Thiocarbohydrazones Based on Adamantane and Ferrocene as Efficient Corrosion Inhibitors for Hydrochloric Acid Pickling of C-Steel
Abstract
:1. Introduction
2. Experimental Part
2.1. Instrument, Solutions, and Materials
2.2. Synthesis of N′-(Adamantan-2-Ylidene)Hydrazinecarbothiohydrazide (4) and 2-(Ferrocenyl-1-Ylidene) Hydrazinecarbothiohydrazide (5)
2.3. Experimental Setup and Corrosion Measurements
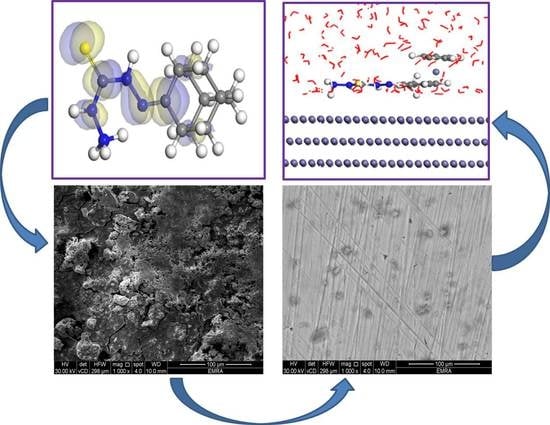
2.4. Surface Characterization
2.5. Theoretical Studies (DFT Calculations and MC Simulation)
3. Results and Discussions
3.1. Structure Configuration of the As-Prepared Compounds
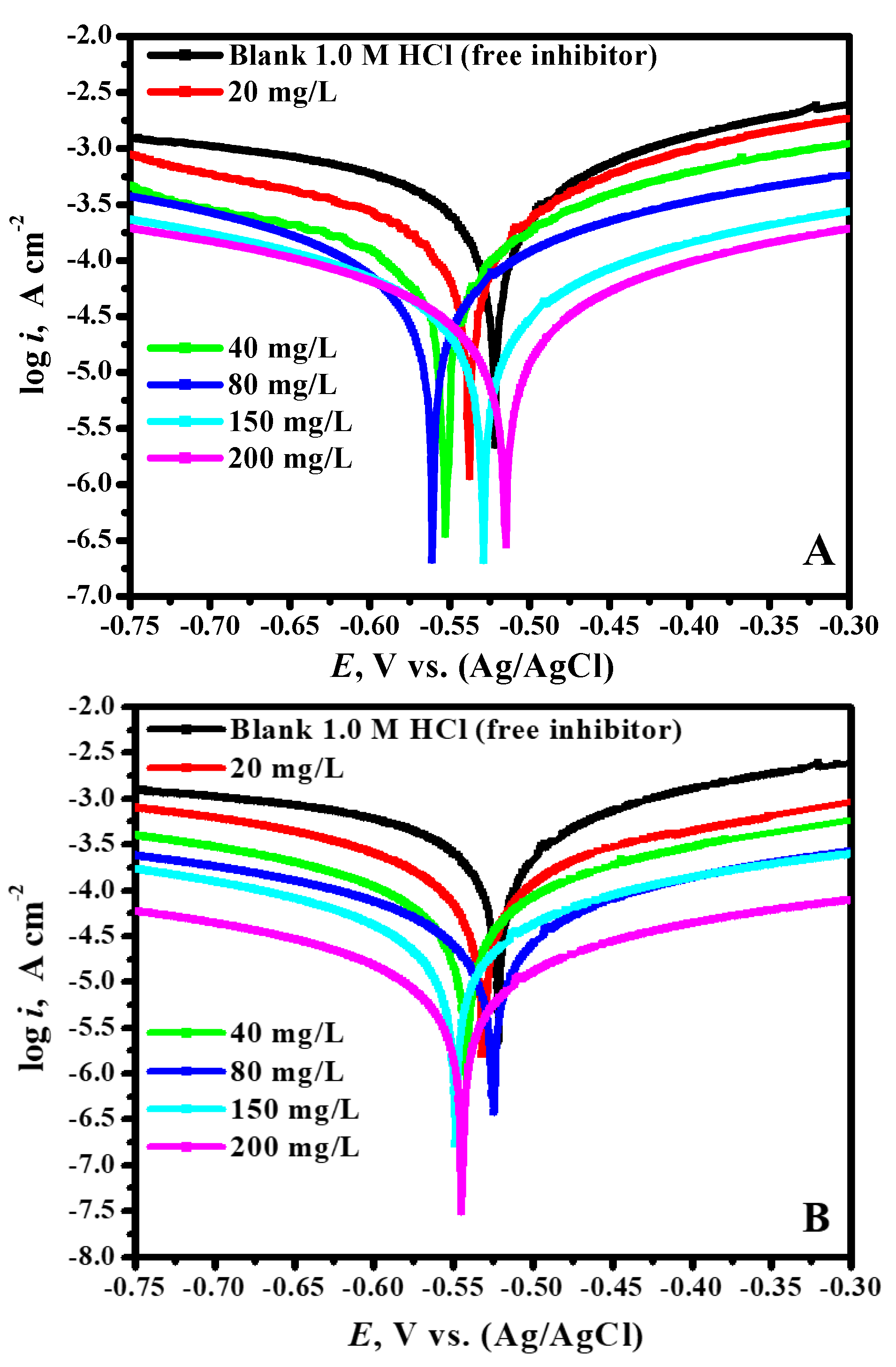
3.2. OCP vs. Time and PDP Studies
3.3. EIS Studies
3.4. Adsorption Considerations
3.5. Surface Analysis (FESEM and FTIR)
3.6. Corrosion Mitigation Mechanism
3.7. DFT Calculations
3.8. MC Simulations
3.9. Comparison of the Protection Capacity of Ad-Th and Fe-Th Inhibitors with Earlier Reports
4. Conclusions
- PDP and EIS measurements revealed an increase in the corrosion inhibition capacity with a rise in the inhibitor dose which reached >97.9% at a dosage of 200 ppm of Fe-Th.
- The inhibitors adsorption on the C-steel followed the isotherm of Langmuir model and the value of indicated the presence of both physical and chemical adsorption modes.
- EIS study designates the occurrence of a one-time constant phenomenon for the inhibitor adsorption in which the polarization resistance increasing with an increment in the inhibitor concentration.
- PDP study exhibited that the adsorption of the inhibitors resulted in a mixed-type with cathodic predominance.
- FESEM measurements showed the appearance of a smoother surface morphology compared to the blank specimen. The FTIR studies supported the adsorption of compound additives on the metallic interface.
- DFT calculations indicate that the protection of C-steel from corrosion is achieved more via the adsorption of protonated Fe-ThH and Ad-ThH forms than that by neutral Fe-Th and Ad-Th forms.
- MC simulation findings displayed that both Fe-Th and Ad-Th molecules adsorb intensely on the iron (110) interface and the inclination of expected binding energies agreed with the empirical protection capacitates.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Shaw, P.; Obot, I.B.; Yadav, M. Functionalized 2-hydrazinobenzothiazole with carbohydrates as a corrosion inhibitor: Electrochemical, XPS, DFT and Monte Carlo simulation studies. Mater. Chem. Front. 2019, 3, 931–940. [Google Scholar] [CrossRef]
- El-Lateef, H.M.A.; Alnajjar, A.O. Enhanced the protection capacity of poly(o-toluidine) by synergism with zinc or lanthanum additives at C-steel/HCl interface: A combined DFT, molecular dynamic simulations and experimental methods. J. Mol. Liq. 2020, 303, 112641. [Google Scholar] [CrossRef]
