Chitosan, Nisin, Silicon Dioxide Nanoparticles Coating Films Effects on Blueberry (Vaccinium myrtillus) Quality
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Fruit material and Treatments
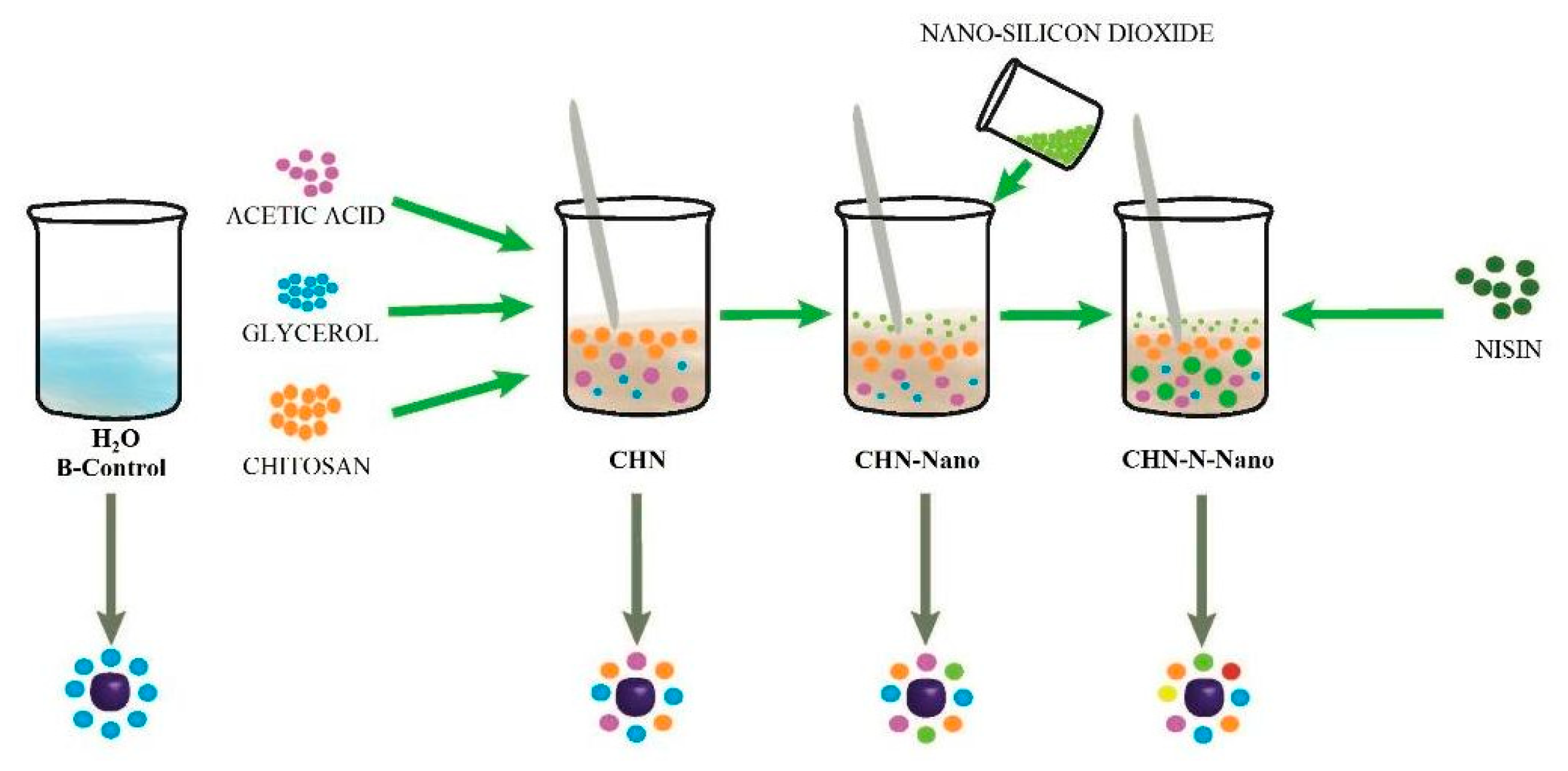
2.3. Films Preparations
2.4. Measurement of Fruit Quality
2.5. Measurement of Fruit Oxidation
2.6. In-Vivo Microbiological Analyses
2.7. Statistical Analyses
3. Results and Discussion
3.1. Physicochemical Parameters
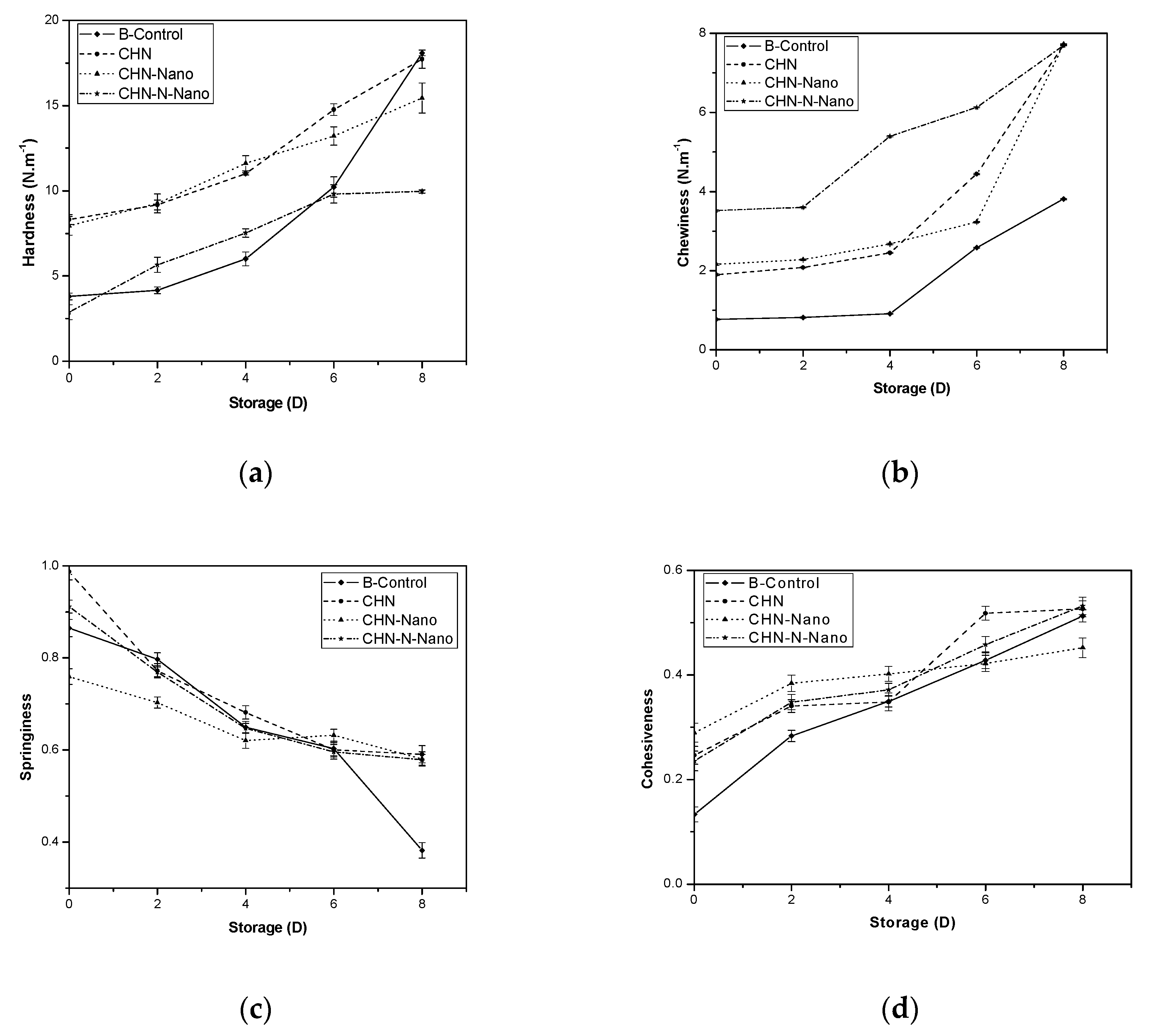
3.1.1. Texture Profile Analysis (TPA).
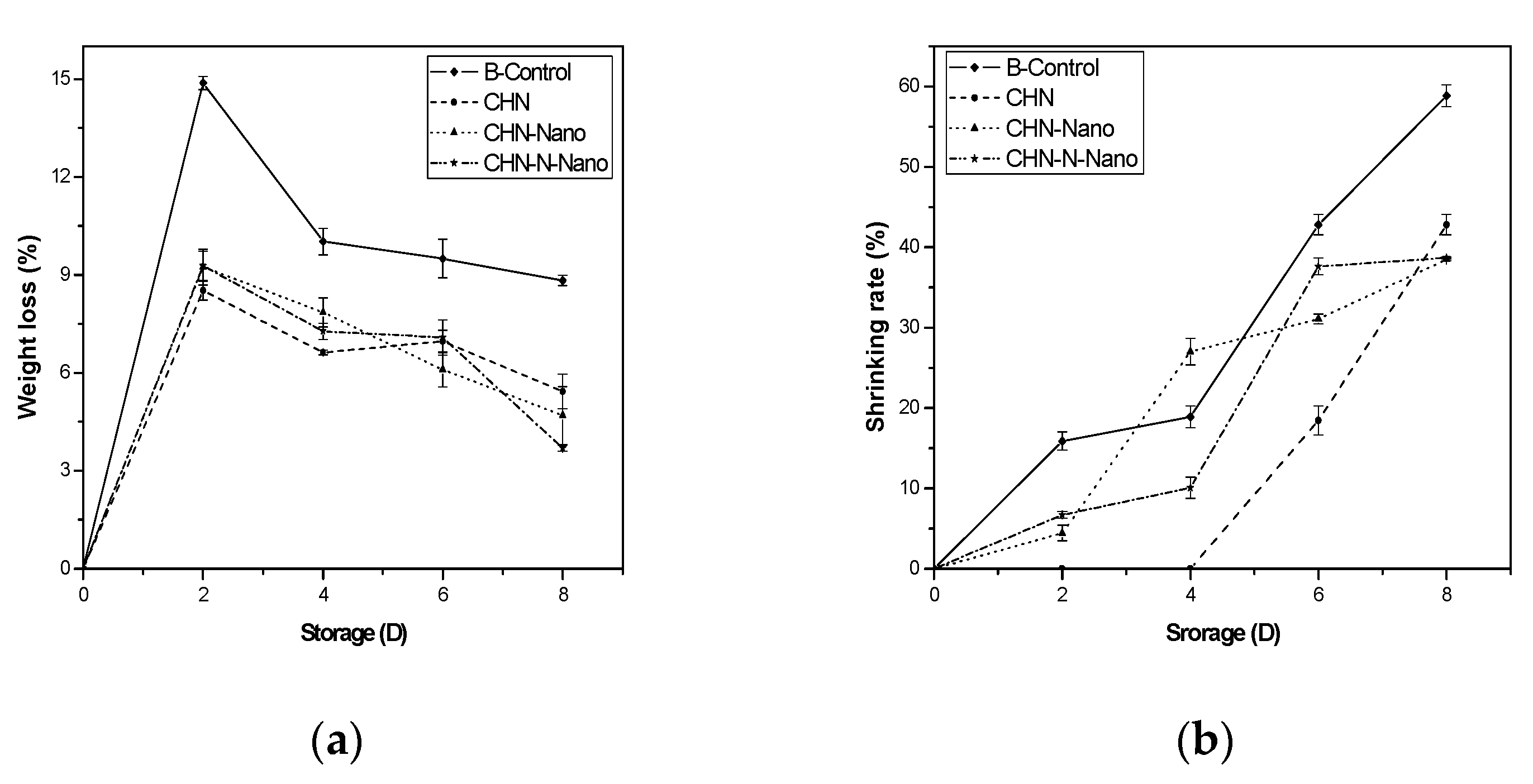
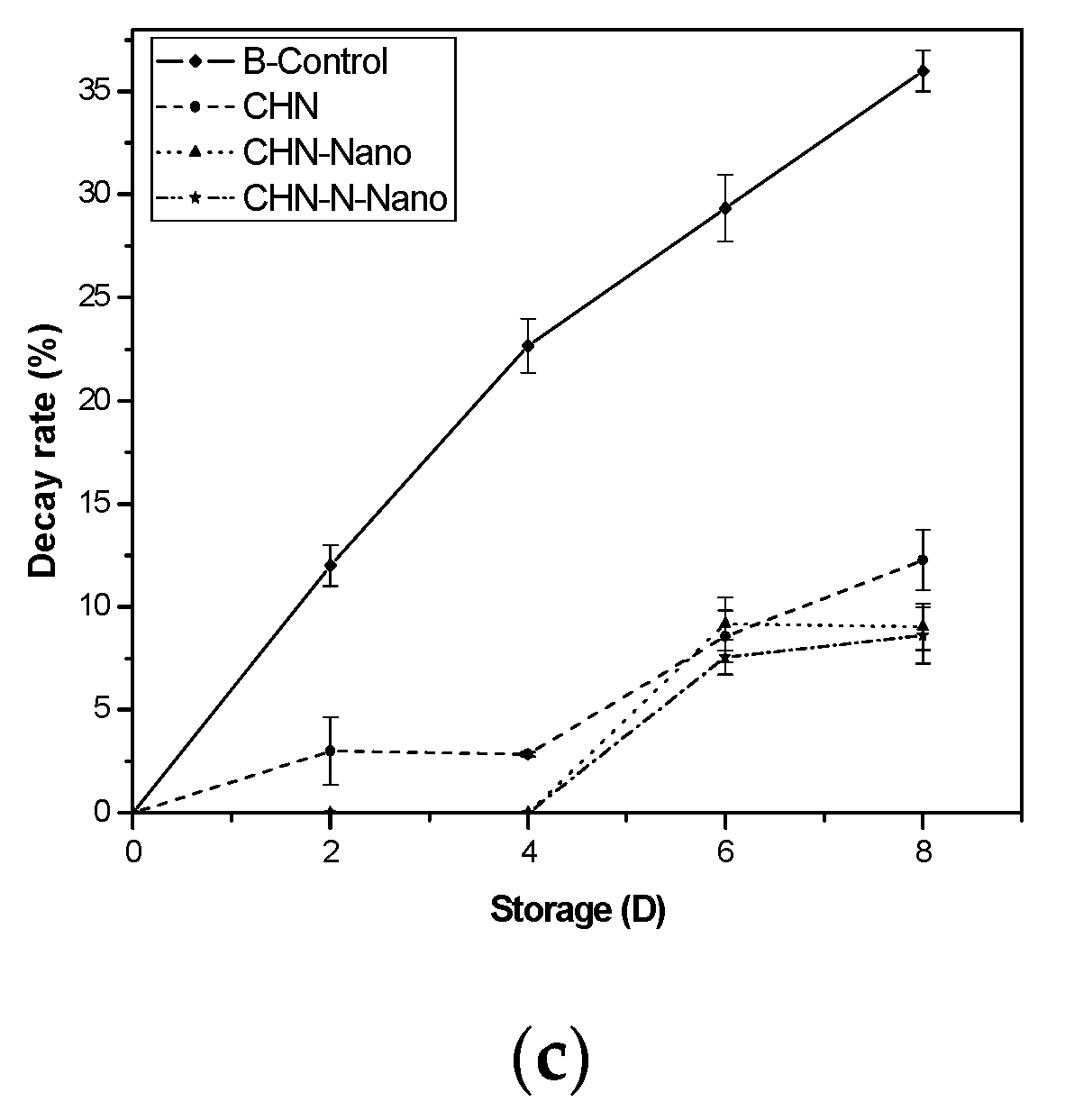
3.1.2. Weight Loss, Shrinking and Decay Rates
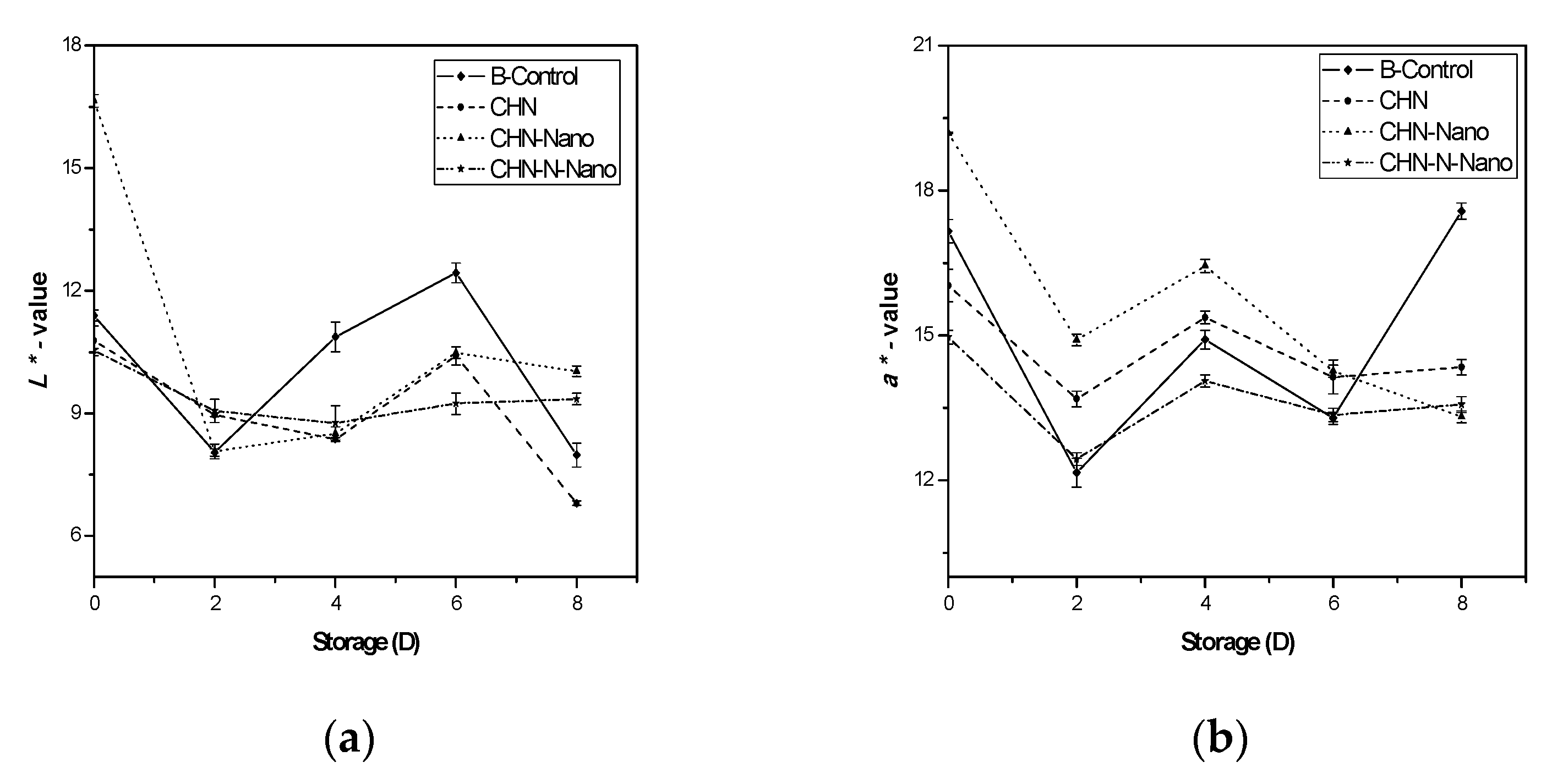
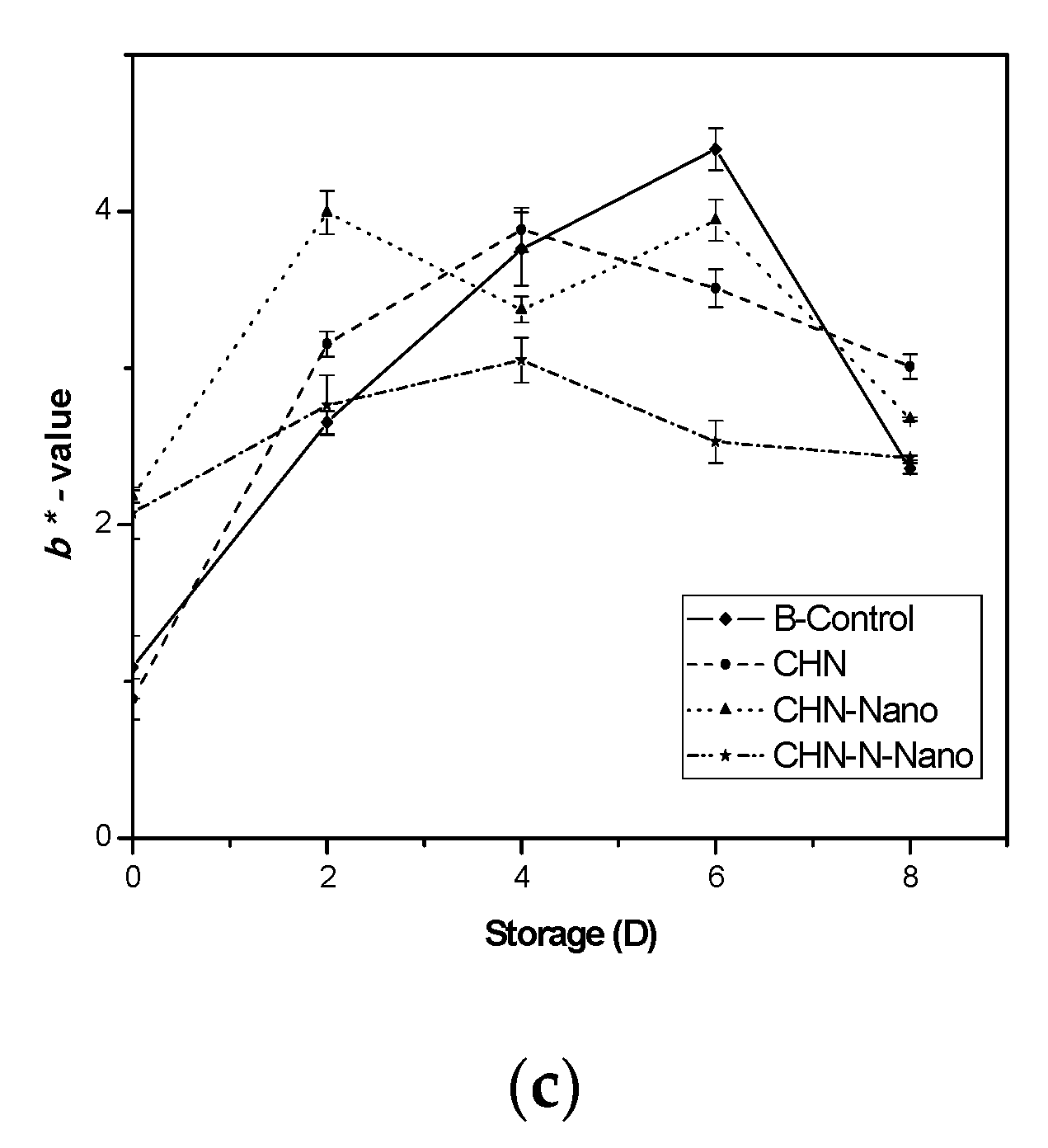
3.1.3. Color Index
3.2. Ph, Vit. c, SSC, TAA and SSC/TAA Ratio
3.3. Oxidation Browning Enzyme (PPO, POD) and TAC
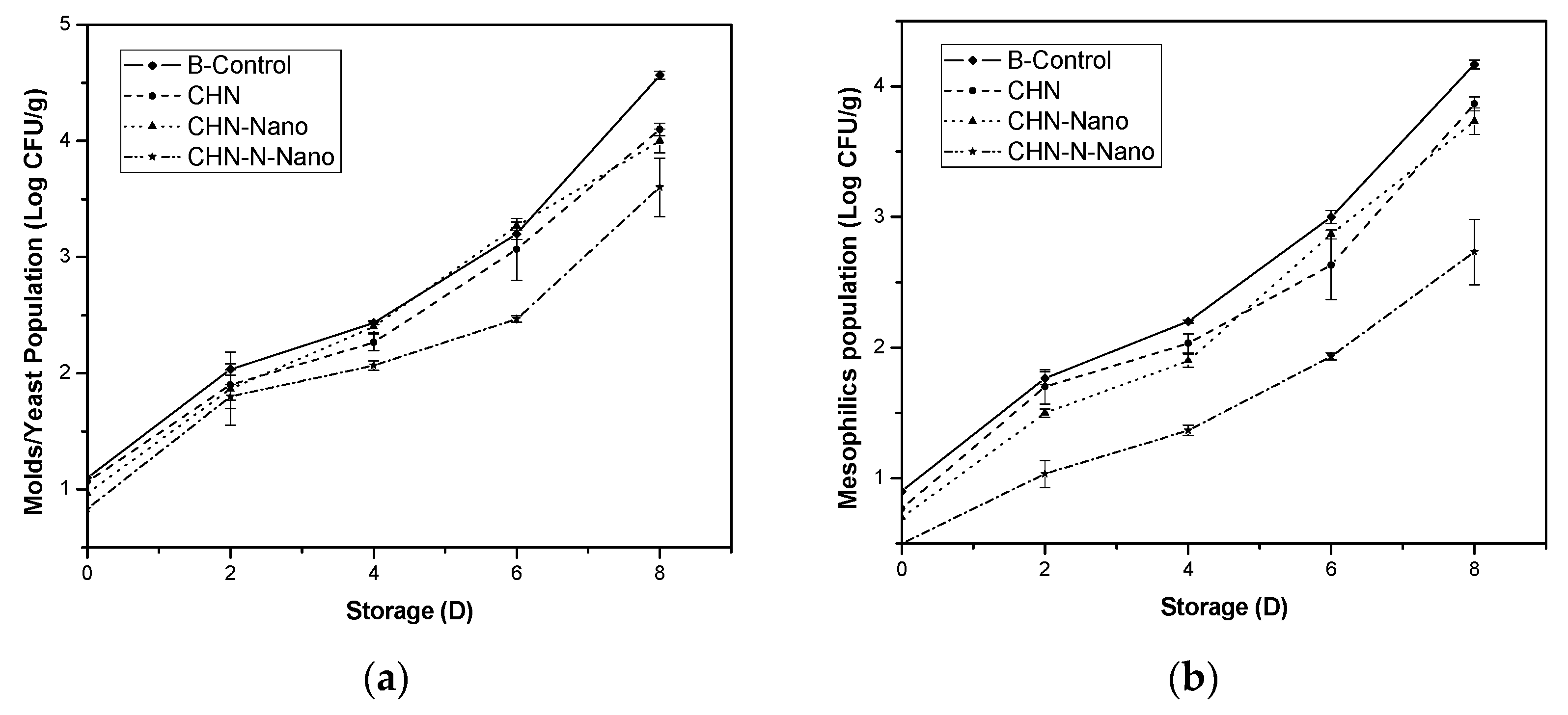
3.4. Antimicrobial Effect of Chitosan, Nisin, Silicon Dioxide Nanoparticles Coatings on Blueberries
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Liato, V.; Hammami, R.; Aïder, M. Infuence of electro-activated solutions of weak organic acid salts on microbial and overall appearance of blueberries during storage. Food Microbiol. 2017, 64, 56–64. [Google Scholar] [CrossRef] [PubMed]
