Self-Assembly of Self-Cleaning Polystyrene/Styrene-Butadiene-Styrene Films with Well-Ordered Micro-Structures
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Porous Films Formation
2.3. Sample Characterization
3. Results and Discussion
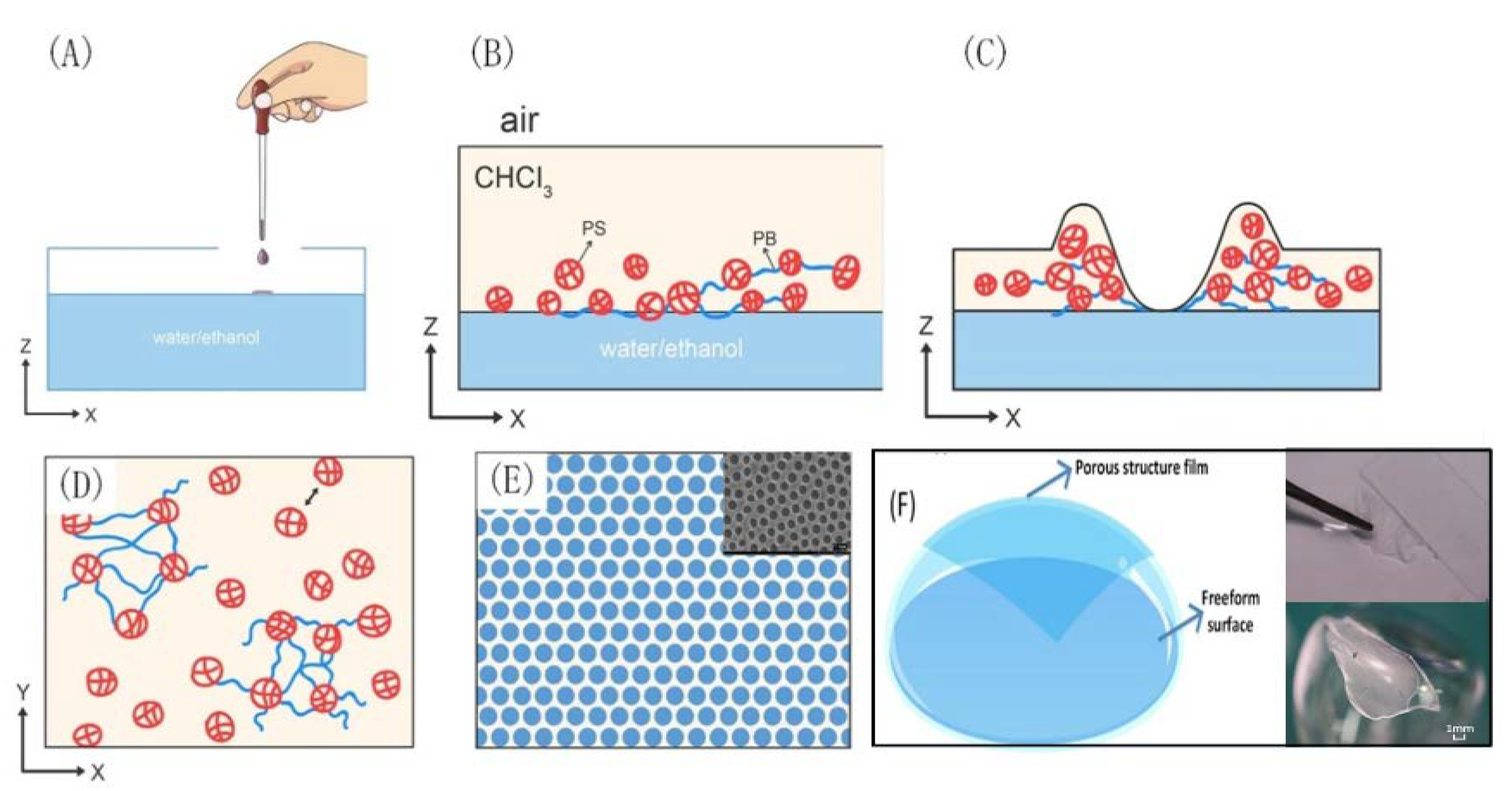
3.1. Formation Mechanism