- Singh, A.K.; Thakur, S.; Pani, B.; Ebenso, E.E.; Quraishi, M.A.; Pandey, A.K. 2-Hydroxy-N′-((Thiophene-2-yl)methylene) benzohydrazide: Ultrasound-assisted synthesis and corrosion inhibition study. ACS Omega 2018, 3, 4695–4705. [Google Scholar] [CrossRef] [PubMed]
- Finsgar, M.; Jackson, J. Application of corrosion inhibitors for steels in acidic media for the oil and gas industry: A review. Corros. Sci. 2014, 86, 17–41. [Google Scholar] [CrossRef] [Green Version]
- Chafai, N.; Chafaa, S.; Benbouguerra, K.; Hellal, A.; Mehri, M. Synthesis, spectral analysis, anti-corrosive activity and theoretical study of an aromatic hydrazone derivative. J. Mol. Struct. 2019, 1181, 83–92. [Google Scholar] [CrossRef]
- Al-Shihry, S.S.; Sayed, A.R.; El-lateef, H.M.A. Design and assessment of a novel poly (urethane-semicarbazides) containing thiadiazoles on the backbone of the polymers as inhibitors for steel pipelines corrosion in CO2-saturated oilfield water. J. Mol. Struct. 2020, 1201, 127223. [Google Scholar] [CrossRef]
- Qiang, Y.; Li, H.; Lan, X. Self-assembling anchored film basing on two tetrazole derivatives for application to protect copper in sulfuric acid environment. J. Mater. Sci. Technol. 2020, 52, 63–71. [Google Scholar] [CrossRef]
- Kassim, K.; Hamali, M.A.; Yamin, B. A new alternative synthesis of salicylaldazine via microwave irradiation method. J. Chem. 2019, 2019, 9546373. [Google Scholar] [CrossRef]
- Wang, J.; Wang, Y.-T.; Fang, Y.; Lu, Y.-L.; Li, M.-X. Tin thiocarbonohydrazone complexes: Synthesis, crystal structures and biological evaluation. Toxicol. Res. 2019, 8, 862–867. [Google Scholar] [CrossRef]
- Muglu, H.; Cavus, M.S.; Bakir, T.; Yakan, H. Synthesis, characterization, quantum chemical calculations and antioxidant activity of new bis-isatin carbohydrazone and thiocarbohydrazone derivatives. J. Mol. Struct. 2019, 1196, 819–827. [Google Scholar] [CrossRef]
- Assaleh, M.H.; Bozic, A.R.; Bjelogrlic, S.; Milosevic, M.; Simic, M.; Marinkovic, A.D.; Cvijetic, I.N. Water-induced isomerism of salicylaldehyde and 2-acetylpyridine mono- and bis-(thiocarbohydrazones) improves the antioxidant activity: Spectroscopic and DFT study. Struct. Chem. 2019, 30, 2447–2457. [Google Scholar] [CrossRef]
- Baashen, M.A. A simple and efficient process for the synthesis of novel heterocycles containing benzofuran moiety using thiocarbohydrazide as a precursor. J. Heterocycl. Chem. 2019, 56, 1953–1957. [Google Scholar] [CrossRef]
- Srividya, L.; Reddy, A.; Rama, N. Antidiabetic activity of 1-(4-chlorobenzylidene)-5-(2-oxoindolin-3-ylidene) thiocarbohydrazone in chick model. Asian J. Biol. Sci. 2017, 10, 126–130. [Google Scholar] [CrossRef] [Green Version]
- Prasad, D.; Prasad, A.; Singh, A.K.R.; Verma, U.N. Physio chemical studies of Cu(II) and Zn(II) complexes with schiff bases of thiocarbohydrazide. J. Chemtracks 2017, 19, 343–346. [Google Scholar]
- Gabr, M.T.; El-Gohary, N.S.; El-Bendary, E.R.; Ni, N.; Shaaban, M.I.; El-Kerdawy, M.M. Microwave-assisted synthesis, antimicrobial, antiquorum-sensing and cytotoxic activities of a new series of isatin-β-thiocarbohydrazones. Synth. Commun. 2018, 48, 2899–2911. [Google Scholar] [CrossRef]
