Galvanic Corrosion Due to a Heterogeneous Sulfate Reducing Bacteria Biofilm
Abstract
:1. Introduction
2. Experimental
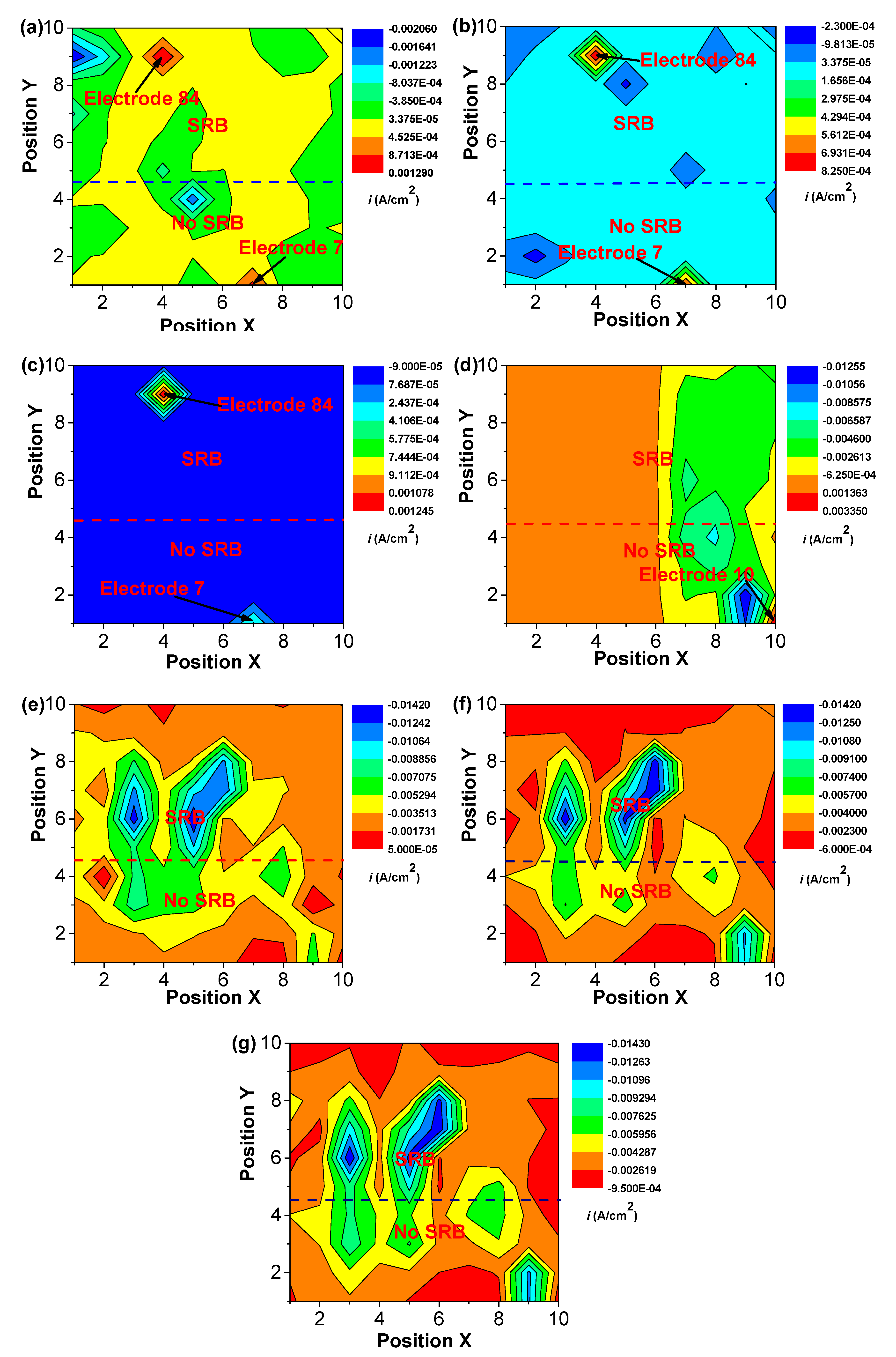
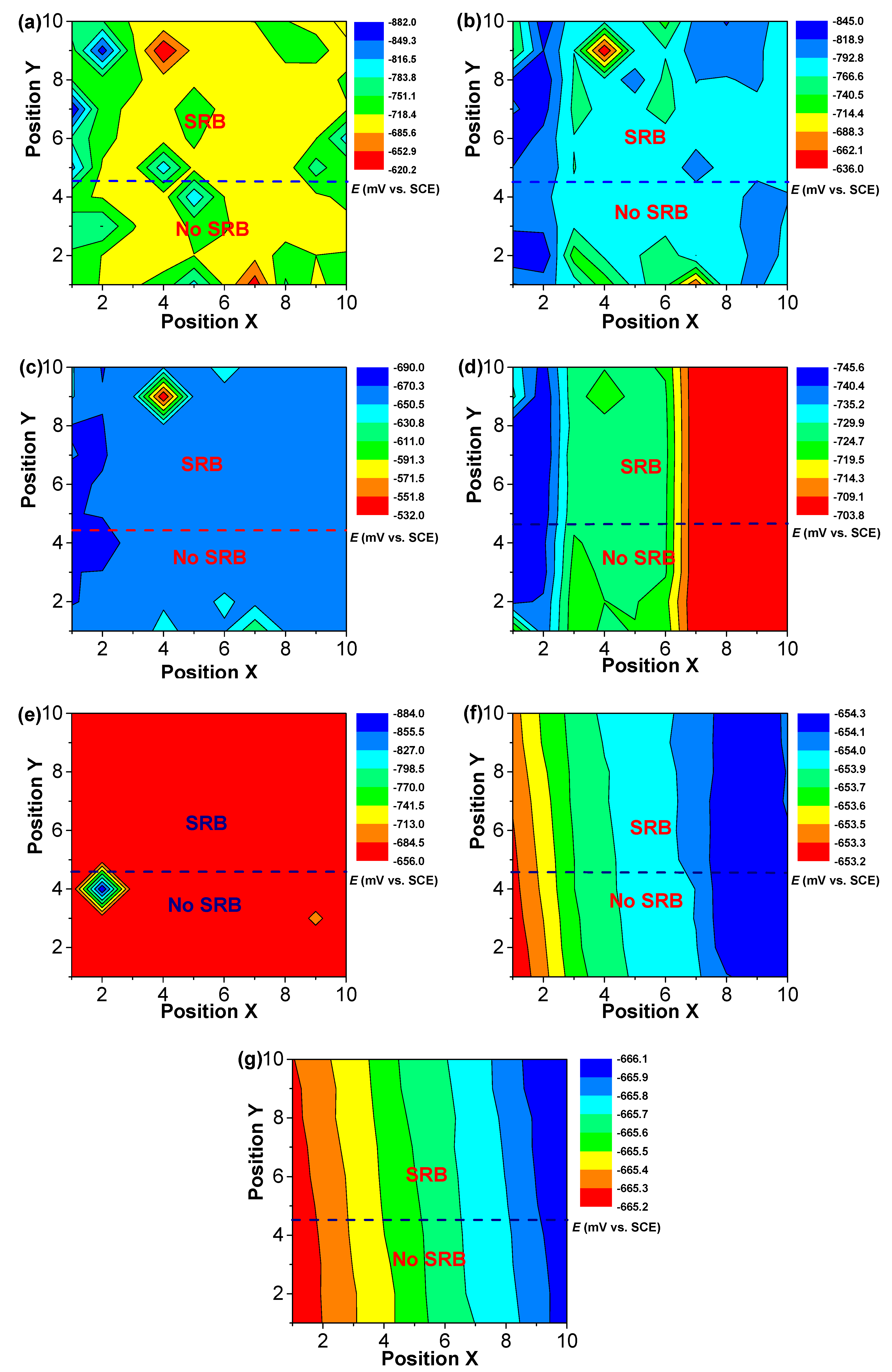
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Zhang, P.; Xu, D.; Li, Y.; Yang, K.; Gu, T. Electron mediators accelerate the microbiologically influenced corrosion of 304 stainless steel by the Desulfovibrio vulgaris biofilm. Bioelectrochemistry 2015, 101, 14–21. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Cheng, Y.F. Mechanistic aspects of microbially influenced corrosion of X52 pipeline steel in a thin layer of soil solution containing sulphate-reducing bacteria under various gassing conditions. Corros. Sci. 2018, 133, 178–189. [Google Scholar] [CrossRef]
- Liu, H.; Xu, D.; Yang, K.; Liu, H.; Cheng, Y.F. Corrosion of antibacterial Cu-bearing 316L stainless steels in the presence of sulfate reducing bacteria. Corros. Sci. 2018, 132, 46–55. [Google Scholar] [CrossRef]
- Mehanna, M.; Basseguy, R.; Delia, M.-L.; Bergel, A. Role of direct microbial electron transfer in corrosion of steels. Electrochem. Commun. 2009, 11, 568–571. [Google Scholar] [CrossRef] [Green Version]
- Jia, R.; Tan, J.L.; Jin, P.; Blackwood, D.J.; Xu, D.; Gu, T. Effects of biogenic H2S on the microbiologically influenced corrosion of C1018 carbon steel by sulfate reducing Desulfovibrio vulgaris biofilm. Corros. Sci. 2018, 130, 1–11. [Google Scholar] [CrossRef]
- Anandkumar, B.; George, R.P.; Maruthamuthu, S.; Parvathavarthini, N.; Mudali, U.K. Corrosion characteristics of sulfate-reducing bacteria (SRB) and the role of molecular biology in SRB studies: An overview. Corros. Rev. 2016, 34, 41–63. [Google Scholar] [CrossRef]
