Biomimetic Calcium Phosphate Coating as a Drug Delivery Vehicle for Bone Tissue Engineering: A Mini-Review
Abstract
:1. Introduction
2. Biomimetic Calcium Phosphate Coatings
2.1. Biomimetic Calcium Phosphate Coating Technique
2.2. Features of Biomimetic Calcium Phosphate Coatings
2.2.1. Thickness of Coatings
2.2.2. Composition and Geometry of Coatings
2.2.3. The Mechanism of Binding and Release of Drugs Incorporated into Coatings
2.2.4. In Vivo Degradation of Coatings
2.2.5. The Preservation of Osteoinductive Efficacy of Drugs Incorporated into Coatings
3. Biomimetic Calcium Phosphate Coatings as Drug Carriers
3.1. Bone Morphogenetic Protein 2
3.2. Vascular Endothelial Growth Factor
3.3. Antibacterial Drugs
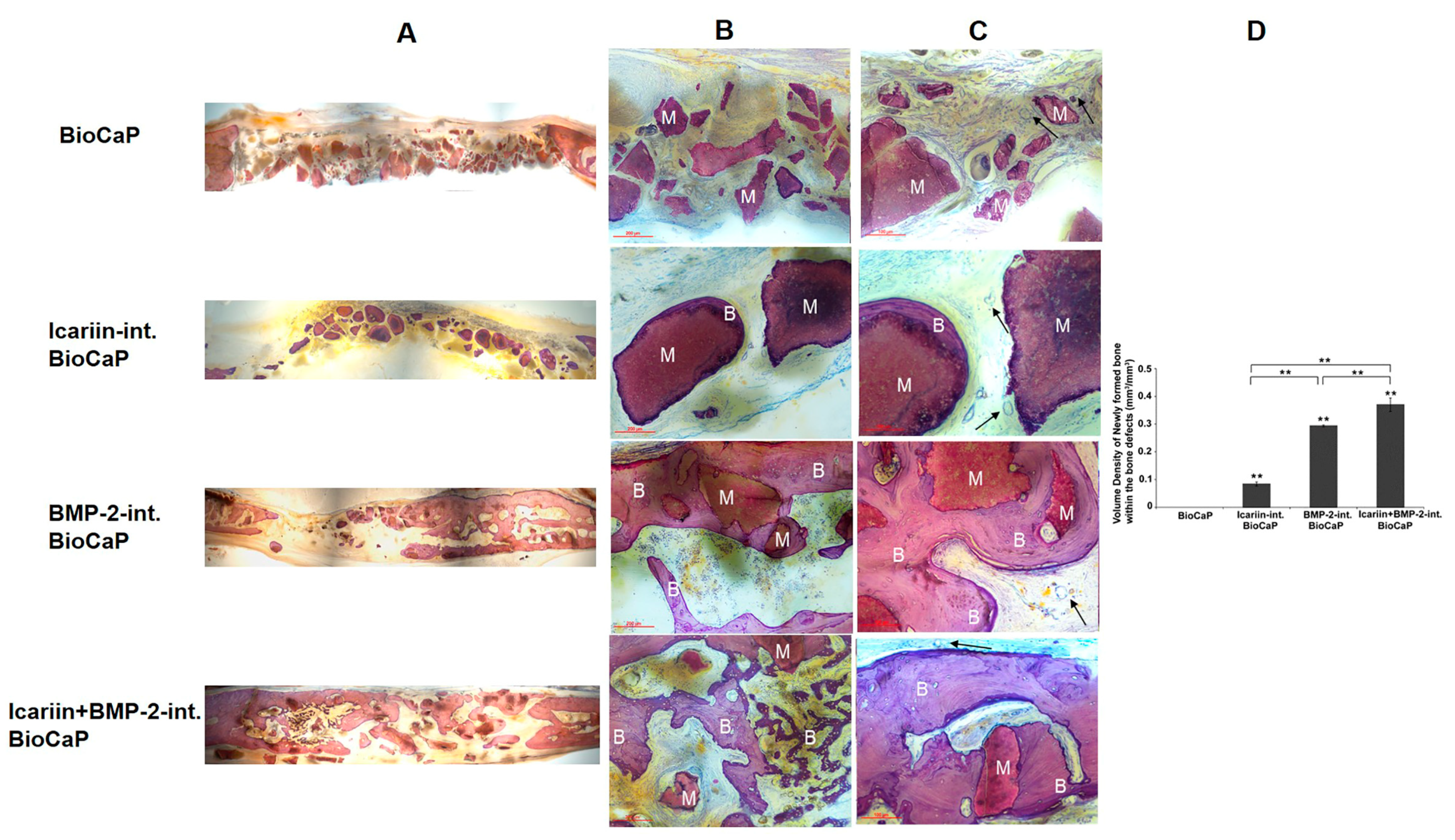
3.4. Icariin
3.5. Parathyroid Hormone
4. Delivering Multicomponent Molecules in Sequence
5. Suppress the Foreign-Body Reactivity
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Huang, Q.; Xu, S.; Ouyang, Z.; Yang, Y.; Liu, Y. Multi-scale nacre-inspired lamella-structured Ti–Ta composites with high strength and low modulus for load-bearing orthopedic and dental applications. Mater. Sci. Eng. C 2021, 118, 111458. [Google Scholar] [CrossRef]
- Tuchman, A.; Brodke, D.S.; Youssef, J.A.; Meisel, H.-J.; Dettori, J.R.; Park, J.-B.; Yoon, S.T.; Wang, J.C. Iliac crest bone graft versus local autograft or allograft for lumbar spinal fusion: A systematic review. Glob. Spine J. 2016, 6, 592–606. [Google Scholar] [CrossRef] [Green Version]
- Erten, E.; Arslan, Y.E. The great harmony in translational medicine: Biomaterials and stem cells. Adv. Exp. Med. Biol. 2018, 1119, 21–39. [Google Scholar] [CrossRef] [PubMed]
- Bose, S.; Roy, M.; Bandyopadhyay, A. Recent advances in bone tissue engineering scaffolds. Trends Biotechnol. 2012, 30, 546–554. [Google Scholar] [CrossRef] [PubMed]
- Alexandrova, R.; Abudalleh, A.; Andonova-lilova, B.; Zhivkova, T.; Mitrenga, P.; Dyakova, L.; Alexandrov, O.; Dinev, D.; Spasova, S.; Sigurjonsson, O. Bone tissue engineering. In Proceedings of the IX Workshop on Biological Activity of Metals, Synthetic Compounds and Natural Products, Sofia, Bulgaria, 26–28 November 2014. [Google Scholar]
- Albrektsson, T.; Johansson, C. Osteoinduction, osteoconduction and osseointegration. Eur. Spine J. 2001, 10 (Suppl. 2), S96–S101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El-Banna, A.; Bissa, M.W.; Khurshid, Z.; Zohaib, S.; Asiri, F.Y.I.; Zafar, M.S. 4—Surface modification techniques of dental implants. In Dental Implants; Woodhead Publishing: Duxford, UK, 2020; pp. 49–68. ISBN 978-0-12-819586-4l. [Google Scholar]
- Siebers, M.C.; Walboomers, X.F.; Leeuwenburgh, S.C.; Wolke, J.G.; Jansen, J.A. Electrostatic spray deposition (ESD) of calcium phosphate coatings, an in vitro study with osteoblast-like cells. Biomaterials 2004, 25, 2019–2027. [Google Scholar] [CrossRef] [PubMed]
- Graziani, G.; Bianchi, M.; Sassoni, E.; Russo, A.; Marcacci, M. Ion-substituted calcium phosphate coatings deposited by plasma-assisted techniques: A review. Mater. Sci. Eng. C 2017, 74, 219–229. [Google Scholar] [CrossRef] [PubMed]
- Asri, R.I.M.; Harun, W.S.W.; Hassan, M.A.; Ghani, S.A.C.; Buyong, Z. A review of hydroxyapatite-based coating techniques: Sol–gel and electrochemical depositions on biocompatible metals. J. Mech. Behav. Biomed. Mater. 2016, 57, 95–108. [Google Scholar] [CrossRef] [Green Version]
- Surmeneva, M.A.; Chaikina, M.V.; Zaikovskiy, V.I.; Pichugin, V.F.; Buck, V.; Prymak, O.; Epple, M.; Surmenev, R.A. The structure of an RF-magnetron sputter-deposited silicate-containing hydroxyapatite-based coating investigated by high-resolution techniques. Surf. Coat. Technol. 2013, 218, 39–46. [Google Scholar] [CrossRef]
- Qadir, M.; Li, Y.; Wen, C. Ion-substituted calcium phosphate coatings by physical vapor deposition magnetron sputtering for biomedical applications: A review. Acta Biomater. 2019, 89, 14–32. [Google Scholar] [CrossRef]
- Kazimierczak, P.; Przekora, A. Osteoconductive and osteoinductive surface modifications of biomaterials for bone regeneration: A concise review. Coatings 2020, 10, 971. [Google Scholar] [CrossRef]
- Oyane, A.; Nakamura, M.; Sakamaki, I.; Shimizu, Y.; Miyata, S.; Miyaji, H. Laser-assisted wet coating of calcium phosphate for surface-functionalization of PEEK. PLoS ONE 2018, 13, e0206524. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Najeeb, S.; Khurshid, Z.; Matinlinna, J.P.; Siddiqui, F.; Nassani, M.Z.; Baroudi, K. Nanomodified peek dental implants: Bioactive composites and surface modification—A review. Int. J. Dent. 2015, 2015, 381759. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wernike, E.; Hofstetter, W.; Liu, Y.; Wu, G.; Sebald, H.J.; Wismeijer, D.; Hunziker, E.B.; Siebenrock, K.A.; Klenke, F.M. Long-term cell-mediated protein release from calcium phosphate ceramics. J. Biomed. Mater. Res. A 2010, 92, 463–474. [Google Scholar] [CrossRef]
