Fabrication and Characterization of Ceramic Coating on Al7075 Alloy by Plasma Electrolytic Oxidation in Molten Salt
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
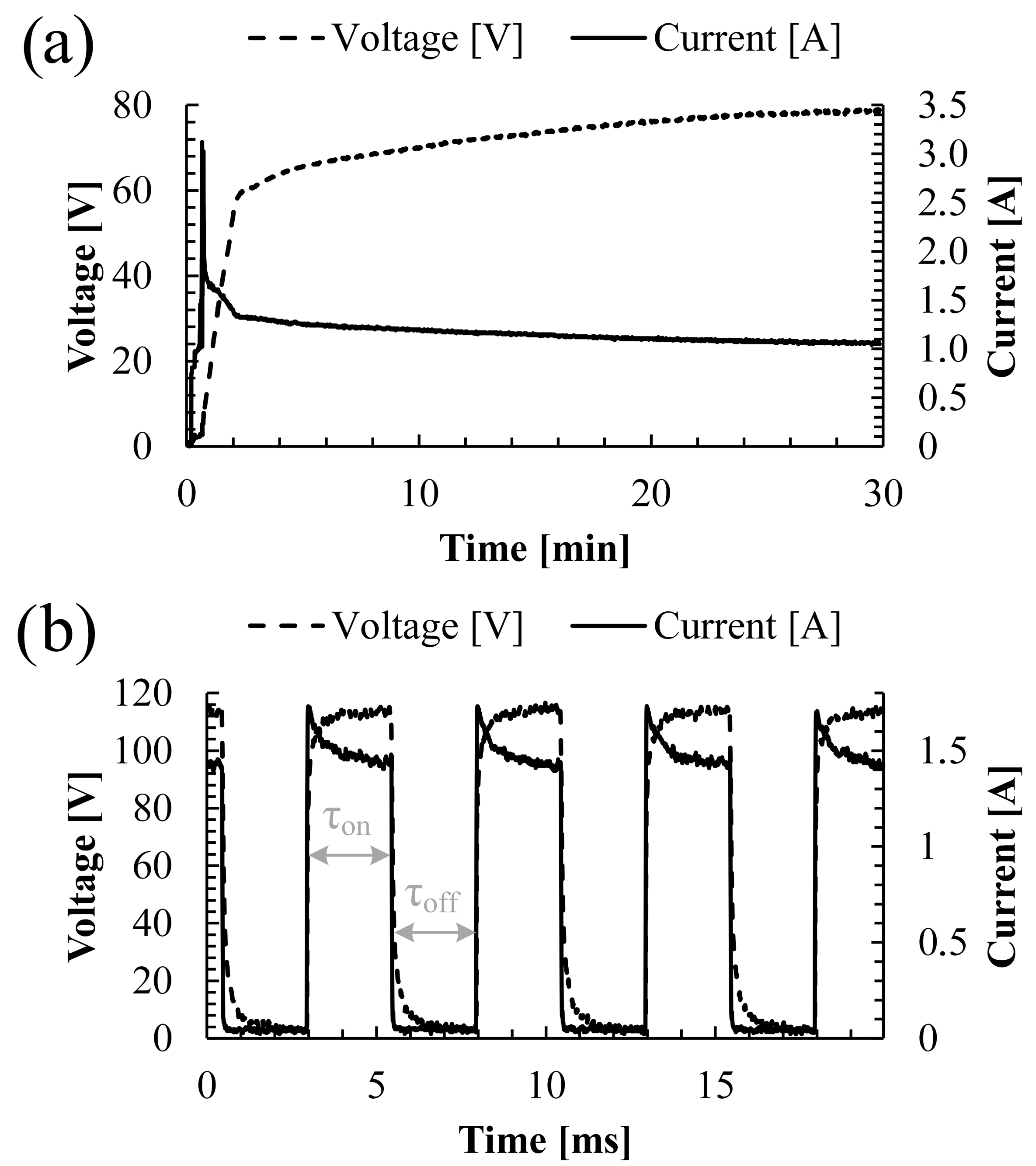
3.1. PEO Processing
3.2. Phase and Chemical Composition Characterization
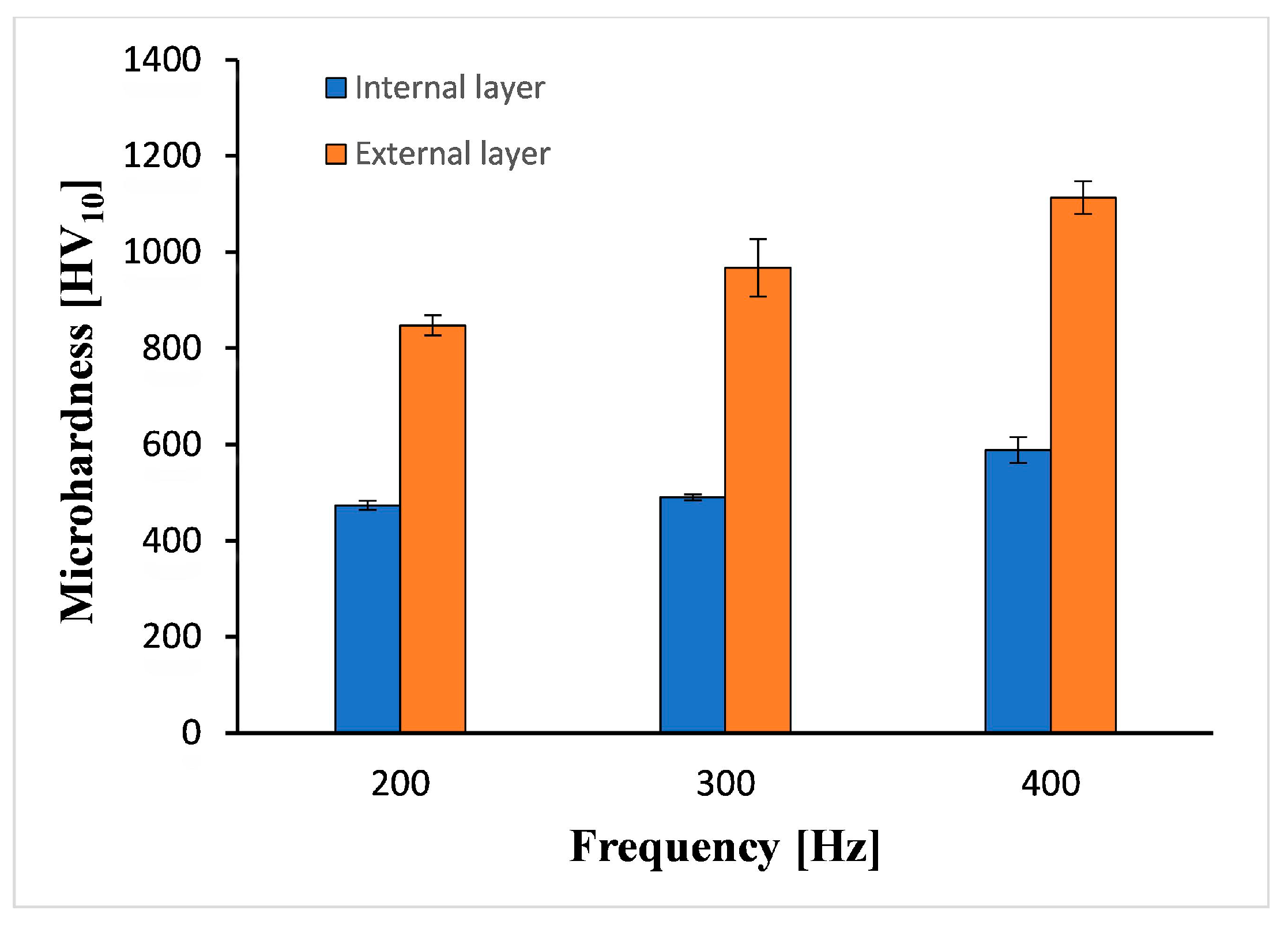
3.3. Coating Characterization
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Stojadinović, S.; Vasilić, R.; Petković, M.; Kasalica, B.; Belča, I.; Zekić, A.; Zeković, L. Characterization of the plasma electrolytic oxidation of titanium in sodium metasilicate. Appl. Surf. Sci. 2013, 265, 226–233. [Google Scholar] [CrossRef]
- Lu, X.; Blawert, C.; Kainer, K.U.; Zheludkevich, M.L. Investigation of the formation mechanisms of plasma electrolytic oxidation coatings on Mg alloy AM50 using particles. Elect. Acta 2016, 196, 680–691. [Google Scholar] [CrossRef]
- Dehnavi, V.; Liu, X.Y.; Luan, B.L.; Shoesmith, D.W.; Rohani, S. Phase transformation in plasma electrolytic oxidation coatings on 6061 aluminum alloy. Surf. Coat. Technol. 2014, 251, 106–114. [Google Scholar] [CrossRef]
- Rokosz, K.; Hryniewicz, T.; Matýsek, D.; Raaen, S.; Valíček, J.; Dudek, L.; Harničárová, M. SEM, EDS and XPS analysis of the coatings obtained on titanium after plasma electrolytic oxidation in electrolytes containing copper nitrate. Materials 2016, 9, 318. [Google Scholar] [CrossRef]
- Yerokhin, A.L.; Nie, X.; Leyland, A.; Matthews, A. Characterization of oxide films produced by plasma electrolytic oxidation of a Ti–6Al–4V alloy. Surf. Coat. Technol. 2000, 130, 195–206. [Google Scholar] [CrossRef]
- Park, S.Y.; Choe, H.C. Functional element coatings on Ti-alloys for biomaterials by plasma electrolytic oxidation. Thin Solid Films 2020, 699, 137896. [Google Scholar] [CrossRef]