- María, F.; Liliana, M.; María, R. An approach to improve the safety and quality of ready-to-eat blueberries. J. Food Saf. 2018, 39, e12602. [Google Scholar]
- Karolina, K.; Iwona, S.; Małgorzata, G.; Marta, M.; Katarzyna, P.; Andrzej, C. Effect of Pullulan Coating on Postharvest Quality and Shelf-Life of Highbush Blueberry (Vaccinium corymbosum L.). Materials 2017, 10, 965. [Google Scholar]
- Sun, X.; Narciso, J.; Wang, Z.; Ference, C.; Bai, J.; Zhou, K. Effects of chitosan-essential oil coatings on safety and quality of fresh blueberries. J. Food Sci. 2014, 79, 955–960. [Google Scholar] [CrossRef]
- Cantín, C.M.; Palou, L.; Bremer, V.; Michailides, T.J.; Crisosto, C.H. Evaluation of the use of sulfur dioxide to reduce postharvest losses on dark and green figs. Postharvest Biol. Technol. 2011, 59, 150–158. [Google Scholar] [CrossRef]
- Chiabrando, V.; Peano, C.; Beccaro, G.; Bounous, G.; Rolle, L. Postharvest quality of highbush blueberry (Vacciniumcorymbosum L.) cultivars in relation to storage methods. Acta Hortic. 2006, 715, 545–551. [Google Scholar] [CrossRef]