- Zanje, S.B.; Suryavanshi, V.J.; Kokare, A.N.; Ghare, A.A.; Kamble, G.S.; Kamble, P.N.; Anuse, M.A. 2-nitrobenzaldehyde thiocarbohydrazone assisted precise extraction spectrophotometric method for the determination of ruthenium(III) in alloy and catalysts. J. Anal. Chem. 2018, 73, 438–451. [Google Scholar] [CrossRef]
- Duryodhan, P.W. Liquid-liquid extraction and spectrophotometric determination of tellurium (IV) by using thio-ligand: Analysis of alloys and chalcogenides. Res. J. Chem. Environ. 2017, 21, 13–23. [Google Scholar]
- Elkanzi, N.A.A.; Morsy, N.M.; Aly, A.A.; el Malah, T.; Shawky, A.M. Green chemistry: Microwave-assisted facile synthesis of 6-imino-1,3,4-thiadiazenes from reaction of thiocarbohydrazones with malononitrile dimer. J. Sulfur Chem. 2016, 37, 114–121. [Google Scholar] [CrossRef]
- Biswas, S.; Gangopadhyay, M.; Barman, S.; Sarkar, J.; Singh, N.D. Simple and efficient coumarin-based colorimetric and fluorescent chemosensor for F-detection: An ON1-OFF-ON2 fluorescent assay. Sens. Actuators B Chem. 2016, 222, 823–828. [Google Scholar] [CrossRef]
- Kaya, Y.; Ercag, A.; Koca, A. Synthesis, structures, electrochemical studies and antioxidant activities of cis-dioxomolybdenum(VI) complexes of the new bisthiocarbohydrazones. J. Mol. Struct. 2015, 1102, 117–126. [Google Scholar] [CrossRef]
- Chafiq, M.; Chaouiki, A.; Al-Hadeethi, M.R.; Ali, I.H.; Mohamed, S.K.; Toumiat, K.; Salghi, R. Naproxen-based hydrazones as effective corrosion inhibitors for mild steel in 1.0 M HCl. Coatings 2020, 10, 700. [Google Scholar] [CrossRef]
- Khamaysa, O.M.A.; Selatnia, I.; Zeghache, H.; Lgaz, H.; Sid, A.; Chung, M.; Benahmed, M.; Gherraf, N.; Mosset, P. Enhanced corrosion inhibition of carbon steel in HCl solution by a newly synthesized hydrazone derivative: Mechanism exploration from electrochemical, XPS, and computational studies. J. Mol. Liq. 2020, 315, 113805. [Google Scholar] [CrossRef]
- Chaouiki, A.; Chafiq, M.; Lgaz, H.; Al-Hadeethi, M.R.; Ali, I.H.; Masroor, S.; Chung, M. Green corrosion inhibition of mild steel by hydrazone derivatives in 1.0 M HCl. Coatings 2020, 10, 640. [Google Scholar] [CrossRef]
- Lgaz, H.; Salghi, R.; Masroor, S.; Kim, S.-H.; Kwon, C.; Kim, S.Y.; Yang, Y.-J.; Chung, M. Assessing corrosion inhibition characteristics of hydrazone derivatives on mild steel in HCl: Insights from electronic-scale DFT and atomic-scale molecular dynamics. J. Mol. Liq. 2020, 308, 112998. [Google Scholar] [CrossRef]
- El-Lateef, H.M.A. Corrosion inhibition characteristics of a novel salycilidene isatin hydrazine sodium sulfonate on carbon steel in HCl and a synergistic nickel ions additive: A combined experimental and theoretical perspective. Appl. Surf. Sci. 2020, 501, 144237. [Google Scholar] [CrossRef]
