thickness of two layers: I-ZnO nanorods, p-Cu2O and its influence on the carriers transport mechanism of the p-Cu2O/i-ZnO nanorods/n-IGZO heterojunction. SpringerPlus 2016, 5, 710. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Makhlouf, H.; Weber, M.; Messaoudi, O.; Tingry, S.; Moret, M.; Briot, O.; Chtoutou, R.; Bechelany, M. Study of Cu2O/ZnO nanowires heterojunction designed by combining electrodeposition and atomic layer deposition. Appl. Surf. Sci. 2017, 426, 301–306. [Google Scholar] [CrossRef]
- Balaraju, J.N.; Raman, N.; Manikandanath, N.T. Nanocrystalline electroless nickel poly-alloy deposition: Incorporation of W and Mo. Trans. IMF 2014, 92, 169–176. [Google Scholar] [CrossRef]
- Guo, Z.; Keong, K.G.G.; Sha, W. Crystallisation and phase transformation behaviour of electroless nickel phosphorus platings during continuous heating. J. Alloys Compd. 2003, 358, 112–119. [Google Scholar] [CrossRef]
- Mukhopadhyay, A.; Barman, T.K.; Sahoo, P. Wear and friction characteristics of electroless Ni-B-W coatings at different operating temperatures. Mater. Res. Express 2018, 5, 26526. [Google Scholar] [CrossRef]
- Lee, G.H. Relationship between crystal structure and photoluminescence properties of ZnO films formed by oxidation of metallic Zn. Electron. Mater. Lett. 2010, 6, 155–159. [Google Scholar] [CrossRef]
- Lim, K.; Abdul Hamid, M.; Shamsudin, R.; Al-Hardan, N.H.; Mansor, I.; Chiu, W. Temperature-Driven Structural and Morphological Evolution of Zinc Oxide Nano-Coalesced Microstructures and Its Defect-Related Photoluminescence Properties. Materials (Basel) 2016, 9, 300. [Google Scholar] [CrossRef]
- Bengas, R.; Lahmar, H.; Redha, K.M.; Mentar, L.; Azizi, A.; Schmerber, G.; Dinia, A. Electrochemical synthesis of n-type ZnS layers on p-Cu2O/n-ZnO heterojunctions with different deposition temperatures. RSC Adv. 2019, 9, 29056–29069. [Google Scholar] [CrossRef]
- Elsayed, E.M.; Shalan, A.E.; Rashad, M.M. Preparation of ZnO nanoparticles using electrodeposition and co-precipitation techniques for dye-sensitized solar cells applications. J. Mater. Sci. Mater. Electron. 2014, 25, 3412–3419. [Google Scholar] [CrossRef]
- Chen, S.; Lin, L.; Liu, J.; Lv, P.; Wu, X.; Zheng, W.; Qu, Y.; Lai, F. An electrochemical constructed p-Cu2O/n-ZnO heterojunction for solar cell. J. Alloys Compd. 2015, 644, 378–382. [Google Scholar] [CrossRef]
- Li, Z.; Jia, M.; Doble, S.; Hockey, E.; Yan, H.; Avenoso, J.P.; Bodine, D.; Zhang, Y.; Ni, C.; Newberg, J.T.; et al. Energy Band Architecture of a Hierarchical ZnO/Au/CuxO Nanoforest by Mimicking Natural Superhydrophobic Surfaces. ACS Appl. Mater. Interfaces 2019, 11, 40490–40502. [Google Scholar] [CrossRef] [PubMed]
- Iencinella, D.; Centurioni, E.; Grazia Busana, M. Thin-film solar cells on commercial ceramic tiles. Sol. Energy Mater. Sol. Cells 2009, 93, 206–210. [Google Scholar] [CrossRef]



| Solar Cell | JSC (μA/cm2) | VOC (μV) |
|---|---|---|
| Cu2O/ZnO/CSITO | 1.51 | 544.032 |
| Cu2O/ZnO/CS30 | 39.92 | 383.229 |
| Cu2O/ZnO/CS300 | 27.25 | 532.648 |
| Cu2O/ZnO/CS300-160 | 939.96 | 454.338 |
| Cu2O/ZnO/CS300-400 | 4.64 | 620.602 |
| ELD Condition of ZnO | JSC (μA/cm2) | VOC (μV) |
|---|---|---|
| −0.6 V; 30 min | 52.48 | 713.53 |
| −0.7 V; 30 min | 719.75 | 659.27 |
| −0.8 V; 30 min | 939.96 | 454.34 |
| −0.8 V; 60 min | 1440 | 760.23 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rosas-Laverde, N.M.; Pruna, A.I.; Cembrero, J.; Busquets-Mataix, D. Electrodeposition of ZnO/Cu2O Heterojunctions on Ni-Mo-P Electroless Coating. Coatings 2020, 10, 935. https://doi.org/10.3390/coatings10100935
Rosas-Laverde NM, Pruna AI, Cembrero J, Busquets-Mataix D. Electrodeposition of ZnO/Cu2O Heterojunctions on Ni-Mo-P Electroless Coating. Coatings. 2020; 10(10):935. https://doi.org/10.3390/coatings10100935
Chicago/Turabian StyleRosas-Laverde, Nelly Maria, Alina Iuliana Pruna, Jesus Cembrero, and David Busquets-Mataix. 2020. "Electrodeposition of ZnO/Cu2O Heterojunctions on Ni-Mo-P Electroless Coating" Coatings 10, no. 10: 935. https://doi.org/10.3390/coatings10100935
APA StyleRosas-Laverde, N. M., Pruna, A. I., Cembrero, J., & Busquets-Mataix, D. (2020). Electrodeposition of ZnO/Cu2O Heterojunctions on Ni-Mo-P Electroless Coating. Coatings, 10(10), 935. https://doi.org/10.3390/coatings10100935