- Biase, P.D.; Capanna, R. Clinical applications of BMPs. Injury 2005, 36, S43–S46. [Google Scholar] [CrossRef]
- Nandi, S.K.; Kundu, B.; Mukherjee, J.; Mahato, A.; Datta, S.; Balla, V.K. Converted marine coral hydroxyapatite implants with growth factors: In vivo bone regeneration. Mater. Sci. Eng. C 2015, 49, 816–823. [Google Scholar] [CrossRef]
- Lin, X.; Hunziker, E.B.; Liu, T.; Hu, Q.; Liu, Y. Enhanced biocompatibility and improved osteogenesis of coralline hydroxyapatite modified by bone morphogenetic protein 2 incorporated into a biomimetic coating. Mater. Sci. Eng. C 2019, 96, 329–336. [Google Scholar] [CrossRef]
- Liu, Y.; Enggist, L.; Kuffer, A.F.; Buser, D.; Hunziker, E.B. The influence of BMP-2 and its mode of delivery on the osteoconductivity of implant surfaces during the early phase of osseointegration. Biomaterials 2007, 28, 2677–2686. [Google Scholar] [CrossRef]
- Haidar, Z.S.; Hamdy, R.C.; Tabrizian, M. Delivery of recombinant bone morphogenetic proteins for bone regeneration and repair. Part A: Current challenges in BMP delivery. Biotechnol. Lett. 2009, 31, 1817–1824. [Google Scholar] [CrossRef]
- Halloran, D.; Durbano, H.W.; Nohe, A. Bone morphogenetic protein-2 in development and bone homeostasis. J. Dev. Biol. 2020, 8, 19. [Google Scholar] [CrossRef]
- Park, S.-Y.; Kim, K.-H.; Kim, S.; Lee, Y.-M.; Seol, Y.-J. BMP-2 Gene delivery-based bone regeneration in dentistry. Pharmaceutics 2019, 11, 393. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Y.; Huse, R.O.; de Groot, K.; Buser, D.; Hunziker, E.B. Delivery mode and efficacy of BMP-2 in association with implants. J. Dent. Res. 2007, 86, 84–89. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; de Groot, K.; Hunziker, E.B. Osteoinductive implants: The mise-en-scène for drug-bearing biomimetic coatings. Ann. Biomed. Eng. 2004, 32, 398–406. [Google Scholar] [CrossRef] [PubMed]
- Kokubo, T.; Kushitani, H.; Sakka, S.; Kitsugi, T.; Yamamuro, T. Solutions able to reproduce in vivo surface-structure changes in bioactive glass-ceramic A-W. J. Biomed. Mater. Res. 1990, 24, 721–734. [Google Scholar] [CrossRef] [PubMed]
- Kokubo, T.; Ito, S.; Huang, Z.T.; Hayashi, T.; Sakka, S.; Kitsugi, T.; Yamamuro, T. Ca, P-rich layer formed on high-strength bioactive glass-ceramic A-W. J. Biomed. Mater. Res. 1990, 24, 331–343. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Wang, L.; Jiang, X.; Rowe, D.; Wei, M. Biomimetic CaP coating incorporated with parathyroid hormone improves the osseointegration of titanium implant. J. Mater. Sci. Mater. Med. 2012, 23, 2177–2186. [Google Scholar] [CrossRef]
- Hagi, T.T.; Wu, G.; Liu, Y.; Hunziker, E.B. Cell-mediated BMP-2 liberation promotes bone formation in a mechanically unstable implant environment. Bone 2010, 46, 1322–1327. [Google Scholar] [CrossRef]
- Jacobs, E.E.; Gronowicz, G.; Hurley, M.M.; Kuhn, L.T. Biomimetic calcium phosphate/polyelectrolyte multilayer coatings for sequential delivery of multiple biological factors. J. Biomed. Mater. Res. A 2017, 105, 1500–1509. [Google Scholar] [CrossRef] [Green Version]
- Alhamdi, J.R.; Peng, T.; Al-Naggar, I.M.; Hawley, K.L.; Spiller, K.L.; Kuhn, L.T. Controlled M1-to-M2 transition of aged macrophages by calcium phosphate coatings. Biomaterials 2019, 196, 90–99. [Google Scholar] [CrossRef]
- Liu, Y.; Hunziker, E.B.; Layrolle, P.; De Bruijn, J.D.; De Groot, K. Bone morphogenetic protein 2 incorporated into biomimetic coatings retains its biological activity. Tissue Eng. 2004, 10, 101–108. [Google Scholar] [CrossRef]
- Stigter, M.; de Groot, K.; Layrolle, P. Incorporation of tobramycin into biomimetic hydroxyapatite coating on titanium. Biomaterials 2002, 23, 4143–4153. [Google Scholar] [CrossRef]
- Lin, X.; de Groot, K.; Wang, D.; Hu, Q.; Wismeijer, D.; Liu, Y. A review paper on biomimetic calcium phosphate coatings. Open Biomed. Eng. J. 2015, 9, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Layrolle, P.; de Bruijn, J.; van Blitterswijk, C.; de Groot, K. Biomimetic coprecipitation of calcium phosphate and bovine serum albumin on titanium alloy. J. Biomed. Mater. Res. 2001, 57, 327–335. [Google Scholar] [CrossRef]
- Qu, H.; Wei, M. The effect of temperature and initial pH on biomimetic apatite coating. J. Biomed. Mater. Res. B Appl. Biomater. 2008, 87, 204–212. [Google Scholar] [CrossRef] [PubMed]
- Zhou, R.; Xu, W.; Chen, F.; Qi, C.; Lu, B.-Q.; Zhang, H.; Wu, J.; Qian, Q.-R.; Zhu, Y.-J. Amorphous calcium phosphate nanospheres/polylactide composite coated tantalum scaffold: Facile preparation, fast biomineralization and subchondral bone defect repair application. Colloids Surf. B 2014, 123, 236–245. [Google Scholar] [CrossRef]
- Wen, H.B.; Moradian-Oldak, J. Modification of calcium–phosphate coatings on titanium by recombinant amelogenin. J. Biomed. Mater. Res. 2003, 64A, 483–490. [Google Scholar] [CrossRef]