- El-Lateef, H.M.A.; Khalaf, M.M. Novel dispersed Tl2O3-SiO2/polyaniline nanocomposites: In-sit polymerization, characterization and enforcement as a corrosion protective layer for carbon-steel in acidic chloride medium. Colloids Surf. A 2019, 573, 95–111. [Google Scholar] [CrossRef]
- Tantawy, A.H.; Soliman, K.A.; El-Lateef, H.M.A. Novel synthesized cationic surfactants based on natural piper nigrum as sustainable-green inhibitors for steel pipeline corrosion in CO2-3.5% NaCl: DFT, Monte Carlo simulations and experimental approaches. J. Clean. Prod. 2020, 250, 119510. [Google Scholar] [CrossRef]
- Eid, A.M.; Shaaban, S.; Shalabi, K. Tetrazole-based organoselenium bi-functionalized corrosion inhibitors during oil well acidizing: Experimental, computational studies, and SRB bioassay. J. Mol. Liq. 2020, 298, 111980–111998. [Google Scholar] [CrossRef]
- Kaya, S.; Guo, L.; Kaya, C.; Tüzün, B.; Obot, I.B.; Touir, R.; Islam, N. Quantum chemical and molecular dynamic simultion studies for the prediction of inhibition efficiencies of some piperidine derivatives on the corrosion of iron. J. Taiwan Inst. Chem. Eng. 2016, 65, 522–529. [Google Scholar] [CrossRef]
- Gao, G.; Liang, C. Electrochemical and DFT studies of β-amino-alcohols as corrosion inhibitors for brass. Electrochim. Acta 2007, 52, 4554–4559. [Google Scholar] [CrossRef]
- Khalaf, M.M.; Tantawy, A.H.; Soliman, K.A.; El-Lateef, H.M.A. Cationic gemini-surfactants based on waste cooking oil as new ‘green’ inhibitors for N80-steel corrosion in sulphuric acid: A combined empirical and theoretical approaches. J. Mol. Struct. 2020, 1203, 127442. [Google Scholar] [CrossRef]
- Saleh, M.M.; Mahmoud, M.G.; Abd El-Lateef, H.M. Comparative study of synergistic inhibition of mild steel and pure iron by 1- hexadecylpyridinium chloride and bromide ions. Corros. Sci. 2019, 154, 70–79. [Google Scholar] [CrossRef]
- Abd El-Lateef, H.M.; Mohamed, I.M.A.; Zhu, J.-H.; Khalaf, M.M. An efficient synthesis of electrospun TiO2-nanofibers/Schiff base phenylalanine composite and its inhibition behavior for C-steel corrosion in acidic chloride environments. J. Taiwan Inst. Chem. Eng. 2020, 112, 306–321. [Google Scholar] [CrossRef]
- Masroor, S.; Mobin, M.; Alam, M.J.; Ahmad, S. The novel iminium surfactant p-benzylidene benzyldodecyl iminium chloride as a corrosion inhibitor for plain carbon steel in 1 M HCl: Electrochemical and DFT evaluation. RSC Adv. 2017, 7, 23182–23196. [Google Scholar] [CrossRef] [Green Version]
- Gadow, S.H.S.; Motawea, M.M. Investigation of the corrosion inhibition of carbon steel in hydrochloric acid solution by using ginger roots extract. RSC Adv. 2017, 7, 24576–24588. [Google Scholar] [CrossRef] [Green Version]
- Meng, Y.; Ning, W.; Xu, B.; Yang, W.; Zhang, K.; Chen, Y.; Li, L.; Liu, X.; Zheng, J.; Zhang, Y. Inhibition of mild steel corrosion in hydrochloric acid using two novel pyridine Schiff base derivatives: A comparative study of experimental and theoretical results. RSC Adv. 2017, 7, 43014–43029. [Google Scholar] [CrossRef] [Green Version]