- Gnedenkov, S.V.; Khrisanfova, O.A.; Zavidnaya, A.G.; Sinebrukhov, S.L.; Gordienko, P.S.; Iwatsubo, S.; Matsui, A. Composition and adhesion of protective coatings on aluminum. Surf. Coat. Technol. 2001, 145, 146–151. [Google Scholar] [CrossRef]
- Asquith, D.; Yerokhin, A.; James, N.; Yates, J.; Matthews, A. Evaluation of residual stress development at the interface of plasma electrolytically oxidized and cold-worked aluminum. Metall. Mater. Trans. A 2013, 44, 4461–4465. [Google Scholar] [CrossRef]
- Simchen, F.; Sieber, M.; Kopp, A.; Lampke, T. Introduction to plasma electrolytic oxidation—An overview of the process and applications. Coatings 2020, 10, 628. [Google Scholar] [CrossRef]
- Lou, H.-R.; Tsai, D.-S.; Chou, C.-C. Correlation between defect density and corrosion parameter of electrochemically oxidized aluminum. Coatings 2019, 10, 20. [Google Scholar] [CrossRef] [Green Version]
- Yang, K.; Zeng, J.; Huang, H.; Chen, J.; Cao, B. A Novel self-adaptive control method for plasma electrolytic oxidation processing of aluminum alloys. Materials 2019, 12, 2744. [Google Scholar] [CrossRef] [Green Version]
- Terleeva, O.P.; Slonova, A.I.; Rogov, A.B.; Matthews, A.; Yerokhin, A. Wear resistant coatings with a high friction coefficient produced by plasma electrolytic oxidation of al alloys in electrolytes with basalt mineral powder additions. Materials 2019, 12, 2738. [Google Scholar] [CrossRef] [Green Version]
- Sobolev, A.; Kossenko, A.; Borodianskiy, K. Study of the effect of current pulse frequency on Ti–6Al–4V alloy coating formation by micro arc oxidation. Materials 2019, 12, 3983. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sobolev, A.; Kossenko, A.; Zinigrad, M.; Borodianskiy, K. Comparison of plasma electrolytic oxidation coatings on Al alloy created in aqueous solution and molten salt electrolytes. Surf. Coat. Technol. 2018, 344, 590–595. [Google Scholar] [CrossRef]