- Budnicka, M.; Szymaniak, M.; Kołbuk, D.; Ruśkowski, P.; Gadomska-Gajadhur, A. Biomineralization of poly-l-lactide spongy bone scaffolds obtained by freeze-extraction method. J. Biomed. Mater. Res. Part B Appl. Biomater. 2020, 108, 868–879. [Google Scholar] [CrossRef]
- Liu, Y.; de Groot, K.; Hunziker, E.B. BMP-2 liberated from biomimetic implant coatings induces and sustains direct ossification in an ectopic rat model. Bone 2005, 36, 745–757. [Google Scholar] [CrossRef]
- Wu, G.; Liu, Y.; Iizuka, T.; Hunziker, E.B. Biomimetic coating of organic polymers with a protein-functionalized layer of calcium phosphate: The surface properties of the carrier influence neither the coating characteristics nor the incorporation mechanism or release kinetics of the protein. Tissue Eng. Part C Methods 2010, 16, 1255–1265. [Google Scholar] [CrossRef]
- Hagi, T.T.; Enggist, L.; Michel, D.; Ferguson, S.J.; Liu, Y.; Hunziker, E.B. Mechanical insertion properties of calcium-phosphate implant coatings. Clin. Oral Implants Res. 2010, 21, 1214–1222. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, X.; Liu, Y.; Jin, X.; Fan, C.; Ye, H.; Ou, M.; Lv, L.; Wu, G.; Zhou, Y. Bi-functionalization of a calcium phosphate-coated titanium surface with slow-release simvastatin and metronidazole to provide antibacterial activities and pro-osteodifferentiation capabilities. PLoS ONE 2014, 9, e97741. [Google Scholar] [CrossRef] [Green Version]
- Yu, X.; Qu, H.; Knecht, D.A.; Wei, M. Incorporation of bovine serum albumin into biomimetic coatings on titanium with high loading efficacy and its release behavior. J. Mater. Sci. Mater. Med. 2009, 20, 287–294. [Google Scholar] [CrossRef]
- Teng, F.; Wei, L.; Yu, D.; Deng, L.; Zheng, Y.; Lin, H.; Liu, Y. Vertical bone augmentation with simultaneous implantation using deproteinized bovine bone block functionalized with a slow delivery of BMP-2. Clin. Oral Implants Res. 2020, 31, 215–228. [Google Scholar] [CrossRef]
- Stigter, M.; Bezemer, J.; de Groot, K.; Layrolle, P. Incorporation of different antibiotics into carbonated hydroxyapatite coatings on titanium implants, release and antibiotic efficacy. J. Control. Release 2004, 99, 127–137. [Google Scholar] [CrossRef] [PubMed]
- Ooya, T.; Eguchi, M.; Ozaki, A.; Yui, N. Carboxyethylester-polyrotaxanes as a new calcium chelating polymer: Synthesis, calcium binding and mechanism of trypsin inhibition. Int. J. Pharm. 2002, 242, 47–54. [Google Scholar] [CrossRef]
- Liu, T.; Wu, G.; Zheng, Y.; Wismeijer, D.; Everts, V.; Liu, Y. Cell-mediated BMP-2 release from a novel dual-drug delivery system promotes bone formation. Clin. Oral Implants Res. 2014, 25, 1412–1421. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Hunziker, E.B.; Zheng, Y.; Wismeijer, D.; Liu, Y. Functionalization of deproteinized bovine bone with a coating-incorporated depot of BMP-2 renders the material efficiently osteoinductive and suppresses foreign-body reactivity. Bone 2011, 49, 1323–1330. [Google Scholar] [CrossRef]
- Wu, G.; Liu, Y.; Iizuka, T.; Hunziker, E.B. The effect of a slow mode of BMP-2 delivery on the inflammatory response provoked by bone-defect-filling polymeric scaffolds. Biomaterials 2010, 31, 7485–7493. [Google Scholar] [CrossRef]
- Liu, Y.; Schouten, C.; Boerman, O.; Wu, G.; Jansen, J.A.; Hunziker, E.B. The kinetics and mechanism of bone morphogenetic protein 2 release from calcium phosphate-based implant-coatings. J. Biomed. Mater. Res. A 2018, 106, 2363–2371. [Google Scholar] [CrossRef]
- De Witte, T.-M.; Fratila-Apachitei, L.E.; Zadpoor, A.A.; Peppas, N.A. Bone tissue engineering via growth factor delivery: From scaffolds to complex matrices. Regen. Biomater. 2018, 5, 197–211. [Google Scholar] [CrossRef] [Green Version]
- Hunziker, E.B.; Enggist, L.; Kuffer, A.; Buser, D.; Liu, Y. Osseointegration: The slow delivery of BMP-2 enhances osteoinductivity. Bone 2012, 51, 98–106. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Tabassum, A.; Wu, G.; Deng, L.; Wismeijer, D.; Liu, Y. Bone regeneration in critical-sized bone defect enhanced by introducing osteoinductivity to biphasic calcium phosphate granules. Clin. Oral Implants Res. 2017, 28, 251–260. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Lin, X.; Liu, T.; Deng, L.; Huang, Y.; Liu, Y. Osteogenic enhancement between icariin and bone morphogenetic protein 2: A potential osteogenic compound for bone tissue engineering. Front. Pharmacol. 2019, 10, 201. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Zheng, Y.; Wu, G.; Wismeijer, D.; Pathak, J.L.; Liu, Y. BMP2-coprecipitated calcium phosphate granules enhance osteoinductivity of deproteinized bovine bone, and bone formation during critical-sized bone defect healing. Sci. Rep. 2017, 7, 41800. [Google Scholar] [CrossRef] [PubMed]
- Wei, L.; Teng, F.; Deng, L.; Liu, G.; Luan, M.; Jiang, J.; Liu, Z.; Liu, Y. Periodontal regeneration using bone morphogenetic protein 2 incorporated biomimetic calcium phosphate in conjunction with barrier membrane: A pre-clinical study in dogs. J. Clin. Periodontol. 2019, 46, 1254–1263. [Google Scholar] [CrossRef] [Green Version]