- Marciales, A.; Peralta, Y.; Haile, T.; Crosby, T.; Wolodko, J. Mechanistic Microbiologically Influenced Corrosion Modeling—A Review. Corros. Sci. 2019, 146, 99–111. [Google Scholar] [CrossRef]
- Gu, T.; Jia, R.; Unsal, T.; Xu, D. Toward a better understanding of microbiologically influenced corrosion caused by sulfate reducing bacteria. J. Mater. Sci. Technol. 2019, 35, 631–636. [Google Scholar] [CrossRef]
- Dong, Z.H.; Shi, W.; Ruan, H.M.; Zhang, G.A. Heterogeneous corrosion of mild steel under SRB-biofilm characterised by electrochemical mapping technique. Corros. Sci. 2011, 53, 2978–2987. [Google Scholar] [CrossRef]
- Yu, L.; Duan, J.; Du, X.; Huang, Y.; Hou, B. Accelerated anaerobic corrosion of electroactive sulfate-reducing bacteria by electrochemical impedance spectroscopy and chronoamperometry. Electrochem. Commun. 2013, 26, 101–104. [Google Scholar] [CrossRef]
- Liu, H.; Zhong, X.; Liu, H.; Cheng, Y.F. Microbiologically-enhanced galvanic corrosion of the steel beneath a deposit in simulated oilfield-produced water containing Desulfotomaculum nigrificans. Electrochem. Commun. 2018, 90, 1–5. [Google Scholar] [CrossRef]
- Liu, H.; Cheng, Y.F. Microbial corrosion of X52 pipeline steel under soil with varied thicknesses soaked with a simulated soil solution containing sulfate-reducing bacteria and the associated galvanic coupling effect. Electrochim. Acta 2018, 266, 312–325. [Google Scholar] [CrossRef]
- Liu, H.; Meng, G.; Li, W.; Liu, H.; Gu, T. Microbiologically influenced corrosion of carbon steel beneath a deposit in CO2-saturated formation water containing Desulfotomaculum nigrificans. Front. Microbiol. 2019, 10, 1298. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Yu, N.; Yang, L.; Guo, X. Galvanic corrosion behavior of deposit-covered and uncovered carbon steel. Corros. Sci. 2014, 86, 202–212. [Google Scholar] [CrossRef]