- Sobolev, A.; Kossenko, A.; Zinigrad, M.; Borodianskiy, K. An investigation of oxide coating synthesized on an aluminum alloy by plasma electrolytic oxidation in molten salt. Appl. Sci. 2017, 7, 889. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.; Gu, Y.; Geng, Y.; Liang, J.; Zhao, J.; Kang, J. Investigating local corrosion behavior and mechanism of MAO coated 7075 aluminum alloy. J. Alloys Compd. 2020, 826, 153976. [Google Scholar] [CrossRef]
- Bahramian, A.; Raeissi, K.; Hakimizad, A. An investigation of the characteristics of Al2O3/TiO2 PEO nanocomposite coating. Appl. Surf. Sci. 2015, 351, 13–26. [Google Scholar] [CrossRef]
- Arunnellaiappan, T.; Arun, S.; Hariprasad, S.; Gowtham, S.; Ravisankar, B.; Rama Krishna, L.; Rameshbabu, N. Fabrication of corrosion resistant hydrophobic ceramic nanocomposite coatings on PEO treated AA7075. Ceram. Int. 2018, 44, 874–884. [Google Scholar] [CrossRef]
- Nie, X.; Meletis, E.; Jiang, J.; Leyland, A.; Yerokhin, A.; Matthews, A. Abrasive wear/corrosion properties and TEM analysis of Al2O3 coatings fabricated using plasma electrolysis. Surf. Coat. Technol. 2002, 149, 245–251. [Google Scholar] [CrossRef]
- Polat, A.; Makaraci, M.; Usta, M. Influence of sodium silicate concentration on structural and tribologicalproperties of microarc oxidation coatings on 2017A aluminum alloy substrate. J. Alloys Compd. 2010, 504, 519–526. [Google Scholar] [CrossRef]
- Brabec, L.; Bohac, P.; Stranyanek, M.; Ctvrtlik, R.; Kocirik, M. Hardness and elastic modulus of silicalite-1 crystal twins. Micropor. Mesopor. Mat. 2006, 94, 226–233. [Google Scholar] [CrossRef]
- Wen, L.; Wang, Y.; Zhou, Y.; Ouyang, J.-H.; Guo, L.; Jia, D. Corrosion evaluation of microarc oxidation coatings formed on 2024 aluminium alloy. Corros. Sci. 2010, 52, 2687–2696. [Google Scholar] [CrossRef]
- Shen, D.; Li, G.; Guo, C.; Zou, J.; Cai, J.; He, D.; Ma, H.; Liu, F. Microstructure and corrosion behavior of micro-arc oxidation coating on 6061 aluminum alloy pre-treated by high-temperature oxidation. Appl. Surf. Sci. 2013, 287, 451–456. [Google Scholar] [CrossRef]