- Wei, L.; Yu, D.; Wang, M.; Deng, L.; Wu, G.; Liu, Y. Dose effects of slow-released bone morphogenetic protein-2 functionalized beta-tricalcium phosphate in repairing critical-sized bone defects. Tissue Eng. Part A 2020, 26, 120–129. [Google Scholar] [CrossRef]
- Schliephake, H.; Scharnweber, D.; Dard, M.; Röbetaler, S.; Sewing, A.; Hüttmann, C. Biological performance of biomimetic calcium phosphate coating of titanium implants in the dog mandible. J. Biomed. Mater. Res. A 2003, 64, 225–234. [Google Scholar] [CrossRef]
- Paul, J.C.; Lonner, B.S.; Vira, S.; Kaye, I.D.; Errico, T.J. Reoperation rates after long posterior spinal fusion: Use of recombinant bone morphogenetic protein in idiopathic and non-idiopathic scoliosis. Spine Deform. 2016, 4, 304–309. [Google Scholar] [CrossRef]
- Begam, H.; Nandi, S.K.; Kundu, B.; Chanda, A. Strategies for delivering bone morphogenetic protein for bone healing. Mater. Sci. Eng. C 2017, 70, 856–869. [Google Scholar] [CrossRef]
- Haubruck, P.; Tanner, M.C.; Vlachopoulos, W.; Hagelskamp, S.; Miska, M.; Ober, J.; Fischer, C.; Schmidmaier, G. Comparison of the clinical effectiveness of bone morphogenic protein (BMP) -2 and -7 in the adjunct treatment of lower limb nonunions. Orthop. Traumatol. Surg. Res. 2018, 104, 1241–1248. [Google Scholar] [CrossRef]
- Liu, T.; Wu, G.; Wismeijer, D.; Gu, Z.; Liu, Y. Deproteinized bovine bone functionalized with the slow delivery of BMP-2 for the repair of critical-sized bone defects in sheep. Bone 2013, 56, 110–118. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Liu, T.; Wu, G.; Li, W.; Feng, X.; Pathak, J.L.; Shi, J. BMP2-functionalized biomimetic calcium phosphate graft promotes alveolar defect healing during orthodontic tooth movement in beagle dogs. Front. Bioeng. Biotechnol. 2020, 8, 517. [Google Scholar] [CrossRef] [PubMed]
- Perez, J.R.; Kouroupis, D.; Li, D.J.; Best, T.M.; Kaplan, L.; Correa, D. Tissue engineering and cell-based therapies for fractures and bone defects. Front. Bioeng. Biotechnol. 2018, 6, 105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferguson, J.; Diefenbeck, M.; McNally, M. Ceramic biocomposites as biodegradable antibiotic carriers in the treatment of bone infections. J. Bone Jt. Infect. 2017, 2, 38–51. [Google Scholar] [CrossRef] [Green Version]
- Kulkarni Aranya, A.; Pushalkar, S.; Zhao, M.; LeGeros, R.Z.; Zhang, Y.; Saxena, D. Antibacterial and bioactive coatings on titanium implant surfaces. J. Biomed. Mater. Res. A 2017, 105, 2218–2227. [Google Scholar] [CrossRef] [Green Version]
- Dreyer, C.H.; Kjaergaard, K.; Ding, M.; Qin, L. Vascular endothelial growth factor for in vivo bone formation: A systematic review. J. Orthop. Transl. 2020, 24, 46–57. [Google Scholar] [CrossRef]
- Wernike, E.; Montjovent, M.O.; Liu, Y.; Wismeijer, D.; Hunziker, E.B.; Siebenrock, K.A.; Hofstetter, W.; Klenke, F.M. VEGF incorporated into calcium phosphate ceramics promotes vascularisation and bone formation in vivo. Eur. Cell Mater. 2010, 19, 30–40. [Google Scholar] [CrossRef]
- Wu, L.; Gu, Y.; Liu, L.; Tang, J.; Mao, J.; Xi, K.; Jiang, Z.; Zhou, Y.; Xu, Y.; Deng, L.; et al. Hierarchical micro/nanofibrous membranes of sustained releasing VEGF for periosteal regeneration. Biomaterials 2020, 227, 119555. [Google Scholar] [CrossRef]
- Wu, Y.; Cao, H.; Yang, Y.; Zhou, Y.; Gu, Y.; Zhao, X.; Zhang, Y.; Zhao, Z.; Zhang, L.; Yin, J. Effects of vascular endothelial cells on osteogenic differentiation of noncontact co-cultured periodontal ligament stem cells under hypoxia. J. Periodontal Res. 2013, 48, 52–65. [Google Scholar] [CrossRef]
- Coory, J.A.; Tan, K.G.; Whitehouse, S.L.; Hatton, A.; Graves, S.E.; Crawford, R.W. The outcome of total knee arthroplasty with and without patellar resurfacing up to 17 years: A report from the australian orthopaedic association national joint replacement registry. J. Arthroplast. 2020, 35, 132–138. [Google Scholar] [CrossRef] [Green Version]
- Eckhardt, S.; Brunetto, P.S.; Gagnon, J.; Priebe, M.; Giese, B.; Fromm, K.M. Nanobio silver: Its interactions with peptides and bacteria, and its uses in medicine. Chem. Rev. 2013, 113, 4708–4754. [Google Scholar] [CrossRef] [Green Version]
- Kang, M.-K.; Lee, S.-B.; Moon, S.-K.; Kim, K.-M.; Kim, K.-N. The biomimetic apatite-cefalotin coatings on modified titanium. Dent. Mater. J. 2012, 31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, M.; Liu, Q.; Jia, Z.; Xu, X.; Shi, Y.; Cheng, Y.; Zheng, Y. Polydopamine-induced nanocomposite Ag/CaP coatings on the surface of titania nanotubes for antibacterial and osteointegration functions. J. Mater. Chem. B 2015, 3, 8796–8805. [Google Scholar] [CrossRef] [PubMed]
- Drugs Home Page. Available online: https://www.drugs.com/dosage/metronidazole.html (accessed on 11 April 2019).