- Esmaeilpour, M.; Sardarian, A.R.; Javidi, J. Schiff base complex of metal ions supported on superparamagnetic Fe3O4@SiO2 nanoparticles: An efficient, selective and recyclable catalyst for synthesis of 1,1-diacetates from aldehydes under solvent-free conditions. Appl. Catal. A Gen. 2012, 445–446, 359–367. [Google Scholar] [CrossRef]
- Alam, R.; Mobin, M.; Aslam, J. Investigation of anti-corrosive properties of poly(aniline-co-2-pyridylamine-co-2,3-xylidine) and its nanocomposite poly(aniline-co-2-pyridylamine-co-2,3-xylidine)/ZnO on mild steel in 0.1 M HCl. Appl. Surf. Sci. 2016, 368, 360–367. [Google Scholar] [CrossRef]
- Singh, A.; Ansari, K.R.; Haque, J.; Dohare, P.; Lgaz, H.; Salghi, R.; Quraishi, M.A. Effect of electron donating functional groups on corrosion inhibition of mild steel in hydrochloric acid: Experimental and quantum chemical study. J. Taiwan Inst. Chem. Eng. 2018, 82, 233–251. [Google Scholar] [CrossRef]
- Qiang, Y.; Zhang, S.; Zhao, H.; Tan, B.; Wang, L. Enhanced anticorrosion performance of copper by novel N-doped carbon dots. Corros. Sci. 2019, 161, 108193. [Google Scholar] [CrossRef]
- Ansari, K.R.; Quraishi, M.A.; Singh, A.; Ramkumar, S.; Obot, I.B. Corrosion inhibition of N80 steel in 15% HCl by pyrazolone derivatives: Electrochemical, surface and quantum chemical studies. RSC Adv. 2016, 6, 24130–24141. [Google Scholar] [CrossRef]
- Li, X.H.; Deng, S.D.; Fu, H.; Mu, G.N. Inhibition by tween-85 of the corrosion of cold rolled steel in 1.0 M hydrochloric acid solution. J. Appl. Electrochem. 2009, 39, 1125–1135. [Google Scholar] [CrossRef]
- Singh, A.; Ansari, K.R.; Quraishi, M.A.; Lgaz, H.; Lin, Y. Synthesis and investigation of pyran derivatives as acidizing corrosion inhibitors for N80 steel in hydrochloric acid: Theoretical and experimental approaches. J. Alloys Compd. 2018, 762, 347–362. [Google Scholar] [CrossRef]
- Ansari, K.R.; Quraishi, M.A.; Singh, A. Schiff’s base of pyridyl substituted triazoles as new and effective corrosion inhibitors for mild steel in hydrochloric acid solution. Corros. Sci. 2014, 79, 5–15. [Google Scholar] [CrossRef]
- Haque, J.; Ansari, K.R.; Srivastava, V.; Quraishi, M.A.; Obot, I.B. Pyrimidine derivatives as novel acidizing corrosion inhibitors for N80 steel useful for petroleum industry: A combined experimental and theoretical approach. J. Ind. Eng. Chem. 2017, 49, 176–188. [Google Scholar] [CrossRef]
- Bentiss, F.; Lebrini, M.; Lagrenée, M. Thermodynamic characterization of metal dissolution and inhibitor adsorption processes in mild steel/2,5-bis(n-thienyl)-1,3,4-thiadiazoles/hydrochloric acid system. Corros. Sci. 2005, 47, 2915–2931. [Google Scholar] [CrossRef]
- Soltani, N.; Salavati, H.; Rasouli, N.; Paziresh, M.; Moghadas, A. Adsorption and corrosion inhibition effect of Schiff base ligands on low carbon steel corrosion in hydrochloric acid solution. Chem. Eng. Commun. 2016, 203, 840–854. [Google Scholar]