- Wu, J.; Wang, P.; Gao, J.; Tan, F.; Zhang, D.; Cheng, Y.; Chen, S. Comparison of water-line corrosion processes in natural and artificial seawater: The role of microbes. Electrochem. Commun. 2017, 80, 9–15. [Google Scholar] [CrossRef]
- Chen, S.; Zhang, D. Corrosion behavior of Q235 carbon steel in air-saturated seawater containing Thalassospira sp. Corros. Sci. 2018, 148, 71–82. [Google Scholar] [CrossRef]
- Liu, H.; Fu, C.; Gu, T.; Zhang, G.; Lv, Y.; Wang, H.; Liu, H. Corrosion behavior of carbon steel in the presence of sulfate reducing bacteria and iron oxidizing bacteria cultured in oilfield produced water. Corros. Sci. 2015, 100, 484–495. [Google Scholar] [CrossRef]
- Homborg, A.; Morales, C.L.; Tinga, T.; De Wit, J.; Mol, J. Detection of microbiologically influenced corrosion by electrochemical noise transients. Electrochim. Acta 2014, 136, 223–232. [Google Scholar] [CrossRef]
- Lopes, F.A.; Perrin, S.; Féron, D. A dual-electrochemical cell to study the biocorrosion of stainless steel. Water Sci. Technol. 2007, 55, 499–504. [Google Scholar] [CrossRef]
- Zheng, B.; Li, K.; Liu, H.; Gu, T. Effects of Magnetic Fields on Microbiologically Influenced Corrosion of 304 Stainless Steel. Ind. Eng. Chem. Res. 2013, 53, 48–54. [Google Scholar] [CrossRef]
- Beese-Vasbender, P.F.; Nayak, S.; Erbe, A.; Stratmann, M.; Mayrhofer, K.J. Electrochemical characterization of direct electron uptake in electrical microbially influenced corrosion of iron by the lithoautotrophic SRB Desulfopila corrodens strain IS4. Electrochim. Acta 2015, 167, 321–329. [Google Scholar] [CrossRef]