- Wang, D.; Liu, Y.; Liu, Y.; Yan, L.; Zaat, S.A.J.; Wismeijer, D.; Pathak, J.L.; Wu, G. A dual functional bone-defect-filling material with sequential antibacterial and osteoinductive properties for infected bone defect repair. J. Biomed. Mater. Res. A 2019, 107, 2360–2370. [Google Scholar] [CrossRef]
- Gürbüz, K.; Yerer, M.B.; Gürbüz, P.; Halıcı, M. Icariin promotes early and late stages of fracture healing in rats. Eklem Hastalik Cerrahisi 2019, 30, 282–288. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Gu, Q.; Chen, M.; Zhang, C.; Chen, S.; Zhao, J. Controlled delivery of icariin on small intestine submucosa for bone tissue engineering. Mater. Sci. Eng. C 2017, 71, 260–267. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.; Pei, F.; Wang, H.; Tan, Z.; Yang, Z.; Kang, P. Icariin: A promising osteoinductive compound for repairing bone defect and osteonecrosis. J. Biomater. Appl. 2015, 30, 290–299. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Ohba, S.; Komiyama, Y.; Shinkai, M.; Chung, U.I.; Nagamune, T. Icariin: A potential osteoinductive compound for bone tissue engineering. Tissue Eng. Part A 2010, 16, 233–243. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, T.; Huang, Y.; Zheng, Y.; Wismeijer, D.; Liu, Y. Osteogenic potential of icariin compared with recombinant human bone morphogenetic protein 2 in vitro: A preliminary study. J. Biomater. Tissue Eng. 2015, 5, 226–233. [Google Scholar] [CrossRef]
- Lombardi, G.; Ziemann, E.; Banfi, G.; Corbetta, S. Physical activity-dependent regulation of parathyroid hormone and calcium-phosphorous metabolism. Int. J. Mol. Sci. 2020, 21, 5388. [Google Scholar] [CrossRef]
- Arrighi, I.; Mark, S.; Alvisi, M.; von Rechenberg, B.; Hubbell, J.A.; Schense, J.C. Bone healing induced by local delivery of an engineered parathyroid hormone prodrug. Biomaterials 2009, 30, 1763–1771. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-H.; Kim, A.R.; Choi, Y.H.; Kim, A.; Sohn, Y.; Woo, G.-H.; Cha, J.-H.; Bak, E.-J.; Yoo, Y.-J. Intermittent PTH administration improves alveolar bone formation in type 1 diabetic rats with periodontitis. J. Transl. Med. 2018, 16, 70. [Google Scholar] [CrossRef] [PubMed]