- Solomon, M.M.; Gerengi, H.; Umoren, S.A. Carboxymethyl cellulose/silver nanoparticles composite: Synthesis, characterization and application as a benign corrosion inhibitor for St37 steel in 15% H2SO4 medium. ACS Appl. Mater. Interfaces 2017, 9, 6376–6389. [Google Scholar] [CrossRef]
- Rastogi, A.; Zivcak, M.; Sytar, O.; Kalaji, H.M.; He, X.; Mbarki, S.; Brestic, M. Impact of metal and metal oxide nanoparticles on plant: A critical review. Front. Chem. 2017, 5, 78. [Google Scholar] [CrossRef] [Green Version]
- Yilmaz, N.; Fitoz, A.; Ergun, Ü.; Emregül, K.C. A combined electrochemical and theoretical study into the effect of 2-((thiazole-2-ylimino)methyl) phenol as a corrosion inhibitor for mild steel in a highly acidic environment. Corros. Sci. 2016, 111, 110–120. [Google Scholar] [CrossRef]
- Abd El-Lateef, H.M.; Shalabi, K.; Tantawy, A.H. Corrosion inhibition of carbon steel in hydrochloric acid solution using newly synthesized urea-based cationic fluorosurfactants: Experimental and computational investigations. New J. Chem. 2020, 44, 17791–17814. [Google Scholar] [CrossRef]
- Ali, A.I.; Mahrous, Y.S. Corrosion inhibition of C-steel in acidic media from fruiting bodies of Melia azedarach L extract and a synergistic Ni2+ additive. RSC Adv. 2017, 7, 23687–23698. [Google Scholar] [CrossRef] [Green Version]
- Wu, Y.; Zhao, W.; Qiang, Y.; Chen, Z.; Wang, L.; Gao, X.; Fang, Z. π-π interaction between fluorinated reduced graphene oxide and acridizinium ionic liquid: Synthesis and anti-corrosion application. Carbon 2020, 159, 292–302. [Google Scholar] [CrossRef]
- Ismail, K.Z. Synthesis and physicochemical studies of metal complexes of ferrocene Schiff base derivatives. Transit. Met. Chem. 1997, 22, 565–569. [Google Scholar] [CrossRef]
- Shalabi, K.; Nazeer, A.A. Ethoxylates nonionic surfactants as promising environmentally safe inhibitors for corrosion protection of reinforcing steel in 3.5 % NaCl saturated with Ca(OH)2 solution. J. Mol. Struct. 2019, 1195, 863–876. [Google Scholar] [CrossRef]
- Oyebamiji, A.K.; Adeleke, B.B. Quantum chemical studies on inhibition activities of 2,3-dihydroxypropyl-sulfanyl derivative on carbon steel in acidic media. Int. J. Corros. Scale Inhib. 2018, 7, 498–508. [Google Scholar]
- Mary, Y.S.; Panicker, C.Y.; Sapnakumari, M.; Narayana, B.; Sarojini, B.K.; Al-Saadi, A.A.; Van Alsenoy, C.; War, J.A. FT-IR, NBO, HOMO–LUMO, MEP analysis and molecular docking study of 1-[3-(4-Fluorophenyl)-5-phenyl-4,5-dihydro-1H-pyrazol-1-yl]ethenone. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2015, 136, 483–493. [Google Scholar] [CrossRef]
- Hachani, S.E.; Necira, Z.; Mazouzi, D.; Nebbache, N. Understanding the inhibition of mild steel corrosion by dianiline schiff bases: A DFT investigation. Acta Chim. Slov. 2018, 65, 183–190. [Google Scholar] [CrossRef] [Green Version]
