Performance of Cu–Ag Thin Films as Diffusion Barrier Layer
Abstract
:1. Introduction
2. Materials and Methods
2.1. Amorphous Geometry Structure
2.2. Procedures for Interface Model Preparation
2.3. Procedure for Diffusion and Deformation
3. Results and Discussion
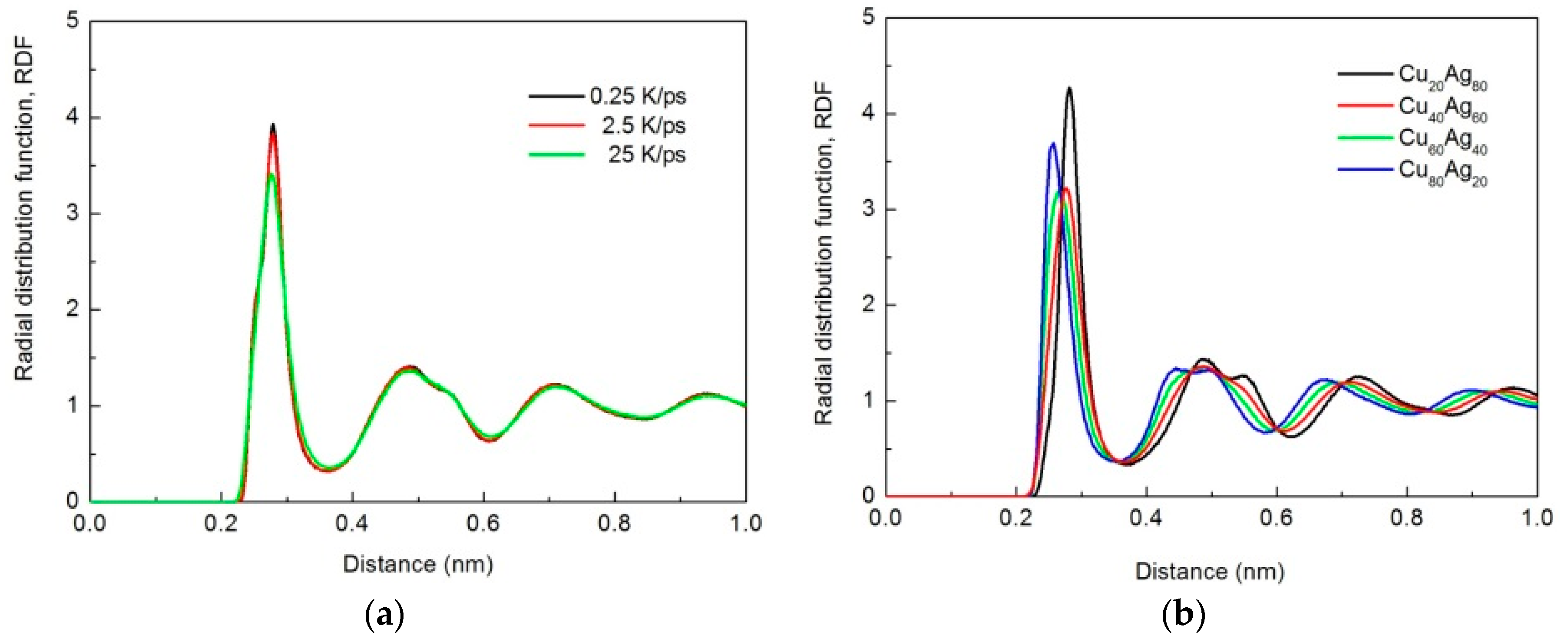
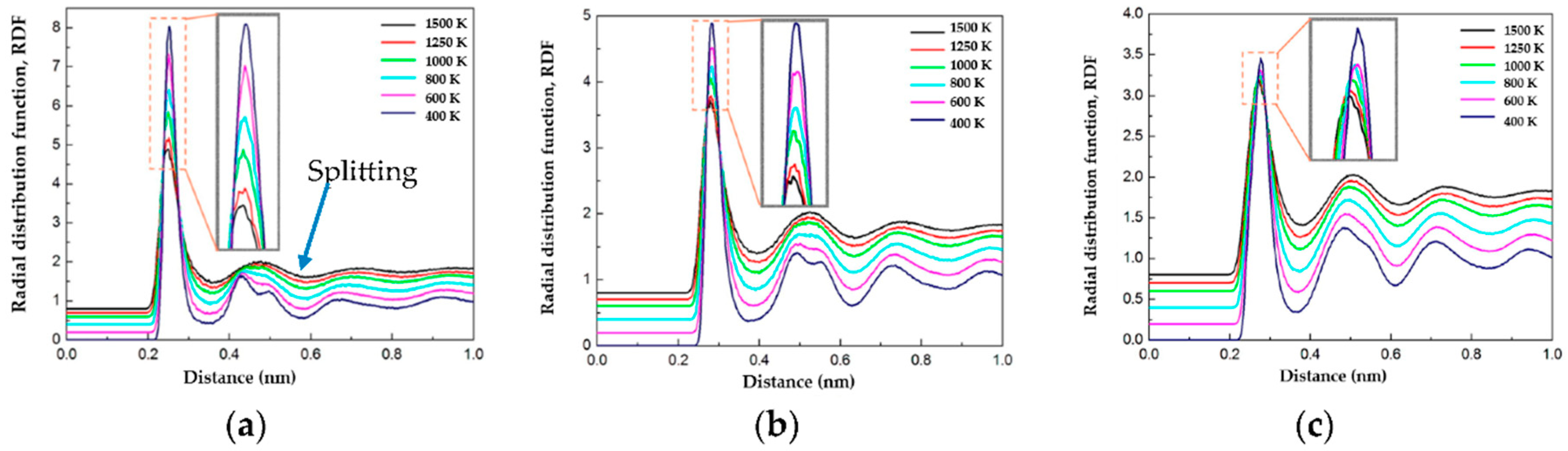
3.1. Radial Distribution Function (RDF)
3.2. Indexed of Glass Forming Ability
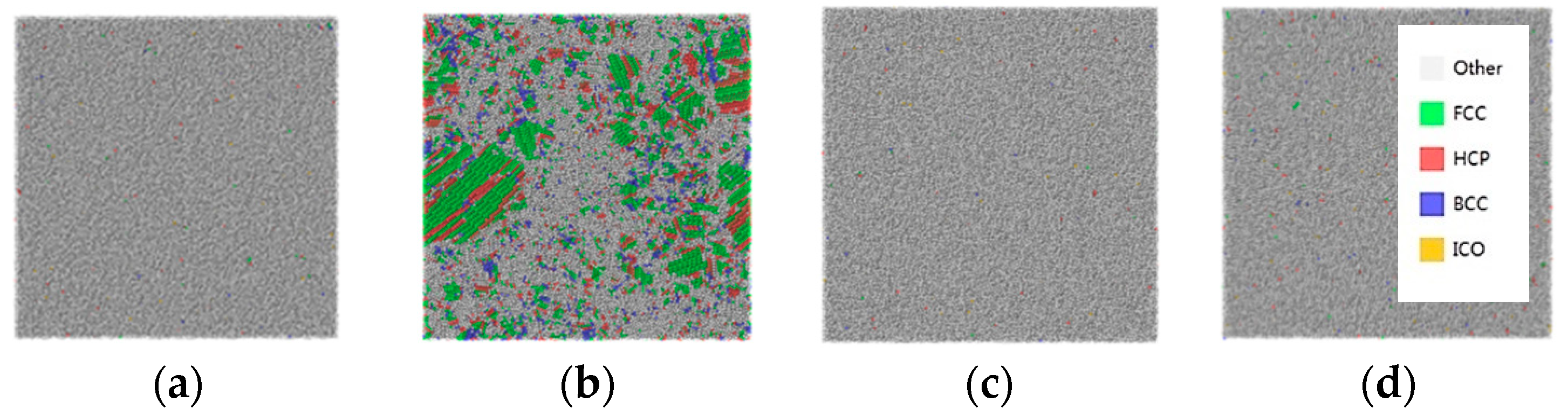
