Structure, Formation, and Biological Interactions of Supported Lipid Bilayers (SLB) Incorporating Lipopolysaccharide
Abstract
1. Introduction
2. Different Methods of Formation of Artificial Lipid Membrane Models
2.1. Liposomes to Lipid Bilayer-Direct Vesicle Fusion
2.2. Monolayers at the Air–Water Interface
2.3. Langmuir–Blodgett Type Approaches
2.4. Spin-Coated Lipid Bilayers and Their Characterization
2.5. Vesicle Fusion Method Leading to Supported Lipid Bilayers (SLBs)
2.6. Self-Spreading of Lipid Layers on Solid Surfaces
3. Lipopolysaccharides in Monolayer Systems
3.1. Lipopolysaccharide Structure
3.2. Formation Conditions of Lipopolysaccharide (LPS) Monolayers
3.3. Structure of LPS Monolayers
- •
- Lipid A
- •
- Rough LPS
- •
- Smooth LPS (Native/Wild-Type LPS)
4. Physical Properties of Supported Lipid Membrane
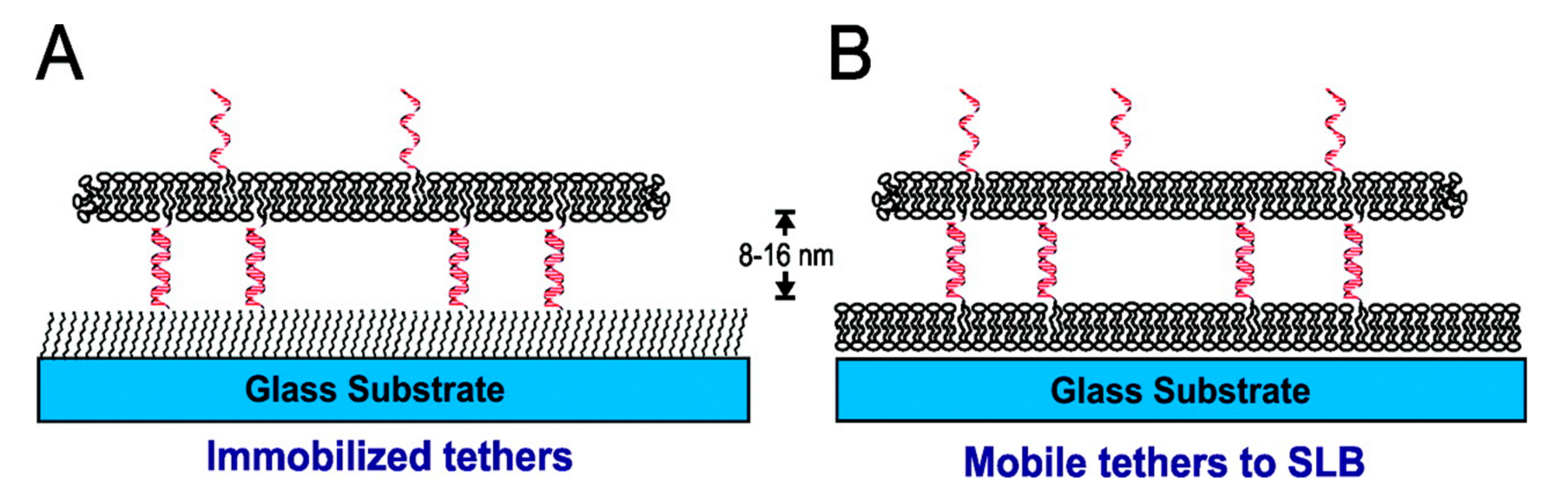
5. Types of Tethered Bilayer Lipid Membranes (t-BLMs)
6. Biological Binding of SLB with LPS
7. Conclusions
Funding
Conflicts of Interest
References
- Siontorou, C.G.; Nikoleli, G.P.; Nikolelis, D.P.; Karapetis, S.K. Artificial Lipid Membranes: Past, Present, and Future. Membranes 2017, 7, 38. [Google Scholar] [CrossRef] [PubMed]
- Edidin, M. Lipids on the frontier: A century of cell-membrane bilayers. Nat. Rev. Mol. Cell Biol. 2003, 4, 414–418. [Google Scholar] [CrossRef] [PubMed]
- Lind, T.K.; Cardenas, M. Understanding the formation of supported lipid bilayers via vesicle fusion-A case that exemplifies the need for the complementary method approach (Review). Biointerphases 2016, 11, 020801. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.S.; Dosoky, N.S.; Williams, J.D. Engineering lipid bilayer membranes for protein studies. Int. J. Mol. Sci. 2013, 14, 21561–21597. [Google Scholar] [CrossRef]
- Mueller, P.; Rudin, D.O.; Tien, H.T.; Wescott, W.C. Methods for the formation of single bimolecular lipid membranes in aqueous solution. J. Phys. Chem. 1963, 67, 534–535. [Google Scholar] [CrossRef]
- Ross, C.; Bean, W.C.S.; Chan, H. Permeability of Lipid Bilayer Membranes to Organic Solutes. J. Gen. Physiol. 1968, 52, 495–508. [Google Scholar]
- Gruner, S.M. Novel Multilayered Lipid Vesicles: Comparison of Physical Characteristics of Multilamellar Liposomes and Stable Plurilamellar Vesicles. Biochemistry 1985, 24, 2833–2842. [Google Scholar] [CrossRef]
- Lombard, J. Once upon a time the cell membranes: 175 years of cell boundary research. Biol. Direct. 2014, 9, 32. [Google Scholar] [CrossRef]
- Tamm, L.K.; McConnell, H.M. Supported phospholipid bilayers. Biophys. J. 1985, 47, 105–113. [Google Scholar] [CrossRef]
- Murray, D.H.; Tamm, L.K.; Kiessling, V. Supported double membranes. J. Struct. Biol 2009, 168, 183–189. [Google Scholar] [CrossRef] [PubMed]
- Plant, A.L. Self-Assembled Phospholipid/Alkanethiol Biomimetic Bilayers on Gold. Langmuir 1993, 9, 2764–2767. [Google Scholar] [CrossRef]
- Lang, H.; Duschl, C.; Vogel, H. A new class of thiolipids for the attachment of lipid bilayers on gold surfaces. Langmuir 1994, 10, 197–210. [Google Scholar] [CrossRef]
- Groves, J.T.; Ulman, N.; Boxer, S.G. Micropatterning Fluid Lipid Bilayers on Solid Supports. Sci. Rep. 1997, 275, 651–653. [Google Scholar] [CrossRef]
- Raguse, B.; Braach-Maksvytis, V.; Cornell, B.A.; King, L.G.; Osman, P.D.J.; Pace, R.J.; Wieczorek, L. Tethered Lipid Bilayer Membranes: Formation and Ionic Reservoir Characterization. Langmuir 1998, 14, 648–659. [Google Scholar] [CrossRef]
- Oudenaarden, A.V.; Boxer, S.G. Brownian Ratchets: Molecular Separations in Lipid Bilayers Supported on Patterned Arrays. Science 1999, 285, 1046–1048. [Google Scholar] [CrossRef] [PubMed]
- Sackmann, E.; Tanaka, M. Supported membranes on soft polymer cushions: Fabrication, characterization and applications. Trends Biotechnol. 2000, 18, 58–64. [Google Scholar] [CrossRef]
- Mennicke, U.; Salditt, T. Preparation of Solid-Supported Lipid Bilayers by Spin-Coating. Langmuir 2002, 18, 8172–8177. [Google Scholar] [CrossRef]
- Richard, C.; Balavoine, F.; Schultz, P.; Ebbesen, T.W.; Mioskowski, C. Supramolecular Self-Assembly of Lipid Derivatives on Carbon Nanotubes. Science 2003, 300, 775–778. [Google Scholar] [CrossRef] [PubMed]
- Mornet, S.P.; Lambert, O.; Duguet, E.; Brisson, A. The Formation of Supported Lipid Bilayers on Silica Nanoparticles Revealed by Cryoelectron Microscopy. Nano Lett. 2005, 5, 281–285. [Google Scholar] [CrossRef]
- Kalyankar, N.D.; Sharma, M.K.; Vaidya, S.V.; Calhoun, D.; Maldarelli, C.; Couzis, A.; Gilchrist, L. Arraying of Intact Liposomes into Chemically Functionalized Microwells. Langmuir 2006, 22, 5403–5411. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, Y.; Jin, G. PEGylated phospholipid membrane on polymer cushion and its interaction with cholesterol. Langmuir 2010, 26, 11140–11144. [Google Scholar] [CrossRef] [PubMed]
- Richter, R.P.; Berat, R.; Brisson, A.R. Formation of Solid-Supported Lipid Bilayers: An Integrated View. Langmuir 2006, 22, 3497–3505. [Google Scholar] [CrossRef] [PubMed]
- Berti, D.; Caminati, G.; Baglioni, P. Functional liposomes and supported lipid bilayers: Towards the complexity of biological archetypes. Phys. Chem. Chem. Phys. 2011, 13, 8769–8782. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Jass, J.; Tjarnhage, T.; Puu, G. From Liposomes to Supported, Planar Bilayer Structures on Hydrophilic and Hydrophobic Surfaces: An Atomic Force Microscopy Study. Biophys. J. 2000, 79, 3153–3163. [Google Scholar] [CrossRef]