- Venugopal, A.; Srinath, J.; Rama Krishna, L.; Ramesh Narayanan, P.; Sharma, S.C.; Venkitakrishnan, P.V. Corrosion and nanomechanical behaviors of plasma electrolytic oxidation coated AA7020-T6 aluminum alloy. Mater. Sci. Eng. A 2016, 660, 39–46. [Google Scholar] [CrossRef]
- Rao, Y.; Wang, Q.; Oka, D.; Ramachandran, C.S. On the PEO treatment of cold sprayed 7075 aluminum alloy and its effects on mechanical, corrosion and dry sliding wear performances thereof. Surf. Coat. Technol. 2020, 383, 125271. [Google Scholar] [CrossRef]
- Evans, U.R. CXL—The passivity of metals. Part, I. The isolation of the protective film. J. Chem. Soc. 1927, 1020–1040. [Google Scholar] [CrossRef]
- Rosenfeld, I.L.; Marshakov, I.K. Mechanism of crevice corrosion. Corrosion 1964, 20, 115t–125t. [Google Scholar] [CrossRef]
- Hoar, T.P.; Mears, D.C.; Rothwell, G.P. The relationships between anodic passivity, brightening and pitting. Corros. Sci. 1965, 5, 279–289. [Google Scholar] [CrossRef]
- McCafferty, E. General relations regarding graph theory and the passivity of binary alloys. J. Electrochem. 2003, 150, B238–B247. [Google Scholar] [CrossRef]
- Lv, D.; Ou, J.; Xue, M.; Wang, F. Stability and corrosion resistance of superhydrophobic surface onoxidized aluminum in NaCl aqueous solution. Appl. Surf. Sci. 2015, 333, 163–169. [Google Scholar] [CrossRef]
- Zhang, B.; Wang, J.; Wu, B.; Guo, X.W.; Wang, Y.J.; Chen, D.; Zhang, Y.C.; Du, K.; Oguzie, E.E.; Ma, X.L. Unmasking chloride attack on the passive film of metals. Nat. Commun. 2018, 9, 2559. [Google Scholar] [CrossRef] [PubMed]








| Chemical Element, wt.% | |||||||
|---|---|---|---|---|---|---|---|
| Zn | Cu | Mg | Cr | Si | Mn | Ti | Al |
| 5.1–6.1 | 1.2–2.0 | 2.1–2.9 | 0.18–0.35 | <0.4 | <0.3 | <0.2 | balance |
| Samples | icorr × 10−7 (A·cm−2) | Ecorr vs. Ag/AgClsat (mV) | βa (mV/dec) | βc (mV/dec) |