3.3. Honeycutt–Anderson (HA) Bond Pair Analysis
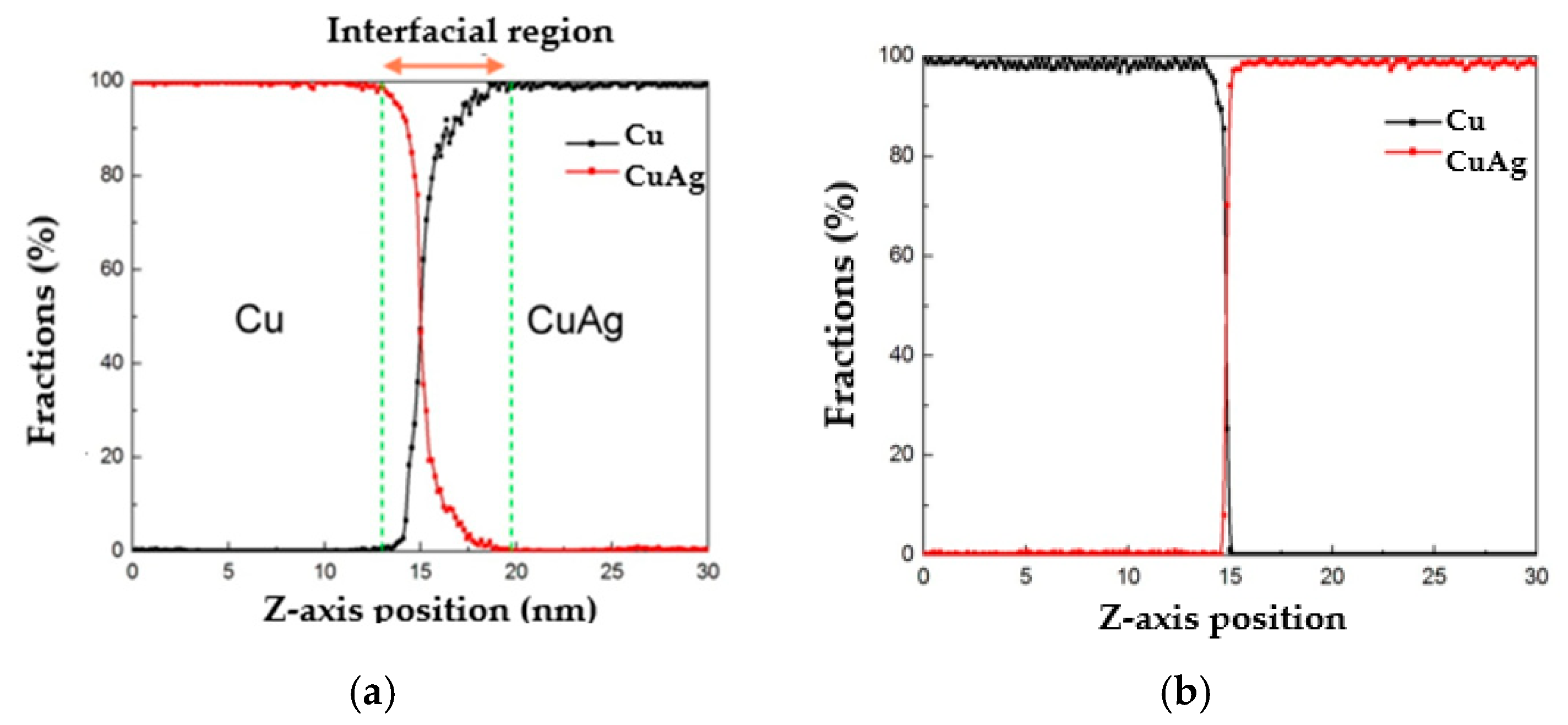
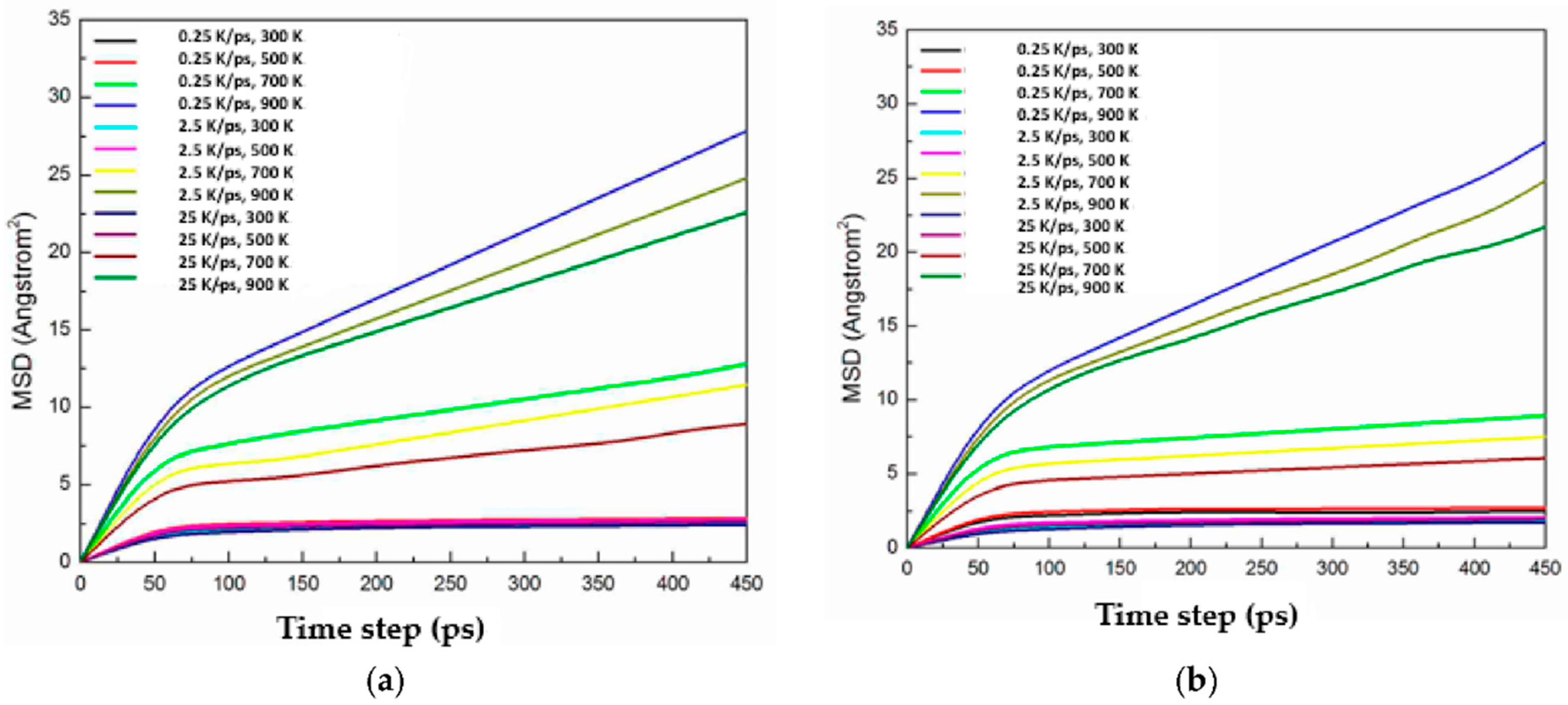
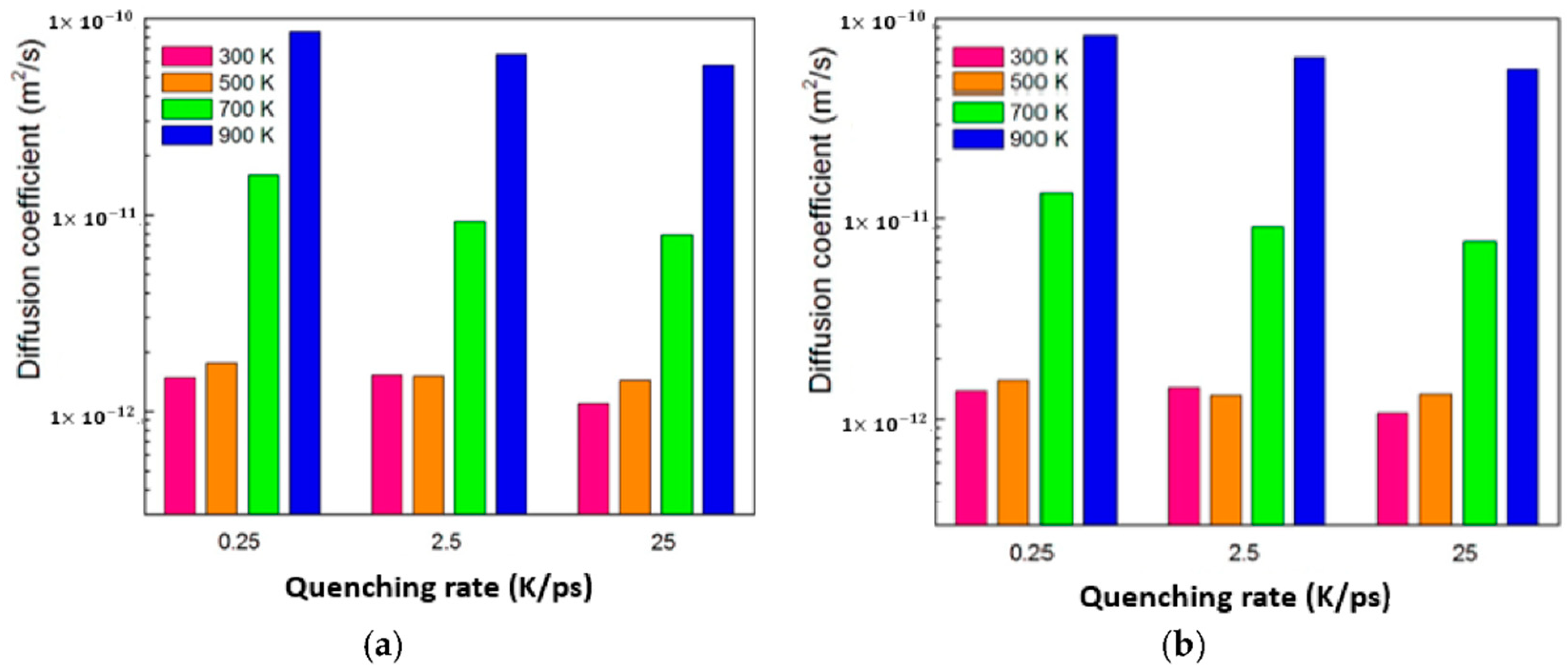
3.4. Diffusion between Cu–Ag and Cu
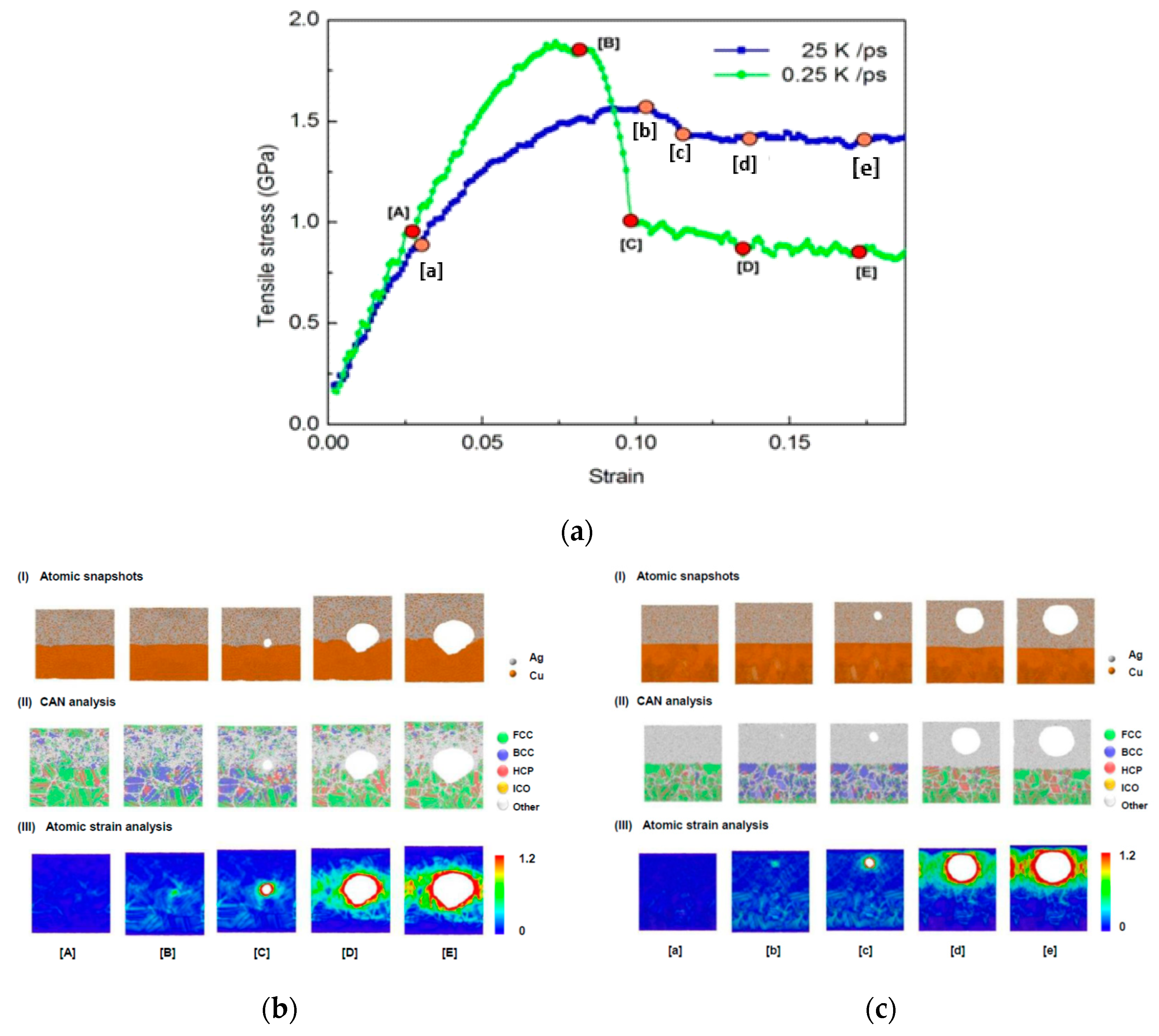
3.5. Tensile Behavior
4. Conclusions
- Cu20Ag80 is 50% amorphous at quenching rate of 0.25 K/ps, whereas Cu40Ag60, Cu60Ag40, and Cu80Ag20 are more than 95% amorphous at quenching rate between 0.25 K/ps and 25 K/ps. In other words, Cu–Ag alloys exhibit excellent GFA except Cu20Ag80.