- Puu, G.; Gustafson, I. Planar lipid bilayers on solid supports from liposomes-factors of importance for kinetics and stability. Biochim. Biophys. Acta 1997, 1327, 149–161. [Google Scholar] [CrossRef]
- Strauss, G.; Ingenito, E.P. Stabilization of Liposome Bilayers to Freezing and Thawing: Effects of Cryoprotective Agents and Membrane Proteins. Cryobiology 1980, 17, 508–515. [Google Scholar] [CrossRef]
- Ye, Q.; Konradi, R.; Textor, M.; Reimhult, E. Liposomes tethered to omega-functional PEG brushes and induced formation of PEG brush supported planar lipid bilayers. Langmuir 2009, 25, 13534–13539. [Google Scholar] [CrossRef]
- Kumar, K.; Isa, L.; Egner, A.; Schmidt, R.; Textor, M.; Reimhult, E. Formation of nanopore-spanning lipid bilayers through liposome fusion. Langmuir 2011, 27, 10920–10928. [Google Scholar] [CrossRef] [PubMed]
- Jing, Y.; Trefna, H.; Persson, M.; Kasemo, B.; Svedhem, S. Formation of supported lipid bilayers on silica: Relation to lipid phase transition temperature and liposome size. Soft Matter 2014, 10, 187–195. [Google Scholar] [CrossRef] [PubMed]
- McConnell, H.M.; Tamm, L.K.; Weis, R.M. Periodic structures in lipid monolayer phase transitions. Proc. Natl. Acad. Sci. USA 1984, 81, 3249–3253. [Google Scholar] [CrossRef]
- von Tscharner, V.; McConnell, H.M. An alternative view of phospholipid phase behavior at the air-water interface. Microscope and film balance studies. Biophys. J. 1981, 36, 409–419. [Google Scholar] [CrossRef]
- Klopfer, K.J.; Vanderlick, T.K. Isotherms of Dipalmitoylphosphatidylcholine (DPPC) Monolayers: Features Revealed and Features Obscured. J. Colloid Interface Sci. 1996, 182, 220–229. [Google Scholar] [CrossRef]
- Brown, R.E.; Brockman, H.L. Using Monomolecular Films to Characterize Lipid Lateral Interactions. In Lipid Rafts; McIntosh, T.J., Ed.; Humana Press: Totowa, NJ, USA, 2007; pp. 41–58. [Google Scholar]
- Ali, S.; Smaby, J.M.; Brockman, H.L.; Brown, R.E. Cholesterol’s interfacial interactions with galactosylceramides. Biochemistry 1994, 33, 2900–2906. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.Y.C.; McConnell, H.M. Quantized symmetry of liquid monolayer domains. J. Phys. Chem. 1993, 97, 9532–9539. [Google Scholar] [CrossRef]
- Rice, P.A.; McConnell, H.M. Critical shape transitions of monolayer lipid domains. Proc. Natl. Acad. Sci. USA 1989, 86, 6445–6448. [Google Scholar] [CrossRef] [PubMed]
- McConnell, H.M. Harmonic shape transitions in lipid monolayer domains. J. Phys. Chem. 1990, 94, 4728–4731. [Google Scholar] [CrossRef]
- Ariga, K.; Hill, J.P. Monolayers at air-water interfaces: From origins-of-life to nanotechnology. Chem. Rec. 2011, 11, 199–211. [Google Scholar] [CrossRef] [PubMed]
- David, V.; Kjaer, K.; Als-Nielsen, J.; Losche, M. Structural properties of phosphatidylcholine in a monolayer at the air/water interface. Biophys. J. 1991, 59, 1325–1332. [Google Scholar]
- Tronin, A.; Dubrovsky, T.; Nicolini, C. Comparative study of Langmuir monolayers of immunoglobines G formed at the air-water interface and covalently immobilized on solid supports. Langmuir 1995, 11, 385–389. [Google Scholar] [CrossRef]
- Faure, M.C.; Bassereau, P.; Carignano, M.A.; Szleifer, I.; Gallot, Y.; Andelman, D. Monolayers of diblock copolymer at the air-water interface: The attractive monomer-surface case. Eur. Phys. J. B 1998, 3, 365–375. [Google Scholar] [CrossRef][Green Version]
- Du, X.; Shi, B.; Liang, Y. N-Octadecanoyl-L-alanine Amphiphile Monolayer at the Air/Water Interface and LB Film Studied by FTIR Spectroscopy. Langmuir 1998, 14, 3631–3636. [Google Scholar] [CrossRef]
- Nandi, N.; Vollhardt, D. Chiral discrimination and recognition in Langmuir monolayers. Curr. Opin. Colloid Interface Sci. 2008, 13, 40–46. [Google Scholar] [CrossRef]
- Nandi, N.; Vollhardt, D. Effect of Molecular Chirality on the Morphology of Biomimetic Langmuir Monolayers. Chem. Rev. 2003, 103, 4033–4076. [Google Scholar] [CrossRef] [PubMed]
- Michinobu, T.; Shinoda, S.; Nakanishi, T.; Hill, J.P.; Fujii, K.; Player, T.N.; Tsukube, H.; Ariga, K. Mechanical Control of Enantioselectivity of Amino Acid Recognition by Cholesterol-Armed Cyclen Monolayer at the Air-Water Interface. J. Am. Chem. Soc. 2006, 128, 14478–14479. [Google Scholar] [CrossRef] [PubMed]
- Ebara, Y.; Okahata, Y. In Situ Surface-Detecting Technique by Using a Quartz-Crystal Microbalance. InteractionmBehaviors of Proteins onto a Phospholipid Monolayer at the Air-Water Interface. Langmuir 1993, 9, 574–576. [Google Scholar] [CrossRef]
- Li, J.; Chen, Z.; Wang, X.; Brezesinski, G.; Mohwald, H. Dynamic observations of the hydrolysis of a DPPC monolayer at the air/water interface catalyzed by phospholipase A2. Angew. Chem. Int. Ed. 2000, 39, 3059–3062. [Google Scholar] [CrossRef]
- Miao, W.; Du, X.; Liang, Y. Molecular Recognition of Nucleolipid Monolayers of 1-(2-Octadecyloxycarbonylethyl)cytosine to Guanosine at the Air-Water Interface and Langmuir-Blodgett Films. Langmuir 2003, 19, 5389–5396. [Google Scholar] [CrossRef]
- Zhao, L.; Feng, S.S. Effects of cholesterol component on molecular interactions between paclitaxel and phospholipid within the lipid monolayer at the air-water interface. J. Colloid Interface Sci. 2006, 300, 314–326. [Google Scholar] [CrossRef]