- Wojda, S.J.; Donahue, S.W. Parathyroid hormone for bone regeneration. J. Orthop. Res. 2018, 36, 2586–2594. [Google Scholar] [CrossRef] [Green Version]
- Yu, X.; Wei, M. Preparation and evaluation of parathyroid hormone incorporated CaP coating via a biomimetic method. J. Biomed. Mater. Res. B Appl. Biomater. 2011, 97, 345–354. [Google Scholar] [CrossRef] [PubMed]
- Sun, P.; Wang, M.; Yin, G.-Y. Endogenous parathyroid hormone (PTH) signals through osteoblasts via RANKL during fracture healing to affect osteoclasts. Biochem. Biophys. Res. Commun. 2020, 525, 850–856. [Google Scholar] [CrossRef]
- Kostenuik, P.; Mirza, F.M. Fracture healing physiology and the quest for therapies for delayed healing and nonunion. J. Orthop. Res. 2017, 35, 213–223. [Google Scholar] [CrossRef] [PubMed]
- Spiller, K.L.; Koh, T.J. Macrophage-based therapeutic strategies in regenerative medicine. Adv. Drug Deliv. Rev. 2017, 122, 74–83. [Google Scholar] [CrossRef]
- Lurier, E.B.; Dalton, D.; Dampier, W.; Raman, P.; Nassiri, S.; Ferraro, N.M.; Rajagopalan, R.; Sarmady, M.; Spiller, K.L. Transcriptome analysis of IL-10-stimulated (M2c) macrophages by next-generation sequencing. Immunobiology 2017, 222, 847–856. [Google Scholar] [CrossRef] [Green Version]
- Chen, Z.; Klein, T.; Murray, R.Z.; Crawford, R.; Chang, J.; Wu, C.; Xiao, Y. Osteoimmunomodulation for the development of advanced bone biomaterials. Mater. Today 2016, 19, 304–321. [Google Scholar] [CrossRef] [Green Version]
- Nathan, K.; Lu, L.Y.; Lin, T.; Pajarinen, J.; Jämsen, E.; Huang, J.-F.; Romero-Lopez, M.; Maruyama, M.; Kohno, Y.; Yao, Z.; et al. Precise immunomodulation of the M1 to M2 macrophage transition enhances mesenchymal stem cell osteogenesis and differs by sex. Bone Jt. Res. 2019, 8, 481–488. [Google Scholar] [CrossRef]
- Gronowicz, G.; Jacobs, E.; Peng, T.; Zhu, L.; Hurley, M.; Kuhn, L.T. Calvarial bone regeneration is enhanced by sequential delivery of FGF-2 and BMP-2 from layer-by-layer coatings with a biomimetic calcium phosphate barrier layer. Tissue Eng. Part A 2017, 23, 1490–1501. [Google Scholar] [CrossRef] [PubMed]
- Klopfleisch, R.; Jung, F. The pathology of the foreign body reaction against biomaterials. J. Biomed. Mater. Res. A 2017, 105, 927–940. [Google Scholar] [CrossRef] [PubMed]