- Giuggioli, N.R.; Girgenti, V.; Peano, C. Qualitative performance and consumer acceptability of starch films for the blueberry modified atmosphere packaging storage. Pol. J. Food Nutr. Sci. 2017, 67, 129–136. [Google Scholar] [CrossRef]
- Mannozzi, C.; Cecchini, J.P.; Tylewicz, U.; Siroli, L.; Patrignani, F.; Lanciotti, R.; Rocculi, P.; Dalla Rosa, M.; Romani, S. Study on the efficacy of edible coatings on quality of blueberry fruits during shelf-life. LWT Food Sci. Technol. 2017, 85, 440–444. [Google Scholar] [CrossRef]
- Abugoch, L.; Tapia, C.; Plasencia, D.; Pastor, A.; Castro-Mandujano, O.; López, L.; Escalona, V.H. Shelf-life of fresh blueberries coating with quinoa protein/chitosan/sunflower oil edible film. J. Sci. Food Agric. 2016, 96, 619–626. [Google Scholar] [CrossRef]
- Kou, X.; He, Y.; Li, Y.; Chen, X.; Feng, Y.; Xue, Z. Effect of abscisic acid (ABA) and chitosan/nano-silica/sodium alginate composite film on the color development and quality of postharvest Chinese winter jujube (Zizyphus jujuba Mill. cv. Dongzao). Food Chem. 2019, 270, 385–394. [Google Scholar] [CrossRef]