- Ramalingam, S.; David Suresh Babu, P.; Periandy, S.; Fereyduni, E. Vibrational investigation, molecular orbital studies and molecular electrostatic potential map analysis on 3-chlorobenzoic acid using hybrid computational calculations. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2011, 84, 210–220. [Google Scholar] [CrossRef]
- Gobara, M.; Saleh, A.; Naeem, I. Synthesis, characterization and application of acrylate-based poly ionic liquid for corrosion protection of C1020 steel in hydrochloric acid solution. Mater. Res. Express 2019, 7, 016517. [Google Scholar] [CrossRef]
- Shalabi, K.; Helmy, A.M.; El-Askalany, A.H.; Shahba, M.M. New pyridiniumbromidemono-cationic surfactant as corrosion inhibitor for carbon steel during chemical cleaning: Experimental and theoretical studies. J. Mol. Liq. 2019, 293, 111480–111494. [Google Scholar] [CrossRef]
- Özcan, M.; Dehri, İ.; Erbil, M. Organic sulphur-containing compounds as corrosion inhibitors for mild steel in acidic media: Correlation between inhibition efficiency and chemical structure. Appl. Surf. Sci. 2004, 236, 155–164. [Google Scholar] [CrossRef]
- Lgaz, H.; Chaouiki, A.; Albayati, M.R.; Salghi, R.; el Aoufir, Y.; Ali, I.H.; Khan, M.I.; Mohamed, S.K.; Chung, I.-M. Synthesis and evaluation of some new hydrazones as corrosion inhibitors for mild steel in acidic media. Res. Chem. Intermed. 2019, 45, 2269–2286. [Google Scholar] [CrossRef]
- Chaouiki, A.; Chafiq, M.; Al-Hadeethi, M.R.; Lgaz, H.; Salghi, R.; AbdelRaheem, S.K.; Ali, I.H.; Ebraheem, S.A.M.; Chung, I.-M.; Mohamed, S.K. Exploring the corrosion inhibition effect of two hydrazone derivatives for mild steel corrosion in 1.0 M HCl solution via electrochemical and surface characterization studies. Int. J. Electrochem. Sci. 2020, 15, 9354–9377. [Google Scholar] [CrossRef]










| Inhibitor Code | Cinh/ mg L−1 | icor ± SD/ µA·cm−2 | −Ecor ± SD/ V (Ag/AgCl) | βa/ mV dec−1 | −βc/ mV dec−1 | θ | ηP/% |
|---|---|---|---|---|---|---|---|
| Blank | 0.0 | 387.4 ± 26 | −0.523 ± 0.035 | 91 | 145 | – | – |
| Ad-Th | 20 | 267.7 ± 17 | −0.537 ± 0.051 | 94 | 145 | 0.309 | 30.9 |
| 40 | 211.5 ± 14 | −0.552 ± 0.043 | 96 | 154 | 0.454 | 45.4 | |
| 80 | 156.9 ± 9 | −0.561 ± 0.053 | 92 | 157 | 0.595 | 59.5 | |
| 150 | 96.4 ± 7 | −0.529 ± 0.047 | 95 | 139 | 0.751 | 75.1 | |
| 200 | 24.8 ± 3 | −0.514 ± 0.049 | 93 | 144 | 0.936 | 93.6 | |
| Fe-Th | 20 | 237.1 ± 21 | −0.530 ± 0.056 | 96 | 157 | 0.388 | 38.8 |
| 40 | 180.1 ± 17 | −0.542 ± 0.040 | 98 | 153 | 0.535 | 53.5 | |
| 80 | 117.8 ± 10 | −0.524 ± 0.039 | 94 | 147 | 0.696 | 69.6 | |
| 150 | 53.1 ± 5 | −0.549 ± 0.051 | 98 | 148 | 0.863 | 86.3 | |
| 200 | 8.1 ± 2 | −0.544 ± 0.046 | 100 | 144 | 0.979 | 97.9 |
| Inhibitor Code | Cinh/mg L−1 | Rs/Ω cm2 | ZCPE | Rp/Ω cm2 | Cdl/µF cm−2 | θ | ηE/% | |
|---|---|---|---|---|---|---|---|---|
| Y0/μΩ−1 sn cm−2 | n | |||||||
| Blank | 0.0 | 0.12 | 152.5 | 0.724 | 18.7 ± 2 | 336.1 | – | – |
| Ad-Th | 20 | 0.18 | 87.1 | 0.873 | 33.5 ± 3 | 206.9 | 0.441 | 44.1 |
| 40 | 0.21 | 75.4 | 0.871 | 53.4 ± 5 | 148.8 | 0.649 | 64.9 | |
| 80 | 0.23 | 53.2 | 0.861 | 92.8 ± 8 | 113.3 | 0.798 | 79.8 | |