- Chen, Q.; Zhang, D.; Li, R.; Liu, H.; Hu, Y. Effect of the spacer group on the behavior of the cationic Gemini surfactant monolayer at the air/water interface. Thin Solid Films 2008, 516, 8782–8787. [Google Scholar] [CrossRef]
- Chen, Q.; Kang, X.; Li, R.; Du, X.; Shang, Y.; Liu, H.; Hu, Y. Structure of the complex monolayer of gemini surfactant and DNA at the air/water interface. Langmuir 2012, 28, 3429–3438. [Google Scholar] [CrossRef]
- Jyoti, A.; Prokop, R.M.; Li, J.; Vollhardt, D.; Kwok, D.Y.; Miller, R.; Mohwald, H.; Neumann, A.W. An investigation of the compression rate dependence on the surface pressure-surface area isotherm for a dipalmitoyl phosphatidylcholine monolayer at the air/water interface. Colloids Surf. A Physicochem. Eng. Asp. 1996, 173–180. [Google Scholar] [CrossRef]
- Patino, J.M.R.; Sanchez, C.C.; Nino, M.R.R. Structural and morphological characteristics of beta-casein monolayers at the air–water interface. Food Hydrocoll. 1999, 13, 401–408. [Google Scholar] [CrossRef]
- Ma, G.; Allen, H.C. DPPC Langmuir Monolayer at the Air-Water Interface: Probing the Tail and Head Groups by Vibrational Sum Frequency Generation Spectroscopy. Langmuir 2006, 22, 5341–5349. [Google Scholar] [CrossRef] [PubMed]
- Dufrêne, Y.F.; Lee, G.U. Advances in the characterization of supported lipid films with the atomic force microscope. Biochim. Biophys. Acta Biomembr. 2000, 1509, 14–41. [Google Scholar] [CrossRef]
- Kurniawan, J.; Ventrici de Souza, J.F.; Dang, A.T.; Liu, G.-Y.; Kuhl, T.L. Preparation and Characterization of Solid-Supported Lipid Bilayers Formed by Langmuir–Blodgett Deposition: A Tutorial. Langmuir 2018, 34, 15622–15639. [Google Scholar] [CrossRef]
- Blodgett, K.B.; Langmuir, I. Built-up films of barium stearate and their optical properties. Phys. Rev. 1937, 51, 964–982. [Google Scholar] [CrossRef]
- Cea, P.; Ballesteros, L.M.; Martín, S. Nanofabrication techniques of highly organized monolayers sandwiched between two electrodes for molecular electronics. Nanofabrication 2014, 1, 96–117. [Google Scholar] [CrossRef]
- Rojewska, M.; Skrzypiec, M.; Prochaska, K. The wetting properties of Langmuir-Blodgett and Langmuir-Schaefer films formed by DPPC and POSS compounds. Chem. Phys. Lipids 2019, 221, 158–166. [Google Scholar] [CrossRef]
- Marfin, Y.S.; Usoltsev, S.D.; Kazak, A.V.; Smirnova, А.I.; Rumyantsev, Е.V.; Molchanov, E.E.; Kuznetsov, V.V.; Chumakov, А.S.; Glukhovskoy, Е.G. Synthesis and spectral properties of preorganized BODIPYs in solutions and Langmuir-Schaefer films. Appl. Surf. Sci. 2017, 424, 228–238. [Google Scholar] [CrossRef]
- Stottrup, B.L.; Veatch, S.L.; Keller, S.L. Nonequilibrium Behavior in Supported Lipid Membranes Containing Cholesterol. Biophys. J. 2004, 86, 2942–2950. [Google Scholar] [CrossRef]
- Deverall, M.A.; Gindl, E.; Sinner, E.K.; Besir, H.; Ruehe, J.; Saxton, M.J.; Naumann, C.A. Membrane lateral mobility obstructed by polymer-tethered lipids studied at the single molecule level. Biophys. J. 2005, 88, 1875–1886. [Google Scholar] [CrossRef] [PubMed]
- Clifton, L.A.; Skoda, M.W.; Daulton, E.L.; Hughes, A.V.; Le Brun, A.P.; Lakey, J.H.; Holt, S.A. Asymmetric phospholipid: Lipopolysaccharide bilayers; a Gram-negative bacterial outer membrane mimic. J. R Soc. Interface 2013, 10, 1–11. [Google Scholar] [CrossRef]
- Clifton, L.A.; Holt, S.A.; Hughes, A.V.; Daulton, E.L.; Arunmanee, W.; Heinrich, F.; Khalid, S.; Jefferies, D.; Charlton, T.R.; Webster, J.R.; et al. An accurate in vitro model of the E. coli envelope. Angew. Chem. Int. Ed. Engl. 2015, 54, 11952–11955. [Google Scholar] [CrossRef]
- Clifton, L.A.; Ciesielski, F.; Skoda, M.W.; Paracini, N.; Holt, S.A.; Lakey, J.H. The Effect of Lipopolysaccharide Core Oligosaccharide Size on the Electrostatic Binding of Antimicrobial Proteins to Models of the Gram Negative Bacterial Outer Membrane. Langmuir 2016, 32, 3485–3494. [Google Scholar] [CrossRef]
- Riegler, H.; Spratte, K. Structural changes in lipid monolayers during the Langmuir-Blodgett transfer due to substrate/monolayer interactions. Thin Solid Films 1992, 210–211, 9–12. [Google Scholar] [CrossRef]
- Simonsen, A.C.; Bagatolli, L.A. Structure of Spin-Coated Lipid Films and Domain Formation in Supported Membranes Formed by Hydration. Langmuir 2004, 20, 9720–9728. [Google Scholar] [CrossRef] [PubMed]
- Dols-Perez, A.; Fumagalli, L.; Simonsen, A.C.; Gomila, G. Ultrathin spin-coated dioleoylphosphatidylcholine lipid layers in dry conditions: A combined atomic force microscopy and nanomechanical study. Langmuir 2011, 27, 13165–13172. [Google Scholar] [CrossRef]
- Krapf, L.; Dezi, M.; Reichstein, W.; Kohler, J.; Oellerich, S. AFM characterization of spin-coated multilayered dry lipid films prepared from aqueous vesicle suspensions. Colloids Surf. B 2011, 82, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Hardy, G.J.; Nayak, R.; Zauscher, S. Model cell membranes: Techniques to form complex biomimetic supported lipid bilayers via vesicle fusion. Curr. Opin. Colloid Interface Sci. 2013, 18, 448–458. [Google Scholar] [CrossRef]
- Lind, T.K.; Cárdenas, M.; Wacklin, H.P. Formation of Supported Lipid Bilayers by Vesicle Fusion: Effect of Deposition Temperature. Langmuir 2014, 30, 7259–7263. [Google Scholar] [CrossRef] [PubMed]
- Jackman, J.A.; Cho, N.-J. Supported Lipid Bilayer Formation: Beyond Vesicle Fusion. Langmuir 2020, 36, 1387–1400. [Google Scholar] [CrossRef] [PubMed]