- Rok, E. Application of Nano-Coating and Chitosan Combination Films on Cantaloupe Preservation. Pak. J. Biol. Sci. 2020, 23, 1037–1043. [Google Scholar]
- Shi, S.; Wang, W.; Liu, L.; Wu, S.; Wei, Y.; Li, W. Effect of chitosan/nano-silica coating on the physicochemical characteristics of longan fruit under ambient temperature. J. Food Eng. 2013, 118, 125–131. [Google Scholar] [CrossRef]
- Qiao, G.; Xiao, Z.; Ding, W.; Rok, A. Effect of Chitosan/Nano-Titanium Dioxide/Thymol and Tween Films on Ready-to-Eat Cantaloupe Fruit Quality. Coatings 2019, 9, 828. [Google Scholar] [CrossRef] [Green Version]
- Ahmad, V.; Jamal, M.S.; Alzohairy, Q.M.S.; Al Karaawi, M.A.; Siddiqui, M. Antimicrobial potential of bacteriocins: In therapy, agriculture and food preservation International. J. Antimicrob. Agents 2017, 49, 1–11. [Google Scholar] [CrossRef]
- Elsabee, M.Z.; Abdou, E.S. Chitosan based edible films and coatings: A review. Mater. Sci. Eng. C 2013, 33, 1819–1841. [Google Scholar] [CrossRef]
- Ícaro, P.; Aline, R.; Paulo, M.; Marcelo, B.; Mírian, R.; Carlos, S. Biodegradable Coatings on Blueberries Postharvest Conservation Refrigerated in a Modified Atmosphere. J. Exp. Agric. Int. 2018, 20, 1–11. [Google Scholar]