| 150 | 0.26 | 40.3 | 0.882 | 169.7 ± 12 | 97.1 | 0.889 | 88.9 | |
| 200 | 0.34 | 30.7 | 0.875 | 230.3 ± 18 | 54.0 | 0.918 | 91.8 | |
| Fe-Th | 20 | 0.36 | 75.6 | 0.867 | 43.8 ± 4 | 163.2 | 0.573 | 57.3 |
| 40 | 0.45 | 65.6 | 0.886 | 75.1 ± 6 | 134.8 | 0.751 | 75.1 | |
| 80 | 0.54 | 46.2 | 0.878 | 221.2 ± 15 | 104.1 | 0.915 | 91.5 | |
| 150 | 0.61 | 34.9 | 0.898 | 297.9 ± 23 | 85.6 | 0.937 | 93.7 | |
| 200 | 0.74 | 26.7 | 0.894 | 464.1 ± 34 | 39.6 | 0.959 | 95.9 | |
| Parameters | Non-Protonated Form | Protonated Form | ||
|---|---|---|---|---|
| Ad-Th | Fe-Th | Ad-ThH | Fe-ThH | |
| EHOMO (eV) | −3.758 | −3.822 | −3.693 | −3.923 |
| ELUMO (eV) | −2.012 | −3.020 | −2.047 | −3.128 |
| ΔE= ELUMO − EHOMO (eV) | 1.746 | 0.802 | 1.646 | 0.795 |
| Dipole moments (µ) debye | 7.246 | 5.849 | 13.13 | 22.57 |
| The number of electrons transferred (ΔN) | 1.215 | 1.786 | 0.976 | 1.453 |
| System | Adsorption Energy/kJ mol−1 | Rigid Adsorption Energy/kJ mol−1 | Deformation Energy /kJ mol−1 | dEads/dNi: kJ mol−1 | dEads/dNi: Water kJ mol−1 |
|---|---|---|---|---|---|
| Fe (110) | −2160.84 | −2185.42 | −526.99 | −751.11 | −75.48 |
| Ad-Th | |||||
| H2O | |||||
| Fe (110) | −3719.59 | −2437.30 | −1282.29 | −1414.63 | −77.87 |
| Fe-Th | |||||
| H2O |
| Inhibitors | Measurement Method | Inhibitor Concentration | Protection Capacity/% | References |
|---|---|---|---|---|
| Naproxen-based hydrazones | PDP and EIS | 0.1–5.0 mmol/L | 80.2–89.2 | [21] |
| Dinitrophenyl hydrazone derivatives | Weight loss measurements | 0.1–5.0 mmol/L | 84.7–91.2 | [22] |
| Propanehydrazide derivatives | PDP | 0.1–5.0 mmol/L | 63.0–84.1 | [23] |
| Four benzohydrazide derivatives | EIS | 5.0 mmol/L | 84–95 | [63] |
| Methoxynaphthalen 2-yl propanehydrazide derivatives | PDP | 0.1–5.0 mmol/L | 74–91 | [64] |
| Ad-Th | PDP | 20–200 mg/L | 30.9–93.6 | This work |
| Fe-Th | PDP | 20–200 mg/L | 38.8–97.9 | This work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sayed, A.R.; El-Lateef, H.M.A. Thiocarbohydrazones Based on Adamantane and Ferrocene as Efficient Corrosion Inhibitors for Hydrochloric Acid Pickling of C-Steel. Coatings 2020, 10, 1068. https://doi.org/10.3390/coatings10111068
Sayed AR, El-Lateef HMA. Thiocarbohydrazones Based on Adamantane and Ferrocene as Efficient Corrosion Inhibitors for Hydrochloric Acid Pickling of C-Steel. Coatings. 2020; 10(11):1068. https://doi.org/10.3390/coatings10111068
Chicago/Turabian StyleSayed, Abdelwahed R., and Hany M. Abd El-Lateef. 2020. "Thiocarbohydrazones Based on Adamantane and Ferrocene as Efficient Corrosion Inhibitors for Hydrochloric Acid Pickling of C-Steel" Coatings 10, no. 11: 1068. https://doi.org/10.3390/coatings10111068
APA StyleSayed, A. R., & El-Lateef, H. M. A. (2020). Thiocarbohydrazones Based on Adamantane and Ferrocene as Efficient Corrosion Inhibitors for Hydrochloric Acid Pickling of C-Steel. Coatings, 10(11), 1068. https://doi.org/10.3390/coatings10111068