- Hamai, C.; Yang, T.; Kataoka, S.; Cremer, P.S.; Musser, S.M. Effect of Average Phospholipid Curvature on Supported Bilayer Formation on Glass by Vesicle Fusion. Biophys. J. 2006, 90, 1241–1248. [Google Scholar] [CrossRef] [PubMed]
- Pace, H.; Simonsson, N.L.; Gunnarsson, A.; Eck, E.; Monson, C.; Geschwindner, S.; Snijder, A.; Höök, F. Preserved Transmembrane Protein Mobility in Polymer-Supported Lipid Bilayers Derived from Cell Membranes. Anal. Chem. 2015, 87, 9194–9203. [Google Scholar] [CrossRef] [PubMed]
- Tabaei, S.R.; Choi, J.-H.; Haw Zan, G.; Zhdanov, V.P.; Cho, N.-J. Solvent-Assisted Lipid Bilayer Formation on Silicon Dioxide and Gold. Langmuir 2014, 30, 10363–10373. [Google Scholar] [CrossRef]
- Gillissen, J.J.J.; Tabaei, S.R.; Cho, N.-J. A phenomenological model of the solvent-assisted lipid bilayer formation method. Phys. Chem. Chem. Phys. 2016, 18, 24157–24163. [Google Scholar] [CrossRef]
- Furukawa, K.; Hibino, H. Self-spreading of Supported Lipid Bilayer on SiO2 Surface Bearing Graphene Oxide. Chem. Lett. 2012, 41, 1259–1261. [Google Scholar] [CrossRef]
- Benes, M.; Billy, D.; Benda, A.; Speijer, H.; Hof, M.; Hermens, W.T. Surface-Dependent Transitions during Self-Assembly of Phospholipid Membranes on Mica, Silica, and Glass. Langmuir 2004, 20, 10129–10137. [Google Scholar] [CrossRef] [PubMed]
- Mundev, D.; Turyan, I. Applications of Self-Assembled Monolayers in Electroanalytical Chemistry. Electroanalysis 1996, 8, 207–213. [Google Scholar]
- Cremer, P.S.; Boxer, S.G. Formation and Spreading of Lipid Bilayers on Planar Glass Supports. J. Phys. Chem. B 1999, 103, 2554–2559. [Google Scholar] [CrossRef]
- Peng, Z.; Shimba, K.; Miyamoto, Y.; Yagi, T. Nanopore lipid bilayer formed by self-spreading method. Electron. Commun. Jpn. 2019, 102, 47–54. [Google Scholar] [CrossRef]
- Furukawa, K.; Sumitomo, K.; Nakashima, H.; Kashimura, Y.; Torimitsu, K. Supported Lipid Bilayer Self-Spreading on a Nanostructured Silicon Surface. Langmuir 2007, 23, 367–371. [Google Scholar] [CrossRef] [PubMed]
- Park, J.Y.; Advincula, R.C. Nanostructuring polymers, colloids, and nanomaterials at the air–water interface through Langmuir and Langmuir–Blodgett techniques. Soft Matter 2011, 7, 9829–9843. [Google Scholar] [CrossRef]
- Gidalevitz, D.; Huang, Z.; Rice, S.A. Protein folding at the air–water interface studied with x-ray reflectivity. Proc. Natl. Acad. Sci. USA 1999, 96, 2608–2611. [Google Scholar] [CrossRef]
- Biswas, S.; Bhattacharjee, D.; Nath, R.K.; Hussain, S.A. Formation of complex Langmuir and Langmuir–Blodgett films of water soluble rosebengal. J. Colloid Interface Sci. 2007, 311, 361–367. [Google Scholar] [CrossRef] [PubMed]
- Savva, M.; Sivakumar, B.; Selvi, B. The Conventional Langmuir Trough Technique as a Convenient Means to Determine the Solubility of Sparingly Soluble Surface Active Molecules: Case Study Cholesterol. Colloids Surf. A Physicochem. Eng. Asp. 2008, 325, 1–6. [Google Scholar] [CrossRef]
- Nie, H.-L.; Dou, X.; Tang, Z.; Jang, H.D.; Huang, J. High-Yield Spreading of Water-Miscible Solvents on Water for Langmuir–Blodgett Assembly. J. Am. Chem. Soc. 2015, 137, 10683–10688. [Google Scholar] [CrossRef]
- Chan, Y.-H.M.; Boxer, S.G. Model membrane systems and their applications. Curr. Opin. Chem. Biol. 2007, 11, 581–587. [Google Scholar] [CrossRef]
- Yeung, S.Y.; Ederth, T.; Pan, G.; Cicenaite, J.; Cárdenas, M.; Arnebrant, T.; Sellergren, B.r. Reversible Self-Assembled Monolayers (rSAMs) as Robust and Fluidic Lipid Bilayer Mimics. Langmuir 2018, 34, 4107–4115. [Google Scholar] [CrossRef]
- Andersson, J.; Köper, I.; Knoll, W. Tethered Membrane Architectures—Design and Applications. Front. Mater. 2018, 5, 55. [Google Scholar] [CrossRef]
- Glazier, R.; Salaita, K. Supported lipid bilayer platforms to probe cell mechanobiology. Biochim. Biophys. Acta Biomembr. 2017, 1859, 1465–1482. [Google Scholar] [CrossRef]
- Adams, P.G.; Swingle, K.L.; Paxton, W.F.; Nogan, J.J.; Stromberg, L.R.; Firestone, M.A.; Mukundan, H.; Montaño, G.A. Exploiting lipopolysaccharide-induced deformation of lipid bilayers to modify membrane composition and generate two-dimensional geometric membrane array patterns. Sci. Rep. 2015, 5, 10331. [Google Scholar] [CrossRef] [PubMed]
- Kaeothip, S.; Paranjape, G.; Terrill, S.E.; Bongat, A.F.G.; Udan, M.L.D.; Kamkhachorn, T.; Johnson, H.L.; Nichols, M.R.; Demchenko, A.V. Development of LPS antagonistic therapeutics: Synthesis and evaluation of glucopyranoside-spacer-amino acid motifs. RSC Adv. 2011, 1, 83–92. [Google Scholar] [CrossRef]
- Jeworrek, C.; Evers, F.; Howe, J.; Brandenburg, K.; Tolan, M.; Winter, R. Effects of specific versus nonspecific ionic interactions on the structure and lateral organization of lipopolysaccharides. Biophys. J. 2011, 100, 2169–2177. [Google Scholar] [CrossRef] [PubMed]
- Le Brun, A.P.; Clifton, L.A.; Halbert, C.E.; Lin, B.; Meron, M.; Holden, P.J.; Lakey, J.H.; Holt, S.A. Structural characterization of a model gram-negative bacterial surface using lipopolysaccharides from rough strains of Escherichia coli. Biomacromolecules 2013, 14, 2014–2022. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, A.D.; Welte, W.; Hofmann, E.; Lindner, B.; Holst, O.; Coulton, J.W.; Diederichs, K. A conserved structural motif for lipopolysaccharide recognition by procaryotic and eucaryotic proteins. Structure 2000, 8, 585–592. [Google Scholar] [CrossRef]