- Sun, Y.; Li, M.; Mitra, S.; Muhammad, R.H.; Debnath, B.; Lu, X.; Jian, H.; Qiu, D. Comparative phytochemical profiles and antioxidant enzyme activity analyses of the southern highbush blueberry (Vaccinium corymbosum) at different developmental stages. Molecules 2018, 23, 2209. [Google Scholar] [CrossRef] [Green Version]
- Pešaković, M.; Karaklajić-Stajić, Ž.; Milenković, S.; Mitrović, O. Biofertilizer affecting yield related characteristics of strawberry (Fragaria ananassa Duch.) and soil micro-organisms. Sci. Hortic. 2013, 150, 238–243. [Google Scholar] [CrossRef]
- Sonja, V.; Biljana, K.; Branka, S.; Flavia, N. Senescence- and drought-related changes in peroxidase and superoxide dismutase isoforms in leaves of Ramonda serbica. J. Exp. Bot. 2006, 57, 1759–1768. [Google Scholar]
- Nunes, M.C.N.; Brecht, J.K.; Morais, A.M.; Sargent, S.A. Possible influences of water loss and polyphenol oxidase activity on anthocyanin content and discolouration in fresh ripe strawberry during storage at 1 °C. J. Food Sci. 2005, 70, 116121. [Google Scholar] [CrossRef]
- Qiu, M.; Wu, C.; Ren, G.; Liang, X.; Wang, X.; Huang, J. Effect of chitosan and its derivatives as antifungal and preservative agents on postharvest green asparagus. Food Chem. 2014, 155, 105–111. [Google Scholar] [CrossRef]