- Javed, M.; Stoddart, P.; Wade, S. Corrosion of carbon steel by sulphate reducing bacteria: Initial attachment and the role of ferrous ions. Corros. Sci. 2015, 93, 48–57. [Google Scholar] [CrossRef]
- AlAbbas, F.M.; Williamson, C.; Bhola, S.M.; Spear, J.R.; Olson, D.L.; Mishra, B.; Kakpovbia, A.E. Influence of sulfate reducing bacterial biofilm on corrosion behavior of low-alloy, high-strength steel (API-5L X80). Int. Biodeterior. Biodegrad. 2013, 78, 34–42. [Google Scholar] [CrossRef]
- Venzlaff, H.; Enning, D.; Srinivasan, J.; Mayrhofer, K.J.; Hassel, A.W.; Widdel, F.; Stratmann, M. Accelerated cathodic reaction in microbial corrosion of iron due to direct electron uptake by sulfate-reducing bacteria. Corros. Sci. 2013, 66, 88–96. [Google Scholar] [CrossRef] [Green Version]
- Xu, D.; Gu, T. Carbon source starvation triggered more aggressive corrosion against carbon steel by the Desulfovibrio vulgaris biofilm. Int. Biodeterior. Biodegrad. 2014, 91, 74–81. [Google Scholar] [CrossRef]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, H.; Liu, H.; Zhang, Y. Galvanic Corrosion Due to a Heterogeneous Sulfate Reducing Bacteria Biofilm. Coatings 2020, 10, 1116. https://doi.org/10.3390/coatings10111116
Liu H, Liu H, Zhang Y. Galvanic Corrosion Due to a Heterogeneous Sulfate Reducing Bacteria Biofilm. Coatings. 2020; 10(11):1116. https://doi.org/10.3390/coatings10111116
Chicago/Turabian StyleLiu, Hongwei, Haixian Liu, and Yuxuan Zhang. 2020. "Galvanic Corrosion Due to a Heterogeneous Sulfate Reducing Bacteria Biofilm" Coatings 10, no. 11: 1116. https://doi.org/10.3390/coatings10111116