|---|---|---|---|---|
| Original | 248.5 | −701.2 | 17 | −324 |
| Sam-200 Hz | 8.9 | −654.7 | 199 | −164 |
| Sam-300 Hz | 7.6 | −605.9 | 207 | −222 |
| Sam-400 Hz | 2.3 | −560.6 | 76 | −457 |
| Samples | Rp (kΩ cm2) | CR (mm/Year) |
|---|---|---|
| Original | 1.5 | 0.3192 |
| Sam-200 Hz | 43.7 | 0.0092 |
| Sam-300 Hz | 61.4 | 0.0083 |
| Sam-400 Hz | 122.9 | 0.0026 |
| Samples | Original | Sam-200 Hz | Sam-300 Hz | Sam-400 Hz |
|---|---|---|---|---|
| Rs (Ω·cm2) | 3.72 | 0.56 | 0.33 | 0.79 |
| CPE1 (F·cm−2·sn−1) | - | 1.35 × 106 | 1.55 × 10-6 | 1.86 × 106 |
| n1 | - | 0.63 | 0.61 | 0.63 |
| R1 (Ω·cm2) | - | 1.21 × 102 | 1.52 × 102 | 2.56 × 102 |
| CPE2 (F·cm−2·sn−1) | 4.27 × 105 | 5.84 × 107 | 1.05 × 108 | 1.62 × 108 |
| n2 | 0.90 | 0.88 | 0.61 | 0.57 |
| R2 (Ω·cm2) | 1.75 × 104 | 5.11 × 104 | 5.30 × 104 | 1.07 × 105 |
| χ2 | 1.01 × 103 | 1.12 × 103 | 1.42 × 103 | 1.25 × 103 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sobolev, A.; Peretz, T.; Borodianskiy, K. Fabrication and Characterization of Ceramic Coating on Al7075 Alloy by Plasma Electrolytic Oxidation in Molten Salt. Coatings 2020, 10, 993. https://doi.org/10.3390/coatings10100993
Sobolev A, Peretz T, Borodianskiy K. Fabrication and Characterization of Ceramic Coating on Al7075 Alloy by Plasma Electrolytic Oxidation in Molten Salt. Coatings. 2020; 10(10):993. https://doi.org/10.3390/coatings10100993
Chicago/Turabian StyleSobolev, Alexander, Tamar Peretz, and Konstantin Borodianskiy. 2020. "Fabrication and Characterization of Ceramic Coating on Al7075 Alloy by Plasma Electrolytic Oxidation in Molten Salt" Coatings 10, no. 10: 993. https://doi.org/10.3390/coatings10100993
APA StyleSobolev, A., Peretz, T., & Borodianskiy, K. (2020). Fabrication and Characterization of Ceramic Coating on Al7075 Alloy by Plasma Electrolytic Oxidation in Molten Salt. Coatings, 10(10), 993. https://doi.org/10.3390/coatings10100993