- Reque, P.M.; Steffens, R.S.; Jablonski, A.; Flôres, S.H.; Rios, A.O.; Jong, E.V. Cold storage of blueberry (Vaccinium spp.) fruits and juice: Anthocyanin stability and antioxidant activity. J. Food Compos. Anal. 2014, 33, 111–116. [Google Scholar] [CrossRef]
- Sogvar, O.B.; Saba, M.K.; Emamifar, A. Aloe vera and ascorbic acid coatings maintain postharvest quality and reduce microbial load of strawberry fruit. Postharvest Biol. Technol. 2016, 114, 29–35. [Google Scholar] [CrossRef]
- Pavel, C.; Gabor, Z.; Dilyana, G.; Kamen, D.; Katia, V. Improvement of Texture Profile Attributes of Cooked Sausage Type “Krenvirsh”. Bulg. J. Agric. Sci. Agric. Acad. 2017, 23, 338–347. [Google Scholar]
- Yaman, Ö.; Bayo, L. Effects of an edible coating and cold storage on shelf-life and quality of cherries. LWT Food Sci. Technol. 2002, 35, 146–150. [Google Scholar] [CrossRef]
- Forney, C.F. Postharvest issues in blueberry and cranberry and methods to improve market-life. In IX International Vaccinium Symposium 810; Hummer, E.K., Ed.; ISHS Acta Horticulturae: Louvain, Belgium, 2008; pp. 785–798. [Google Scholar]