- Hong-bao, M.; Ma, M.; Yan, Y. Lipopolysaccharide (LPS) and Hypoxia Inducible Factor (HIF)-1alpha Research literatures. Rep. Opin. 2015, 7, 93–112. [Google Scholar]
- Nepal, B.; Stine, K.J. Monolayers of Carbohydrate-Containing Lipids at the Water-Air Interface. In Cell Culture; Radwa, A.M., Ed.; BoD—Books on Demand: London, UK, 2018; pp. 213–241. [Google Scholar]
- Abraham, T.; Schooling, S.R.; Beveridge, T.J.; Katsaras, J. Monolayer Film Behavior of Lipopolysaccharide from Pseudomonas aeruginosa at the Air−Water Interface. Biomacromolecules 2008, 9, 2799–2804. [Google Scholar] [CrossRef] [PubMed]
- Neter, E.; Westphal, O.; Lüderitz, O.; Gorzynski, E.A.; Eichenberger, E. Studies of Enterobacterial Lipopolysaccharides. Effects of Heat and Chemicals on Erythrocyte-Modifying, Antigenic, Toxic and Pyrogenic Properties. J. Immunol. 1956, 76, 377–385. [Google Scholar] [PubMed]
- Neter, E.; Gorzynski, E.A.; Westphal, O.; Lüderitz, O. The Effects of Antibiotics on Enterobacterial Lipopolysaccharides (Endotoxins), Hemagglutination and Hemolysis. J. Immunol. 1958, 80, 66–72. [Google Scholar]
- Rothfield, L.; Horne, R.W. Reassociation of Purified Lipopolysaccharide and Phospholipid of the Bacterial Cell Envelope: Electron Microscopic and Monolayer Studies. J. Bacteriol. 1967, 93, 1705–1721. [Google Scholar] [CrossRef] [PubMed]
- Shands, J.J.; Graham, J.; Nath, K. The morphologic structure of isolated bacterial lipopolysaccharide. J. Mol. Biol. 1967, 25, 15–21. [Google Scholar] [CrossRef]
- Romeo, D.; Girard, A.; Rothfield, L. Reconstitution of a functional membrane enzyme system in a monomolecular film: I. Formation of a mixed monolayer of lipopolysaccharide and phospholipid. J. Mol. Biol. 1970, 53, 475–490. [Google Scholar] [CrossRef]
- Roes, S.; Seydel, U.; Gutsmann, T. Probing the Properties of Lipopolysaccharide Monolayers and Their Interaction with the Antimicrobial Peptide Polymyxin B by Atomic Force Microscopy. Langmuir 2005, 21, 6970–6978. [Google Scholar] [CrossRef]
- Derde, M.; Nau, F.; Lechevalier, V.; Guerin-Dubiard, C.; Paboeuf, G.; Jan, S.; Baron, F.; Gautier, M.; Vie, V. Native lysozyme and dry-heated lysozyme interactions with membrane lipid monolayers: Lateral reorganization of LPS monolayer, model of the Escherichia coli outer membrane. Biochim. Biophys. Acta 2015, 1848, 174–183. [Google Scholar] [CrossRef] [PubMed]
- Clifton, L.A.; Skoda, M.W.; Le Brun, A.P.; Ciesielski, F.; Kuzmenko, I.; Holt, S.A.; Lakey, J.H. Effect of divalent cation removal on the structure of gram-negative bacterial outer membrane models. Langmuir 2015, 31, 404–412. [Google Scholar] [CrossRef]
- Micciulla, S.; Gerelli, Y.; Schneck, E. Structure and Conformation of Wild-Type Bacterial Lipopolysaccharide Layers at Air-Water Interfaces. Biophys. J. 2019, 116, 1259–1269. [Google Scholar] [CrossRef]
- Sondhi, P.; Maruf, M.H.U.; Stine, K.J. Nanomaterials for Biosensing Lipopolysaccharide. Biosensors 2019, 10, 2. [Google Scholar] [CrossRef] [PubMed]
- Carotovora, E.; Fukuoka, S.; Brandenburg, K.; Muller, M.; Lindner, B.; Koch, M.H.J.; Seydel, U. Physico-chemical analysis of lipid A fractions of lipopolysaccharide from Erwinia carotovora in relation to bioactivity. Biochim. Biophys. Acta 2001, 1510, 185–197. [Google Scholar]
- Yu, Z.-W.; Jin, J.; Cao, Y. Characterization of the liquid-expanded to liquid-condensed phase transition of monolayers by means of compressibility. Langmuir 2002, 18, 4530–4531. [Google Scholar] [CrossRef]
- Seydel, U.; Oikawa, M.; Fukase, K.; Kusumoto, S.; Brandenburg, K. Intrinsic conformation of lipid A is responsible for agonistic and antagonistic activity. Eur. J. Biochem. 2000, 267, 3032–3039. [Google Scholar] [CrossRef]
- Luderitz, O.; Staub, A.M.; Westphal, O. Immunochemistry of 0 and R Antigens of Salmonella and Related Enterobacteriaceae. Bacteriol. Rev. 1966, 30, 192–255. [Google Scholar] [CrossRef]
- Parikh, A.N.; Groves, J.T. Materials Science of Supported Lipid Membranes. MRS Bull. 2011, 31, 507–512. [Google Scholar] [CrossRef]
- Pompeo, G.; Girasole, M.; Cricenti, A.; Cattaruzza, F.; Flamini, A.; Prosperi, T.; Generosi, J.; Castellano, A.C. AFM characterization of solid-supported lipid multilayers prepared by spin-coating. Biochim. Biophys. Acta 2005, 1712, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Hianik, T.; Haburcak, M.; Lohner, K.; Prenner, E.; Paltauf, F.; Hermetter, A. Compressibility and density of lipid bilayers composed of polyunsaturated phospholipids and cholestrol. Colloids Surf. Physicochem. Eng. Asp. 1998, 139, 189–197. [Google Scholar] [CrossRef]
- Radler, J.; Strey, H.; Sackmann, E. Phenomenology and kinetis of lipid bilayer spreading on hydrophilic surfaces. Langmuir 1995, 11, 4539–4548. [Google Scholar] [CrossRef]
- Honigmann, A.; Mueller, V.; Hell, S.W.; Eggeling, C. STED microscopy detects and quantifies liquid phase separation in lipid membranes using a new far-red emitting fluorescent phosphoglycerolipid analogue. Faraday Discuss. 2013, 161, 77–89. [Google Scholar] [CrossRef] [PubMed]
- Honigmann, A.; Mueller, V.; Ta, H.; Schoenle, A.; Sezgin, E.; Hell, S.W.; Eggeling, C. Scanning STED-FCS reveals spatiotemporal heterogeneity of lipid interaction in the plasma membrane of living cells. Nat. Commun. 2014, 5, 5412. [Google Scholar] [CrossRef]
- Tero, R. Substrate Effects on the Formation Process, Structure and Physicochemical Properties of Supported Lipid Bilayers. Materials 2012, 5, 2658–2680. [Google Scholar] [CrossRef]