- Velard, F.; Braux, J.; Amedee, J.; Laquerriere, P. Inflammatory cell response to calcium phosphate biomaterial particles: An overview. Acta Biomater. 2013, 9, 4956–4963. [Google Scholar] [CrossRef] [PubMed]
- Knippenberg, M.; Helder, M.N.; Zandieh Doulabi, B.; Wuisman, P.I.J.M.; Klein-Nulend, J. Osteogenesis versus chondrogenesis by BMP-2 and BMP-7 in adipose stem cells. Biochem. Biophys. Res. Commun. 2006, 342, 902–908. [Google Scholar] [CrossRef]
- Tsai, A.T.; Rice, J.; Scatena, M.; Liaw, L.; Ratner, B.D.; Giachelli, C.M. The role of osteopontin in foreign body giant cell formation. Biomaterials 2005, 26, 5835–5843. [Google Scholar] [CrossRef] [PubMed]
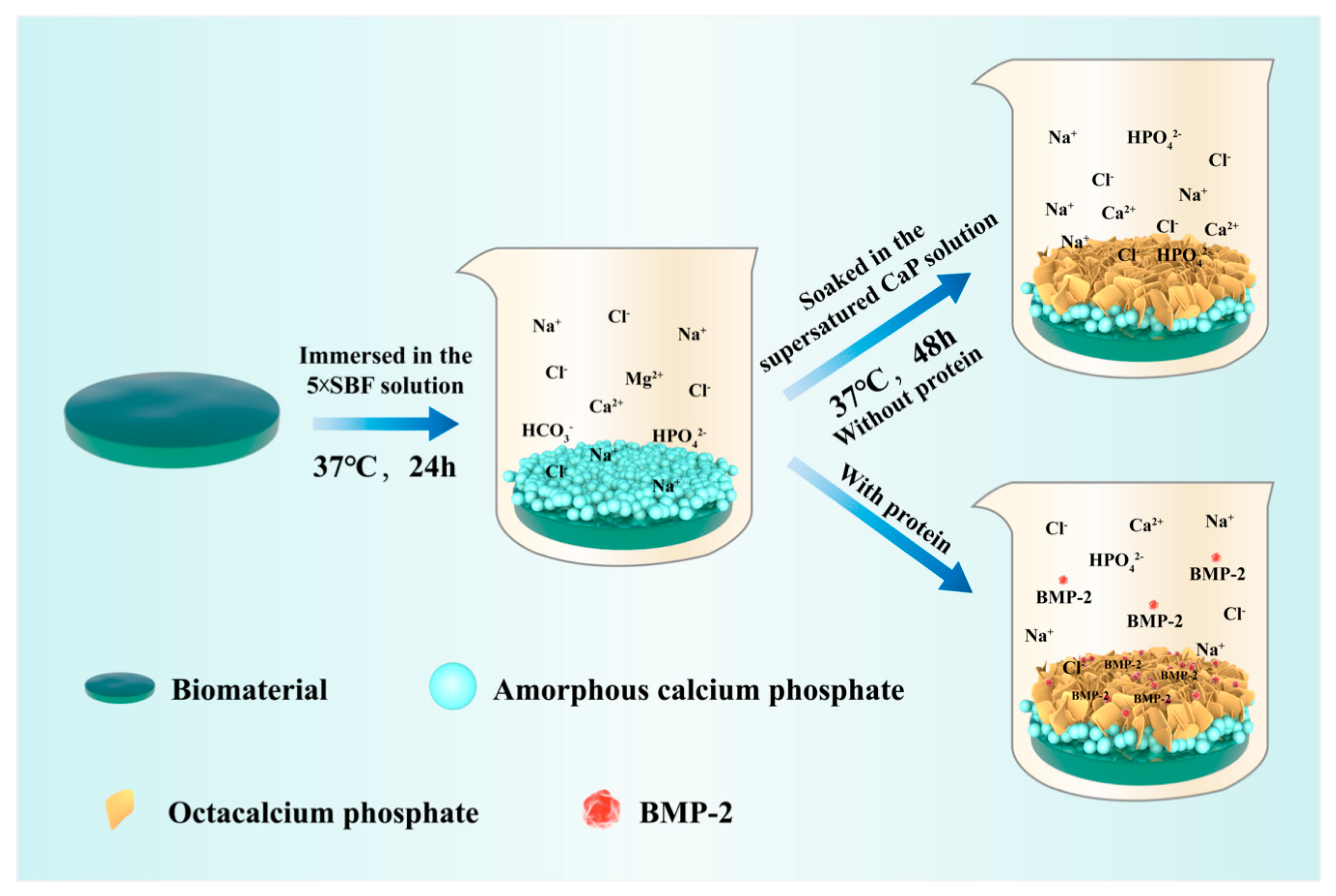
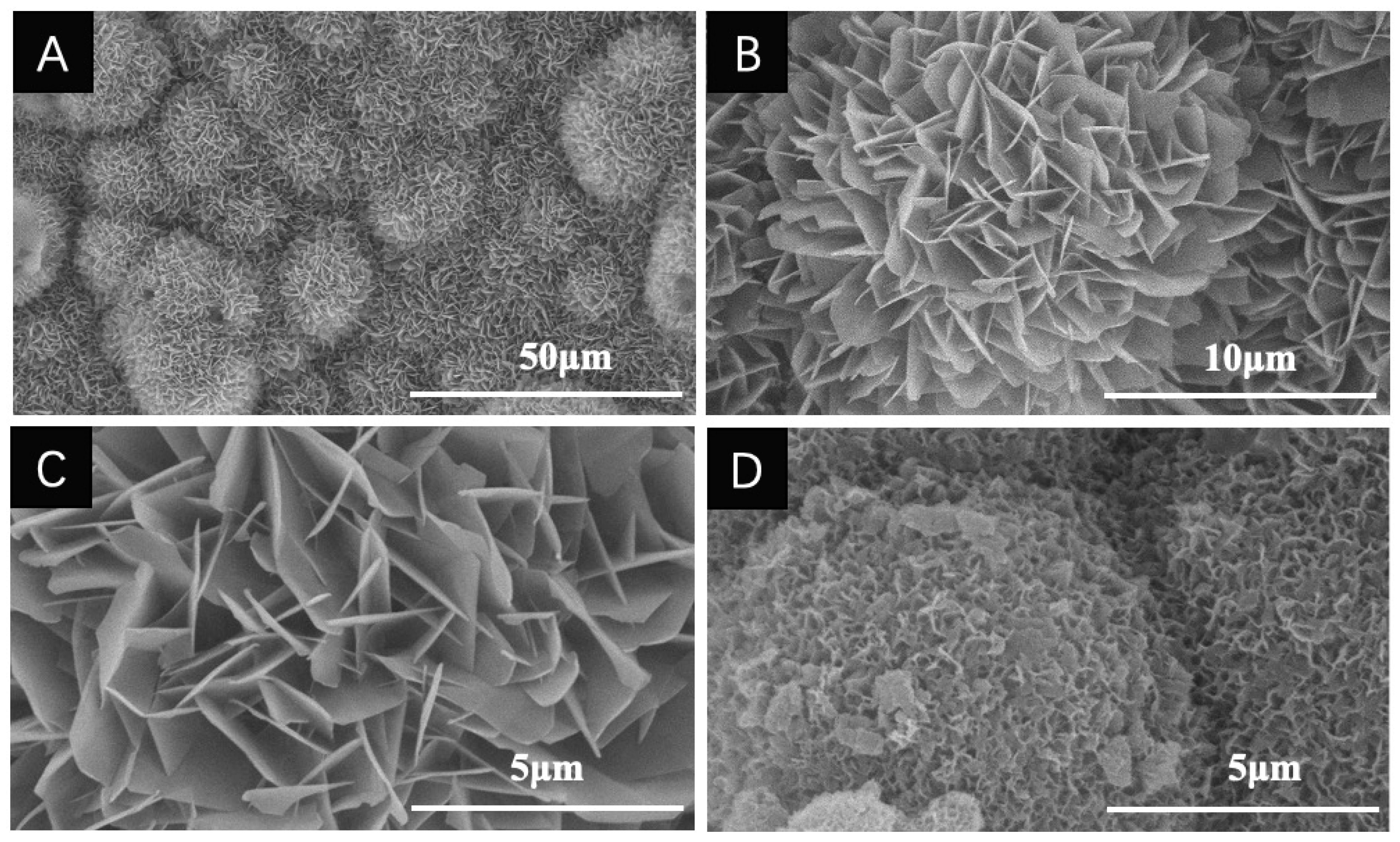
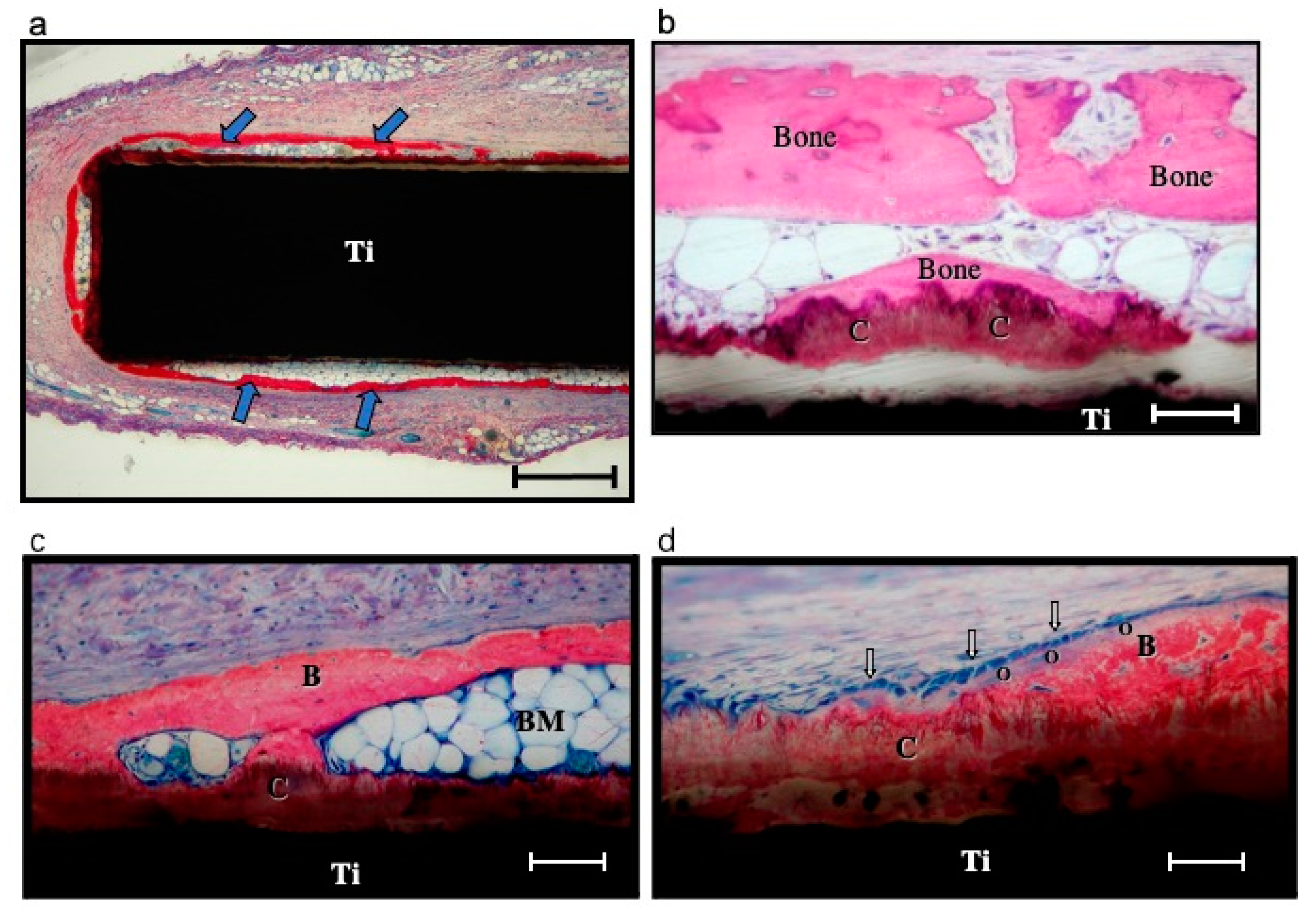
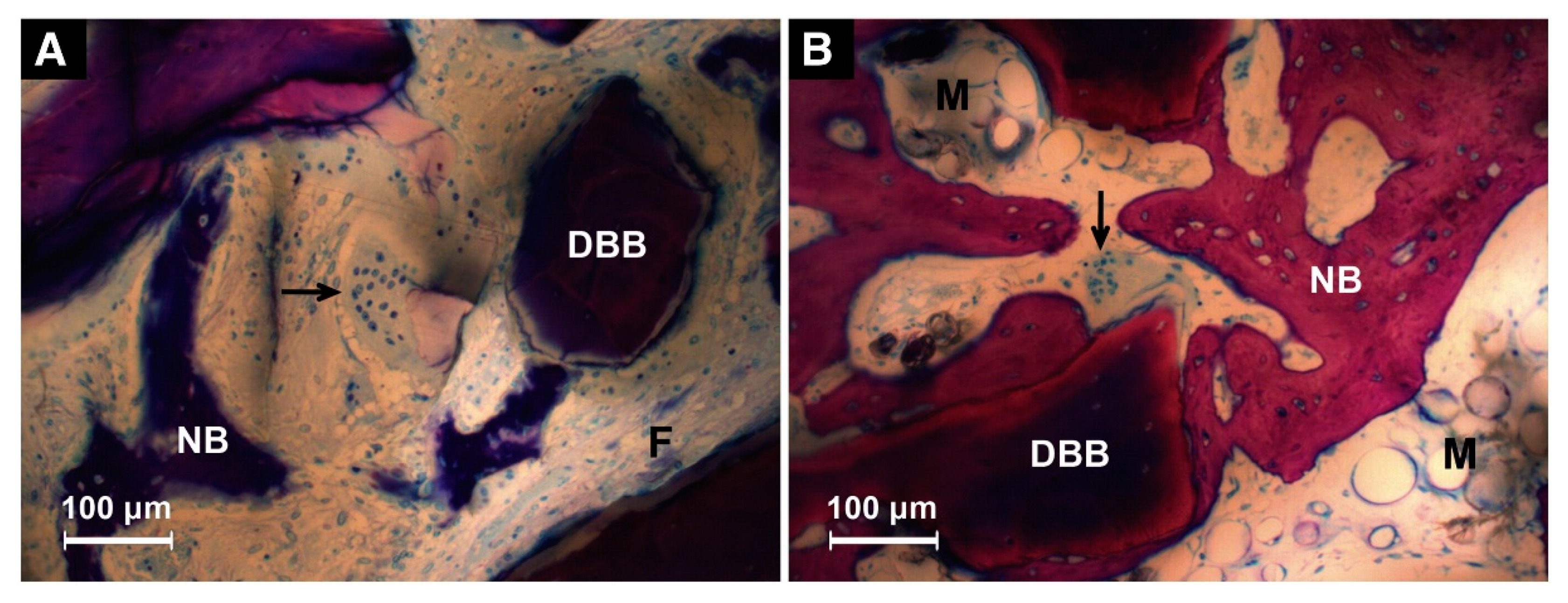
| Solutions | Concentrations of Composed Irons (mM) | Reaction Conditions | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Na+ | K+ | Mg2+ | Ca2+ | Cl− | HPO42− | SO42− | HCO3− | Time | Temperature | pH | |
| SBF | 142.0 | 5.0 | 1.5 | 2.5 | 147.8 | 1.0 | 0.5 | 4.2 | - | - | 7.4 |
| ACP | 733.5 | - | 7.5 | 12.5 | 720.0 | 5.0 | - | 21.0 | 24 h | 37 ℃ | 5.8 |
| OCP | 140.5 | - | - | 4.0 | 144.5 | 2.0 | - | - | 48 h | 37 ℃ | 7.4 |
| Author and Year | Biomaterial Substrates | Bioactive Agent | Animal Model | Observation Period | Osteogenesis | Angiogenesis | Foreign Body Giant Cells |
|---|---|---|---|---|---|---|---|
| Teng F. Clin Oral Implants Res 2020 | DBB | BMP-2 | Mandible of Beagle dogs | 3 months | Enhanced to a similar extent of autologous bone graft | - | - |
| Jiang S. Front. Bioeng. Biotechnol. 2020 | BioCaP granules | BMP-2 | Maxilla of Beagle dog | 8 weeks | Enhanced | Enhanced | Reduced |
| Wei L. Tissue Eng Part A 2020 | β-TCP | BMP-2 | Critical-sized defect of rat cranium | 8 weeks | Enhanced | - | Reduced |
| Lin X. Mater Sci Eng C Mater Biol Appl 2019 | CHA granules | BMP-2 | Dorsal subcutaneous pockets of rats | 5 weeks | Enhanced | - | Reduced |
| Zhang X. Front. Pharmacol. 2019 | BioCaP granules | BMP-2+Icariin | Critical-sized defect of rat cranium | 12 weeks | Enhanced | - | - |