- A diffusion region of 1 to 2 nm at 700 K occurs between copper and Cu40Ag60, or Cu60Ag40, or Cu80Ag20 as quenched within the range from 0.25 to 25 K/ps. However, a diffusion region of approximately 7 to 8 nm takes place at 700 K between copper and Cu20Ag80 quenched by 0.25 K/ps. In other words, the layer with higher ratio of amorphous exhibits a better performance of diffusion barrier.
- Simulation results of tensile test show that the stress–strain curves of Cu–Ag alloys having higher ratio of amorphous (for instance, Cu20Ag80 produced at higher quenching rate 25 K/ps, and other ratios of Cu–Ag alloys at quenching rate 0.25–25 K/ps) are smoother and the behavior is close to the ideal elastic-perfectly-plastic response. The void initiates in the Cu–Ag layer first and then gradually enlarges. This phenomenon indicates that the Cu/Cu–Ag interface is stronger than Cu–Ag alloy. For the Cu20Ag80 alloy produced at slower quenching rate (0.25 K/ps), in which both crystalline and amorphous phases exist together, as the strain increases, its stress gradually reaches a higher maximum and then suddenly drops. This is followed by a steady flow regime during which some serrations are evident. The sudden drops are caused by the formation of voids near the Cu/Cu20Ag80 interface. This phenomenon of sudden drop in stress is different from the crystal metallic of dislocations undergoing slippage along the slip plane.