- Huang, C.-J.; Chang, Y.-C. Construction of Cell–Extracellular Matrix Microenvironments by Conjugating ECM Proteins on Supported Lipid Bilayers. Front. Mater. 2019, 6, 39. [Google Scholar] [CrossRef]
- Subczynski, W.K.; Wisniewska, A. Physical properties of lipid bilayer membranes: Relevance to membrane biological functions. Acta Biochim. Pol. 2000, 47, 613–625. [Google Scholar] [CrossRef] [PubMed]
- Howland, M.C.; Szmodis, A.W.; Sanii, B.; Parikh, A.N. Characterization of physical properties of supported phospholipid membranes using imaging ellipsometry at optical wavelengths. Biophys. J. 2007, 92, 1306–1317. [Google Scholar] [CrossRef] [PubMed]
- Janmey, P.A.; Kinnunen, P.K. Biophysical properties of lipids and dynamic membranes. Trends Cell Biol. 2006, 16, 538–546. [Google Scholar] [CrossRef] [PubMed]
- Lind, T.K.; Skoda, M.W.; Cárdenas, M. Formation and characterization of supported lipid bilayers composed of phosphatidylethanolamine and phosphatidylglycerol by vesicle fusion, a simple but relevant model for bacterial membranes. ACS Omega 2019, 4, 10687–10694. [Google Scholar] [CrossRef] [PubMed]
- Stahelin, R.V. Surface plasmon resonance: A useful technique for cell biologists to characterize biomolecular interactions. Mol. Biol. Cell 2013, 24, 883–886. [Google Scholar] [CrossRef]
- Rossi, C.; Chopineau, J. Biomimetic tethered lipid membranes designed for membrane-protein interaction studies. Eur. Biophys. J. 2007, 36, 955–965. [Google Scholar] [CrossRef]
- Kiselev, M.A.; Lombardo, D. Structural characterization in mixed lipid membrane systems by neutron and X-ray scattering. Biochim. Biophys. Acta Gen. Subj. 2017, 1861, 3700–3717. [Google Scholar] [CrossRef]
- Sut, T.N.; Park, S.; Choe, Y.; Cho, N.-J. Characterizing the Supported Lipid Membrane Formation from Cholesterol-Rich Bicelles. Langmuir 2019, 35, 15063–15070. [Google Scholar] [CrossRef]
- Khan, M.S.; Dosoky, N.S.; Patel, D.; Weimer, J.; Williams, J.D. Lipid Bilayer Membrane in a Silicon Based Micron Sized Cavity Accessed by Atomic Force Microscopy and Electrochemical Impedance Spectroscopy. Biosensors 2017, 7, 26. [Google Scholar] [CrossRef]
- Clausen, M.P.; Sezgin, E.; Bernardino de la Serna, J.; Waithe, D.; Lagerholm, B.C.; Eggeling, C. A straightforward approach for gated STED-FCS to investigate lipid membrane dynamics. Methods 2015, 88, 67–75. [Google Scholar] [CrossRef]
- Sezgin, E.; Levental, I.; Mayor, S.; Eggeling, C. The mystery of membrane organization: Composition, regulation and roles of lipid rafts. Nat. Rev. Mol. Cell Biol. 2017, 18, 361–374. [Google Scholar] [CrossRef]
- Chung, M.; Boxer, S.G. Stability of DNA-Tethered Lipid Membranes with Mobile Tethers. Langmuir 2011, 27, 5492–5497. [Google Scholar] [CrossRef] [PubMed]
- Giess, F.; Friedrich, M.G.; Heberle, J.; Naumann, R.L.; Knoll, W. The protein-tethered lipid bilayer: A novel mimic of the biological membrane. Biophys. J. 2004, 87, 3213–3220. [Google Scholar] [CrossRef] [PubMed]
- Ragaliauskas, T.; Mickevicius, M.; Rakovska, B.; Penkauskas, T.; Vanderah, D.J.; Heinrich, F.; Valincius, G. Fast formation of low-defect-density tethered bilayers by fusion of multilamellar vesicles. Biochim. Biophys. Acta Biomembr. 2017, 1859, 669–678. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.K.; Lee, H.Y.; Kim, P.; Suh, K.Y.; Kawai, T. Nanoarrays of tethered lipid bilayer rafts on poly(vinyl alcohol) hydrogels. Lab Chip 2009, 9, 132–139. [Google Scholar] [CrossRef] [PubMed]
- Krishna, G.; Schulte, J.; Cornell, B.A.; Pace, R.J.; Osman, P.D. Tethered Bilayer Membranes Containing Ionic Reservoirs: Selectivity and Conductance. Langmuir 2003, 19, 2294–2305. [Google Scholar] [CrossRef]
- Keizer, H.M.; Dorvel, B.R.; Andersson, M.; Fine, D.; Price, R.B.; Long, J.R.; Dodabalapur, A.; Koper, I.; Knoll, W.; Anderson, P.A.; et al. Functional ion channels in tethered bilayer membranes--implications for biosensors. Chembiochem. 2007, 8, 1246–1250. [Google Scholar] [CrossRef] [PubMed]
- Maccarini, M.; Watkins, E.B.; Stidder, B.; Alcaraz, J.P.; Cornell, B.A.; Martin, D.K. Nanostructural determination of a lipid bilayer tethered to a gold substrate. Eur. Phys. J. E Soft Matter 2016, 39, 123–131. [Google Scholar] [CrossRef]
- Rebaud, S.; Maniti, O.; Girard-Egrot, A.P. Tethered bilayer lipid membranes (tBLMs): Interest and applications for biological membrane investigations. Biochimie 2014, 107, 135–142. [Google Scholar] [CrossRef] [PubMed]
- Penkauskas, T.; Preta, G. Biological applications of tethered bilayer lipid membranes. Biochimie 2019, 157, 131–141. [Google Scholar] [CrossRef]
- Jackman, J.; Knoll, W.; Cho, N.-J. Biotechnology Applications of Tethered Lipid Bilayer Membranes. Materials 2012, 5, 2637–2657. [Google Scholar] [CrossRef]
- Chan, Y.-H.M.; van Lengerich, B.; Boxer, S.G. Effects of linker sequences on vesicle fusion mediated by lipid-anchored DNA oligonucleotides. Proc. Natl. Acad. Sci. USA 2009, 106, 979–984. [Google Scholar] [CrossRef]
- Andersson, J.; Köper, I. Tethered and Polymer Supported Bilayer Lipid Membranes: Structure and Function. Membranes 2016, 6, 30. [Google Scholar] [CrossRef] [PubMed]
- Naumann, C.A.; Prucker, O.; Lehmann, T.; Rühe, J.; Knoll, W.; Frank, C.W. The Polymer-Supported Phospholipid Bilayer: Tethering as a New Approach to Substrate−Membrane Stabilization. Biomacromolecules 2002, 3, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Peetla, C.; Stine, A.; Labhasetwa, V. Biophysical Interactions with Model Lipid Membranes: Applications in Drug Discovery and Drug Deliver. Mol. Pharm. 2009, 6, 1264–1276. [Google Scholar] [CrossRef]