- Jiang, H.; Sun, Z.; Jia, R.; Wang, X.; Huang, J. Effect of Chitosan as An Antifungal and Preservative Agent on Postharvest Blueberry. J. Food Qual. 2016, 39, 516–523. [Google Scholar] [CrossRef]
- Vieira, J.M.; Flores-López, M.L.; Rodríguez, D.J.; Sousa, M.C.; Vicente, A.A.; Martins, J.T. Effect of chitosan–Aloe vera coating on postharvest quality of blueberry (Vacciniumcorymbosum) fruit. Postharvest Biol. Technol. 2016, 116, 88–97. [Google Scholar] [CrossRef] [Green Version]
- Koh, P.C.; Noranizan, M.A.; NurHanani, Z.A.; Karim, R.; Rosli, S.Z. Application of edible coatings and repetitive pulsed light for shelf life extension of fresh-cut cantaloupe (Cucumismelo L. reticulatus cv. Glamour). Postharvest Biol. Technol. 2017, 129, 64–78. [Google Scholar] [CrossRef]
- Benítez, S.; Achaerandio, I.; Sepulcre, F.; Pujolà, M. Aloe vera based edible coatings improve the quality of minimally processed ‘Hayward’ kiwifruit. Postharvest Biol. Technol. 2013, 81, 29–36. [Google Scholar] [CrossRef]
- Duan, J.Y.; Wu, R.Y.; Strik, B.C.; Zhao, Y.Y. Effect of edible coatings on the quality of fresh blueberries (duke and elliott) under commercial storage conditions. Postharvest Biol. Technol. 2011, 59, 71–79. [Google Scholar] [CrossRef]




© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Eldib, R.; Khojah, E.; Elhakem, A.; Benajiba, N.; Helal, M. Chitosan, Nisin, Silicon Dioxide Nanoparticles Coating Films Effects on Blueberry (Vaccinium myrtillus) Quality. Coatings 2020, 10, 962. https://doi.org/10.3390/coatings10100962
Eldib R, Khojah E, Elhakem A, Benajiba N, Helal M. Chitosan, Nisin, Silicon Dioxide Nanoparticles Coating Films Effects on Blueberry (Vaccinium myrtillus) Quality. Coatings. 2020; 10(10):962. https://doi.org/10.3390/coatings10100962
Chicago/Turabian StyleEldib, Rok, Ebtihal Khojah, Abeer Elhakem, Nada Benajiba, and Mahmoud Helal. 2020. "Chitosan, Nisin, Silicon Dioxide Nanoparticles Coating Films Effects on Blueberry (Vaccinium myrtillus) Quality" Coatings 10, no. 10: 962. https://doi.org/10.3390/coatings10100962
APA StyleEldib, R., Khojah, E., Elhakem, A., Benajiba, N., & Helal, M. (2020). Chitosan, Nisin, Silicon Dioxide Nanoparticles Coating Films Effects on Blueberry (Vaccinium myrtillus) Quality. Coatings, 10(10), 962. https://doi.org/10.3390/coatings10100962