Author Contributions
Funding
Conflicts of Interest
References
- Lim, S.P.S.; Ding, M.Z.; Kawano, M. Chip-to-Wafer (C2W) flip chip bonding for 2.5D high density interconnection on TSV free interposer. In Proceedings of the 19th Electronics Packaging Technology Conference IEEE (EPTC), Singapore, 6–9 December 2017; pp. 1–7. [Google Scholar]
- Murarka, S.P. Multilevel interconnections for ULSI and GSI era. Mater. Sci. Eng. R 1997, 19, 87–151. [Google Scholar] [CrossRef]
- Sorensen, M.R.; Mishin, Y.; Voter, A.F. Diffusion mechanisms in Cu grain boundaries. Phys. Rev. B 2000, 62, 3658–3673. [Google Scholar] [CrossRef] [Green Version]
- Ojovan, M.I.; Lee, W.B.E. Connectivity and glass transition in disordered oxide systems. J. Non-Crystal. Solids 2010, 356, 2534–2540. [Google Scholar] [CrossRef]
- Diyatmika, W.; Chu, J.P.; Yen, Y.; Hsueh, C. Sn whisker mitigation by a thin metallic-glass underlayer in Cu-Sn. Appl. Phys. Lett. 2013, 103, 241912. [Google Scholar] [CrossRef]
- Wu, Y.; Sees, J.A.; Pouraghabagher, C.; Foster, L.A.; Marshall, J.L.; Jacobs, E.G.; Pinizzotto, R.F. The formation and growth of intermetallics in composite solder. J. Electron. Mater. 1993, 22, 769–777. [Google Scholar] [CrossRef]
- Diyatmika, W.; Chu, J.P.; Yen, Y.; Chang, W.; Hsueh, C. Thin film metallic glass as an underlayer for tin whisker mitigation: A room-temperature evaluation. Thin Solid Films 2014, 561, 93–97. [Google Scholar] [CrossRef]
- Muehlbacher, M.; Bochkarev, A.S.; Mendez-Martin, F.; Sartory, B.; Chitu, L.; Popov, M.N.; Puschnig, P.; Spitaler, J.; Ding, H.; Schalk, N.; et al. Cu diffusion in single-crystal and polycrystalline TiN barrier layers: A high-resolution experimental study supported by first-principles calculations. J. Appl. Phys. 2015, 118, 085307. [Google Scholar] [CrossRef]
- Popov, M.N.; Bochkarev, A.S.; Razumovskiy, V.I.; Puschnig, P.; Spitaler, J. Point defects at the Σ5 (012)[100] grain boundary in TiN and the early stages of Cu diffusion: An ab initio study. Acta Mater. 2018, 144, 496–504. [Google Scholar] [CrossRef]
- Sangiovanni, D.G. Copper adatom, admolecule transport, and island nucleation on TiN (001) via ab initio molecular dynamics. Appl. Surf. Sci. 2018, 450, 180–189. [Google Scholar] [CrossRef]
- Wu, W.F.; Tsai, K.C.; Chao, C.G.; Chen, J.C.; Ou, K.L. Novel multilayered Ti/TiN diffusion barrier for Al metallization. J. Electron. Mater. 2005, 34, 1150–1156. [Google Scholar] [CrossRef]
- Lee, C.H.; Wong, Y.M.; Doherty, C.; Tai, K.L.; Lane, E.; Bacon, D.D.; Baiocchi, F.; Katz, A. Study of Ni as a barrier metal in AuSn soldering application for laser chip/submount assembly. J. Appl. Phys. 1992, 72, 3808–3815. [Google Scholar] [CrossRef]
- Chang, C.A. Interactions between Au and Cu across a Ni barrier layer. J. Appl. Phys. 1986, 50, 1220–1222. [Google Scholar] [CrossRef]
- Keller, H.N. Solder connections with a Ni barrier. IEEE Trans. Comp. Hybrids Manufac. Technol. 1986, 9, 433–439. [Google Scholar] [CrossRef]
- Iwamoto, N.; Truong, N.; Lee, E. New metal layers for integrated circuit manufacture: Experimental and modeling studies. Thin Solid Films 2004, 469, 431–437. [Google Scholar] [CrossRef]
- Sung, P.H.; Chen, T.C. Material properties of Zr–Cu–Ni–Al thin films as diffusion barrier layer. Crystals 2020, 10, 540. [Google Scholar] [CrossRef]
- Jen, M.H.R.L.; Liu, C.; Lai, Y.S. Electromigration test on void formation and failure mechanism of FCBGA lead-free solder joints. IEEE Trans. Comp. Pack. Technol. 2009, 32, 79–88. [Google Scholar] [CrossRef]
- Rymaszewski, E.; Walsh, J.; Leehan, G. Semiconductor logic technology in IBM. IBM J. Res. Dev. 1981, 25, 603–616. [Google Scholar] [CrossRef]
- Zhang, Z.; Wong, C. Recent advances in flip-chip underfill: Materials, process, and reliability. IEEE Trans. Adv. Pack. 2004, 27, 515–524. [Google Scholar] [CrossRef]
- An, B.; Kwon, Y.; Oh, J.; Yang, C. Amorphous TaxMnyOz layer as a diffusion barrier for advanced copper interconnects. Sci. Rep. 2019, 9, 20132. [Google Scholar] [CrossRef]
- An, B.; Kwon, Y.; Oh, J.; Lee, C.; Choi, S.; Kim, H.; Lee, M.; Pae, S.; Yang, C. Characteristics of an amorphous carbon layer as a diffusion barrier. ACS Appl. Mater. Inter. 2020, 12, 3104–3113. [Google Scholar] [CrossRef]