- Marques, J.T.; Viana, A.S.; de Almeida, R.F. A biomimetic platform to study the interactions of bioelectroactive molecules with lipid nanodomains. Langmuir 2014, 30, 12627–12637. [Google Scholar] [CrossRef] [PubMed]
- Morandat, S.; Kirat, K.E. Exploring the Properties and Interactions of Supported Lipid Bilayers on the Nanoscale by Atomic Force Microscopy. Microsc. Sci. Technol. Appl. Educ. 2010, 4, 1925–1939. [Google Scholar]
- Horton, M.R.; Reich, C.; Gast, A.P.; Radler, J.O.; Nickel, B. Structure and Dynamics of Crystalline Protein Layers Bound to Supported Lipid Bilayers. Langmuir 2007, 23, 6263–6269. [Google Scholar] [CrossRef]
- Gutsmann, T.; Schromm, A.B.; Koch, M.H.J.; Kusumoto, S.; Fukase, K.; Oikawa, M.; Seydel, U.; Brandenburg, K. Lipopolysaccharide-binding protein-mediated interaction of lipid A from different origin with phospholipid membranes. Phys. Chem. Chem. Phys. 2000, 2, 4521–4528. [Google Scholar] [CrossRef]
- Stromberg, L.R. Differential Interactions of Lipopolysaccharides with Lipid Bilayers: Applications for pathogen detection. Ph.D. Thesis, The University of New Mexico Albuquerque, Albuquerque, NM, USA, 2016. [Google Scholar]
- Kataoka-Hamai, C.; Kaizuka, Y.; Taguchi, T. Binding of Lipopolysaccharide and Cholesterol-Modified Gelatin on Supported Lipid Bilayers: Effect of Bilayer Area Confinement and Bilayer Edge Tension. Langmuir 2016, 32, 1250–1258. [Google Scholar] [CrossRef]
- Singh, S.; Kasetty, G.; Schmidtchen, A.; Malmsten, M. Membrane and lipopolysaccharide interactions of C-terminal peptides from S1 peptidases. Biochim. Biophys. Acta 2012, 1818, 2244–2251. [Google Scholar] [CrossRef]
- Domingues, M.M.; Inacio, R.G.; Raimundo, J.M.; Martins, M.; Castanho, M.A.; Santos, N.C. Biophysical characterization of polymyxin B interaction with LPS aggregates and membrane model systems. Biopolymers 2012, 98, 338–344. [Google Scholar] [CrossRef] [PubMed]
- Hsia, C.-Y.; Chen, L.; Singh, R.R.; DeLisa, M.P.; Daniel, S. A Molecularly Complete Planar Bacterial Outer Membrane Platform. Sci. Rep. 2016, 6, 32715. [Google Scholar] [CrossRef] [PubMed]
- Alam, J.M.; Yamazaki, M. Spontaneous insertion of lipopolysaccharide into lipid membranes from aqueous solution. Chem. Phys. Lipids 2011, 164, 166–174. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |







| Artificial Lipid Membrane Model | Advantages | Disadvantages | Applications | References |
|---|---|---|---|---|
| Vesicle fusion | Planar bilayer that fully coats the solid support | Bilayer formation on limited set of hydrophilic substrates - borosilicate glass, mica, silicon dioxide | Biosensors, drug delivery | [75] |
| Monolayers at air–water interface | Achieving well-controlled surface morphologies, controlled composition, adjustable physical state, stability. | Protein unfolding is observed at the interface, single layer. | - | [83,84] |
| Langmuir-Blodgett type approaches | LB parameters (transfer pressure and mode) can modify the film’s characteristics, ultrathin films of well-controlled composition can be formed | Requirement to measure the surface pressures of monolayers, need of water-immiscible spreading solvent, requires successful transfer to substrate | Molecular electronics, non-linear optics, conducting thin films, biosensors | [85,86,87] |
| Supported lipid monolayers | Ease of preparation, stability, patterning, surface sensitive techniques can be applied as the support stabilizes the membrane, platform to probe receptor signaling events | Incorporation of trans-membrane proteins leads to loss of lateral mobility and function | Excellent platform for sensor and array technologies such as heterogeneous analytical assays for environmental monitoring, drug discovery, and drug testing | [56,88] |
| Self-assembled monolayers | Control over ligand density, homogeneity and orientation, simplicity of formation process | Lacks lateral mobility, an important aspect of cellular membranes | Interaction studies can be done easily | [89] |
| Tethered-lipid membranes | Formed on a variety of substrates, high electrical sealing properties and High stability, incorporation of proteins | Reduced lipid mobility | Biotechnology applications with membrane proteins, particularly biosensing | [88,90] |
| Species Used for LPS Study | Spreading Solvent | Subphase | Isotherm Characteristics | Reference |
|---|---|---|---|---|
| Salmonella entericasv. Minnesota strain R595 | Chloroform:methanol (10:1) | Aqueous subphase, deionized water containing 5mM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES) at pH = 7.0 | R595 LPS and lipid A showed temperature independent transition at about 7 mN·m−1, more distinct for lipid A | [105] |
| E. coli J5 (Rc mutant, ATCC no. 43745) | Chloroform:methanol:water (6:4:1 by volume) | 20 mM sodium phosphate, pH = 7.0 | Visible variation in the slope of the isotherm at 150 and 110 Å2, RcLPS compresses to an area/molecule of ~80 Å2 at surface pressure over 40 mN·m−1 | [95] |
| P. Aeruginosa PAO1 (serotype O5) | Phenol:chloroform:petroleum ether (volume ratio of 2:5:8) | Purified water (18 MΩ cm resistivity) | LPS molecules begin to interact laterally with each other when packing area/molecule is ~4.5 cm2·μg−1 (~0.1 mN·m−1), further compression gives an inflection point in isotherm indicating critical lateral stress (~1.0 mN·m−1) and increased packing at the air–water interface | [99] |