| Wang D. J Biomed Mater Res A 2019 | BioCaP granules | HACC+BMP-2 | Dorsal subcutaneous pockets of rats | 5 weeks | Enhanced | - | Reduced |
| Liu T. Sci Rep 2017 | BioCaP granules+ DBB | BMP-2 | Humerus and femur defect of sheep | 8 weeks | Enhanced to a similar extent of autologous bone graft | Newly formed in early stage | Reduced |
| Yu X. J Mater Sci: Mater Med 2012 | cpTi foil | PTH | Tibia of mice | 4 weeks | Dose-dependently improved osseointegration | - | - |
| Hunziker E.B. Bone 2012 | Dental implants | BMP-2 | Maxilla of Goettingen miniature pigs | 3 weeks | Enhanced | - | - |
| Wu G. Bone 2011 | DBB | BMP-2 | Dorsal subcutaneous pockets of rats | 5 weeks | Enhanced | - | Reduced |
| Hagi T. Bone 2010 | Ethisorb™ discs | BMP-2 | Dorsal subcutaneous pockets of rats | 2 weeks | Enhanced ossification is independent of the mechanical environment. | - | - |
| Wu G. Biomaterials 2010 | Collagen; Ethisorb™; PLGA; Polyactive® | BMP-2 | Dorsal subcutaneous pockets of rats | 5 weeks | Enhanced | - | Reduced |
| Wernike E. Eur Cell Mater 2010 | BCP | VEGF | Critical-sized defect of rat cranium | 28 days | Enhanced | Enhanced | - |
| Liu Y. J Dent Res 2007 | Ti6Al4V discs | BMP-2 | Dorsal subcutaneous pockets of rats | 5 weeks | Enhanced | - | No effect |
| Liu Y. Bone 2005 | Ti6Al4V discs | BMP-2 | Dorsal subcutaneous pockets of rats | 5 weeks | Enhanced | - | Reduced |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, X.; Chen, J.; Liao, Y.; Pathak, J.L.; Li, H.; Liu, Y. Biomimetic Calcium Phosphate Coating as a Drug Delivery Vehicle for Bone Tissue Engineering: A Mini-Review. Coatings 2020, 10, 1118. https://doi.org/10.3390/coatings10111118
Lin X, Chen J, Liao Y, Pathak JL, Li H, Liu Y. Biomimetic Calcium Phosphate Coating as a Drug Delivery Vehicle for Bone Tissue Engineering: A Mini-Review. Coatings. 2020; 10(11):1118. https://doi.org/10.3390/coatings10111118
Chicago/Turabian StyleLin, Xingnan, Jiping Chen, Ying Liao, Janak Lal Pathak, Huang Li, and Yuelian Liu. 2020. "Biomimetic Calcium Phosphate Coating as a Drug Delivery Vehicle for Bone Tissue Engineering: A Mini-Review" Coatings 10, no. 11: 1118. https://doi.org/10.3390/coatings10111118