- Hüger, E.; Strauß, F.; Stahn, J.; Deubener, J.; Bruns, M.; Schmidt, H. In-situ measurement of self-atom diffusion in solids using amorphous germanium as a model system. Sci. Rep. 2018, 8, 17607. [Google Scholar] [CrossRef]
- Ou, K.L.; Wu, W.F.; Chou, C.P.; Chiou, S.Y.; Wu, C.C. Improved TaN barrier layer against Cu diffusion by formation of an amorphous layer using plasma treatment. J. Vac. Sci. Technol. B 2002, 20, 2154–2161. [Google Scholar] [CrossRef]
- Wang, C.; Yiu, W.P.; Chu, J.P.; Shek, C.H.; Hsueh, C.H. Zr–Ti–Ni thin film metallic glass as a diffusion barrier between copper and silicon. J. Mater. Sci. 2015, 50, 2085–2092. [Google Scholar] [CrossRef]
- Ribbe, J.; Schmitz, G.; Divinski, S.V. Grain boundary diffusion of Fe in high-purity copper. Defect Diffus. Forum 2009, 289–292, 211–217. [Google Scholar] [CrossRef]
- Si, C.; Duana, B.; Zhang, Q.; Cai, J.; Wu, W. Microstructure, corrosion-resistance, and wear-resistance properties of subsonic flame sprayed amorphous Fe–Mo–Cr–Co coating with extremely high amorphous rate. J. Mater. Res. Technol. 2020, 9, 3292–3303. [Google Scholar] [CrossRef]
- Ning, W.; Zhai, H.; Xiao, R.; He, D.; Li, W.; Li, X. The corrosion resistance mechanism of Fe-based amorphous coatings synthesised by detonation gun spraying. J. Mater. Eng. Perform. 2020, 29, 3921–3929. [Google Scholar] [CrossRef]
- Tran, A.S.; Fang, T.H. Void growth and coalescence in Cu–Ta metallic glasses using molecular dynamics. Comput. Mater. Sci. 2019, 168, 144–153. [Google Scholar] [CrossRef]
- Tran, A.S.; Fang, T.H. Size effect and interfacial strength in nanolaminated Cu/CuxTa100-x composites using molecular dynamics. Comput. Mater. Sci. 2020, 184, 109890. [Google Scholar] [CrossRef]
- Hsieh, J.; Hung, S. The effect of Cu:Ag atomic ratio on the properties of sputtered Cu–Ag alloy thin films. Materials 2016, 9, 914. [Google Scholar] [CrossRef] [Green Version]
- Wang, K.; Fujita, T.; Chen, M.W.; Nieh, T.G.; Okada, H.; Koyama, K.; Zhang, W.; Inoue, A. Electrical conductivity of a bulk metallic glass composite. Appl. Phys. Lett. 2007, 91, 154101. [Google Scholar] [CrossRef]
- Szymanska, I.B.; Piszczek, P.; Bala, W.; Bartkiewicz, K.; Szlyk, E. Ag/Cu layers grown on Si(111) substrates by thermal inducted chemical vapor deposition. Surf. Coat. Technol. 2007, 201, 9015–9020. [Google Scholar] [CrossRef]
- Wei, M.Z.; Xu, L.J.; Shi, J.; Pan, G.J.; Gao, Z.H.; Meng, M.K. Achieving high strength and high electrical conductivity in Ag/Cu multilayers. Appl. Phys. Lett. 2015, 106, 011604. [Google Scholar] [CrossRef]
- Strehle, S.; Menzel, S.; Wetzig, K.; Bartha, J.W. Microstructure of electroplated Cu(Ag) alloy thin films. Thin Sol. Films 2011, 519, 3522–3529. [Google Scholar] [CrossRef]
- Spolenak, R.; Kraft, O.; Arzt, E. Effects of alloying elements on electromigration. Microelectron. Reliab. 1998, 38, 1015–1020. [Google Scholar] [CrossRef]
- Plimpton, S. Fast parallel algorithms for short-range molecular-dynamics. J. Comput. Phys. 1995, 117, 1–19. [Google Scholar] [CrossRef] [Green Version]
- Stukowski, A. Visualization and analysis of atomistic simulation data with OVITO-the Open Visualization Tool. Model. Simul. Mater. Sci. Eng. 2010, 18, 015012. [Google Scholar] [CrossRef]
- Rassoulinejad-Mousavi, S.M.; Mao, Y.; Zhang, Y. Evaluation of copper, aluminum, and nickel interatomic potentials on predicting the elastic properties. J. Appl. Phys. 2016, 119, 244304. [Google Scholar] [CrossRef]
- Daw, M.S.; Baskes, M.I. Embedded-atom method: Derivation and application to impurities, surfaces, and other defects in metals. Phys. Rev. B 1984, 29, 6443–6453. [Google Scholar] [CrossRef] [Green Version]
- Gupta, P.; Pal, S.; Yedla, N. Molecular dynamics based cohesive zone modeling of Al (metal)–Cu50Zr50 (metallic glass) interfacial mechanical behavior and investigation of dissipative mechanisms. Mater. Des. 2016, 105, 41–50. [Google Scholar] [CrossRef]
- Gear, C.W. Numerical Initial Value Problems in Ordinary Differential Equations; Prentice-Hall: Englewood Cliffs, NJ, USA, 1971. [Google Scholar]
- Mohazzabi, P. Temperature dependence of the elastic constants of copper, gold and silver. J. Phys. Chem. Solids 1985, 46, 147–150. [Google Scholar] [CrossRef]
- Qi, L.; Zhang, H.; Hu, Z. Molecular dynamic simulation of glass formation in binary liquid metal: Cu–Ag using EAM. Intermetallics 2004, 12, 1191–1195. [Google Scholar] [CrossRef]