| Escherichia coli serotype O55:B5 | Liquid phenol:chloroform:petroleum ether (volume ratio of 2:5:8) | 10 mM HEPES in Milli-Q water (18 MΩ cm) at pH = 7.4, which is the low salt subphase. Two additional subphases were made from it by addition of 100 mM NaCl and the other with 100 mM NaCl and 20 mM CaCl2 | Pressure increases monotonically up to 45 mN·m−1 with specific surface areas of 0.68, 0.66, and 0.44 cm2·μg−1 on low salt, Ca2+ free, and Ca2+ loaded subphases, respectively. | [108] |
| Salmonella Minnesota strain R595 | Chloroform:methanol (9:1 vol/vol) | Four different subphases were prepared: 1), a 10 mM phosphate buffer solution at a pH of 7; 2), 100 mM NaCl in 10 mM phosphate buffer solution; 3), a 50 mM CaCl2 solution; and 4), a 50 mM CaCl2 and 100 mM NaCl solution. | A distinct change of the slope of all isotherms is visible at ~150 Å2 molecule−1 At a lateral pressure of 30 mN·m−1, LPS films differ in their lateral compressibilities with the smallest compressibility of ~2.26 × 102 m·mN−1 found for the sample containing the divalent salt, 50 mM CaCl2 and the largest of ~2.90 × 102 m·mN−1 is observed with 100 mM NaCl in the subphase | [94] |
| E. coli K12 | Chloroform:methanol (2:1) | 5 mM HEPES buffer pH = 7.0 | Absence of plateau in the isotherm suggests no phase transition. Slight change in slope at 13.2 mN·m−1 indicating reorientation of molecules at the interface | [106] |
| Techniques | Bilayer Characterization | Surfaces | Reference |
|---|---|---|---|
| Atomic force microscopy (AFM) | Surface roughness determination, investigation of bilayer surface at the nanoscale range in real time and in aqueous environment, directly measure physical properties at high spatial resolution, possibility to modify the film structure in a controlled way | Atomically flat surfaces: mica, silicon, quartz, flat gold | [55] |
| Quartz crystal microbalance with dissipation (QCM-D) | Determines the wet mass of the film, sensitive to unfused vesicles on the surface, real-time monitoring of bilayer formation | Gold, SiO2, mica, metal oxides | [125] |
| Surface plasmon resonance (SPR) spectroscopy | Highly sensitive real-time monitoring of interactions without labeling of analyte or the ligand, optical thickness of the bilayer | Gold, silver, aluminium | [126,127] |
| Small angle neutrons and X-ray scattering (SANS and SAXS) | Non-destructive method for the structural investigation of biomembranes and mixed lipids systems with different topologies | Performed in quartz glass | [128] |
| Fluorescence recovery after photobleaching (FRAP) | Reveals the dynamics of lipids and proteins in the artificial membrane can be studied, fluidity and morphology of SLBs can be compared | Optically transparent substrates | [129] |
| Imaging ellipsometry (IE) | Indirect technique for quantitative characterization of structural and functional properties of SLBs such as thickness, lateral uniformity, phase separation, molecular area, and receptor-protein interaction affinities. Real-time large area imaging with high sensitivity | Oxide substrates | [123] |
| Electrochemical impedance spectroscopy (EIS) | Electrical properties (resistance and capacitance) of lipid bilayer membranes, formation process in real-time, stability of the membrane | Gold, silicon | [130] |
| Stimulated emission depletion (STED)-with fluorescence correlation spectroscopy (FCC) | Fast molecular dynamics with single-molecule sensitivity, nanoscale membrane organization, can disclose complex cellular signaling events | Gold, SiO2, mica, metal oxides | [131] |
| Anchoring Groups/Spacer Unit | Advantages | Reference |
|---|---|---|
| DNA | Flexible, facilitates docking, allows spacing between vesicles after docking to probe the effect of distance on fusion of vesicles | [143] |
| Thiols | Increase membrane hydration and ion transport without reducing bilayer impedance, enable functional incorporation of membrane proteins | [144] |
| His-tagged Protein | Imparts intramolecular flexibility | [134] |
| Polymer | Successfully incorporate a range of proteins in a functional form, minimizes negative substrate effects such as, defect formation, and decreased lateral mobility | [145] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sondhi, P.; Lingden, D.; Stine, K.J. Structure, Formation, and Biological Interactions of Supported Lipid Bilayers (SLB) Incorporating Lipopolysaccharide. Coatings 2020, 10, 981. https://doi.org/10.3390/coatings10100981
Sondhi P, Lingden D, Stine KJ. Structure, Formation, and Biological Interactions of Supported Lipid Bilayers (SLB) Incorporating Lipopolysaccharide. Coatings. 2020; 10(10):981. https://doi.org/10.3390/coatings10100981
Chicago/Turabian StyleSondhi, Palak, Dhanbir Lingden, and Keith J. Stine. 2020. "Structure, Formation, and Biological Interactions of Supported Lipid Bilayers (SLB) Incorporating Lipopolysaccharide" Coatings 10, no. 10: 981. https://doi.org/10.3390/coatings10100981
APA StyleSondhi, P., Lingden, D., & Stine, K. J. (2020). Structure, Formation, and Biological Interactions of Supported Lipid Bilayers (SLB) Incorporating Lipopolysaccharide. Coatings, 10(10), 981. https://doi.org/10.3390/coatings10100981