- Qi, Y.; Çağın, T.; Kimura, Y.; Goddard, W.A., III. Molecular-dynamics simulations of glass formation and crystallization in binary liquid metals: Cu–Ag and Cu–Ni. Phys. Rev. B 1999, 59, 3527–3533. [Google Scholar] [CrossRef] [Green Version]
- Honeycutt, J.; Andersen, H.C. Molecular-dynamics study of melting and freezing of small Lennard-Jones clusters. J. Phys. Chem. 1987, 91, 4950–4963. [Google Scholar] [CrossRef]
- Rangsu, L.; Jiyong, L.; Kejun, D.; Caixing, Z.; Hairong, L. Formation and evolution properties of clusters in a large liquid metal system during rapid cooling processes. Mater. Sci. Eng. B 2002, 94, 141–148. [Google Scholar] [CrossRef]
- Dong, K.; Liu, R.; Yu, A.; Zou, R.; Li, J. Simulation study of the evolution mechanisms of clusters in a large-scale liquid Al system during rapid cooling processes. J. Phys. 2003, 15, 743–753. [Google Scholar] [CrossRef]
- Liu, R.S.; Dong, K.J.; Li, J.Y.; Yu, A.B.; Zou, R.P. Formation and description of nano-clusters formed during rapid solidification processes in liquid metals. J. Non-Crystal. Solids 2005, 351, 612–617. [Google Scholar] [CrossRef]
- Liu, R.S.; Dong, K.J.; Tian, Z.A.; Liu, H.R.; Peng, P.; Yu, A.B. Formation and magic number characteristics of clusters formed during solidification processes. J. Phys. 2007, 19, 196103. [Google Scholar] [CrossRef]
- Liu, H.R.; Liu, R.S.; Zhang, A.L.; Hou, Z.Y.; Wang, X.; Tian, Z.A. A simulation study of microstructure evolution during solidification process of liquid metal Ni. Chin. Phys. 2007, 16, 3747–3753. [Google Scholar]
- Chen, S.; Soh, A.; Ke, F. Molecular dynamics modeling of diffusion bonding. Scrip. Mater. 2005, 52, 1135–1140. [Google Scholar] [CrossRef] [Green Version]
- Meunier, M. Diffusion coefficients of small gas molecules in amorphous cis-1, 4-polybutadiene estimated by molecular dynamics simulations. J. Chem. Phys. 2005, 123, 134906. [Google Scholar] [CrossRef]
- Ju, S.P.; Wu, T.Y.; Liu, S.H. Mechanical and dynamical behaviors of ZrSi and ZrSi2 bulk metallic glasses: A molecular dynamics study. J. Appl. Phys. 2015, 117, 105103. [Google Scholar] [CrossRef]
- Faupel, F.; Frank, W.; Macht, M.P.; Mehrer, H.; Naundorf, V.; Rätzke, K.; Schober, H.R.; Sharma, S.K.; Teichler, H. Diffusion in metallic glasses and supercooled melts. Rev. Mod. Phys. 2003, 75, 237–280. [Google Scholar] [CrossRef] [Green Version]
- Todorov, T.; Sutton, A. Force and conductance jumps in atomic-scale metallic contacts. Phys. Rev. B 1996, 54, R14234. [Google Scholar] [CrossRef] [PubMed]
- Yamakov, V.; Wolf, D.; Phillpot, S.R.; Mukherjee, A.K.; Gleiter, H. Dislocation processes in the deformation of nanocrystalline aluminium by molecular-dynamics simulation. Nat. Mater. 2002, 1, 45–48. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Ma, E. Atomic-level structure and structure–property relationship in metallic glasses. Prog. Mater. Sci. 2011, 56, 379–473. [Google Scholar] [CrossRef]
- Shi, Y.; Falk, M.L. Strain localization and percolation of stable structure in amorphous solids. Phys. Rev. Lett. 2005, 95, 095502. [Google Scholar] [CrossRef] [Green Version]
- Albano, F.; Lacevic, N.; Falk, M.L.; Glotzer, S.C. Relating metallic glass mechanical properties to liquid structure. Mater. Sci. Eng. A 2004, 375, 671–674. [Google Scholar] [CrossRef]




| Elements | Methods | C11 (GPa) | C12 (GPa) | C44 (GPa) |
|---|---|---|---|---|
| Cu | Experimental data [38] | 168.4 | 121.4 | 75.4 |
| Calculated data by MD | 168.5 | 120.8 | 75.2 | |
| Error (%) | 0.06 | 0.49 | 0.27 | |
| Ag | Experimental data [42] | 124.8 | 95.2 | 46.0 |
| Calculated data by MD | 126.1 | 94.2 | 46.9 | |
| Error (%) | 1.04 | 1.05 | 1.96 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sung, P.-H.; Chen, T.-C. Performance of Cu–Ag Thin Films as Diffusion Barrier Layer. Coatings 2020, 10, 1087. https://doi.org/10.3390/coatings10111087
Sung P-H, Chen T-C. Performance of Cu–Ag Thin Films as Diffusion Barrier Layer. Coatings. 2020; 10(11):1087. https://doi.org/10.3390/coatings10111087
Chicago/Turabian StyleSung, Po-Hsien, and Tei-Chen Chen. 2020. "Performance of Cu–Ag Thin Films as Diffusion Barrier Layer" Coatings 10, no. 11: 1087. https://doi.org/10.3390/coatings10111087
APA StyleSung, P.-H., & Chen, T.-C. (2020). Performance of Cu–Ag Thin Films as Diffusion Barrier Layer. Coatings, 10(11), 1087. https://doi.org/10.3390/coatings10111087