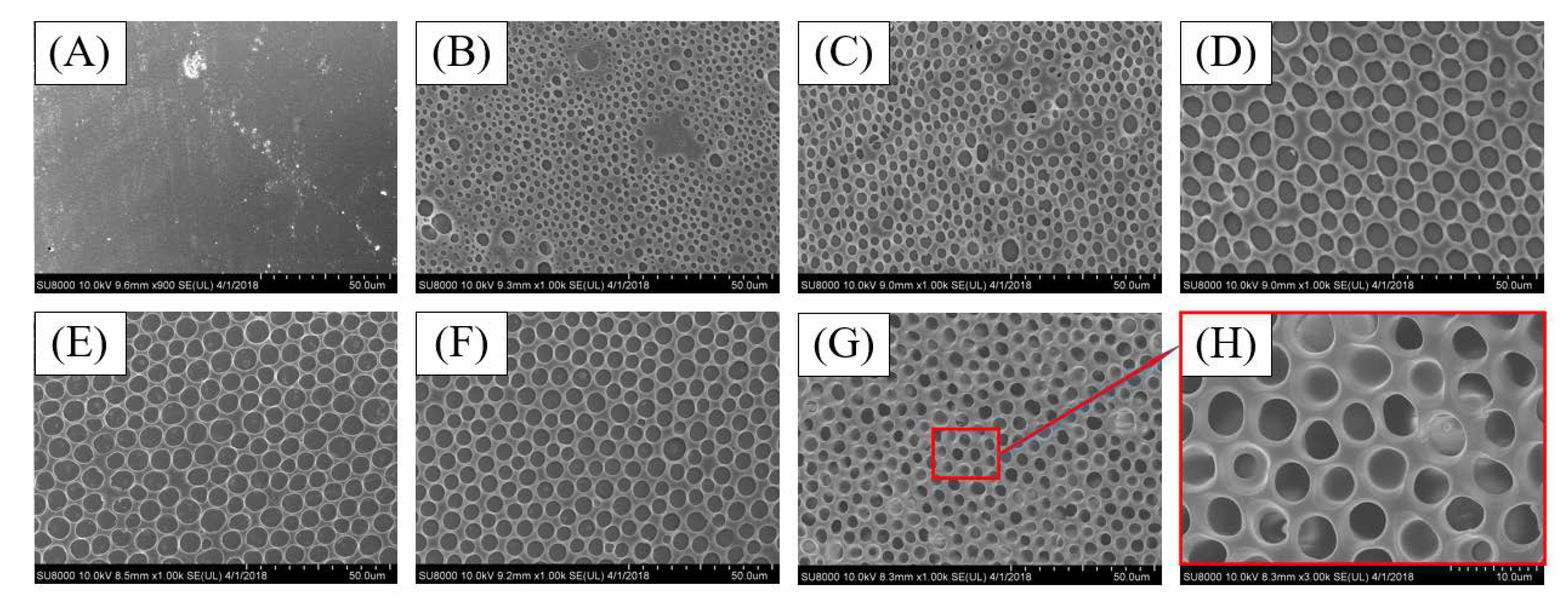
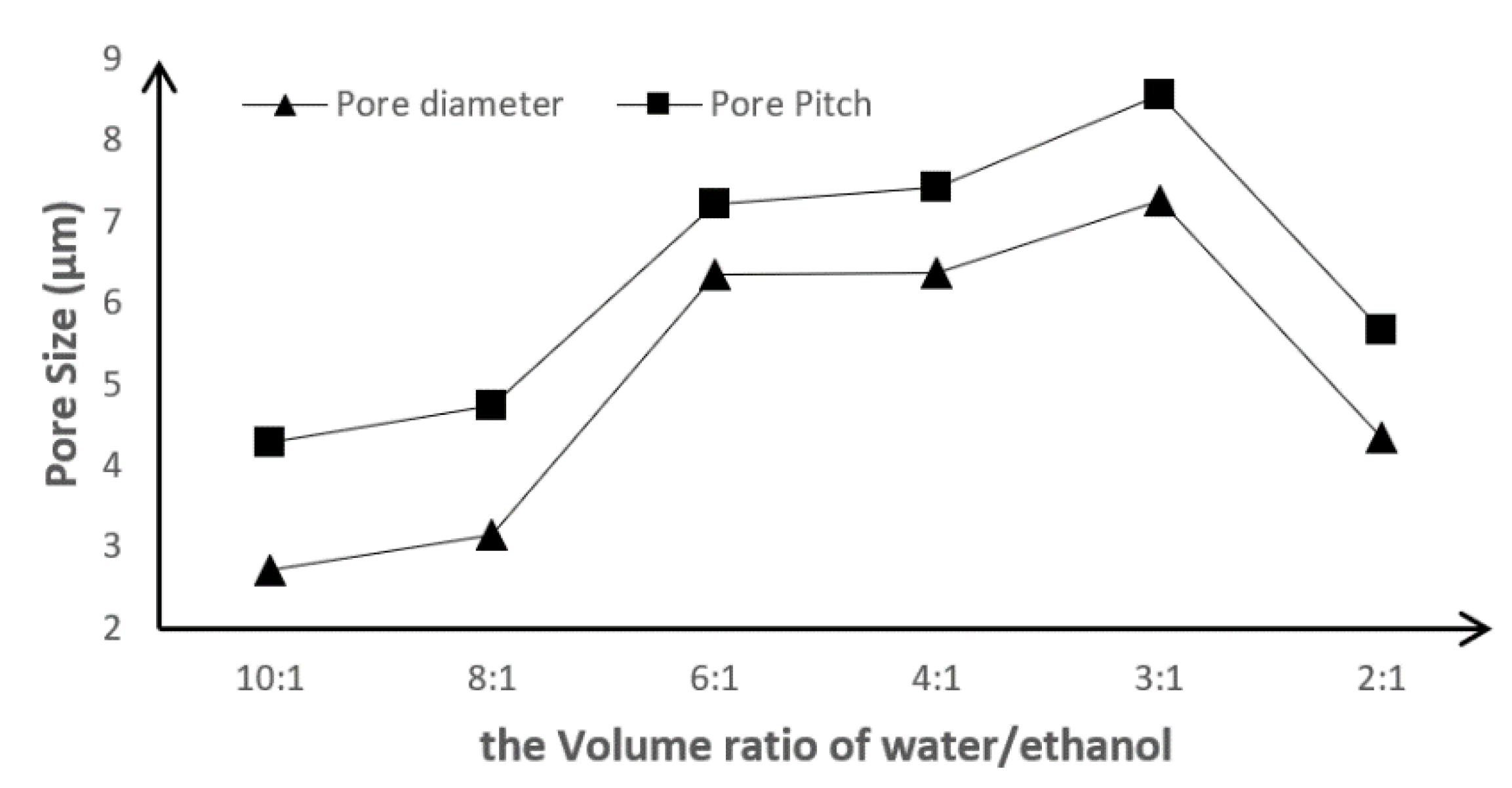
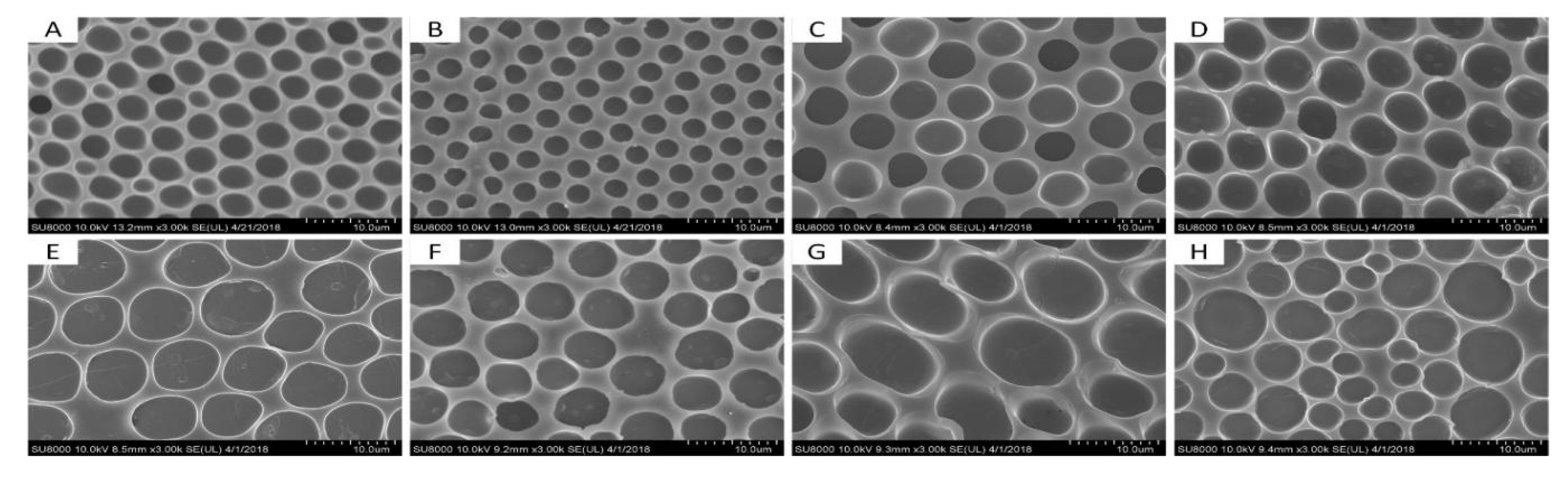
3.2. Influence of Volume Ratio of the Water/Ethanol
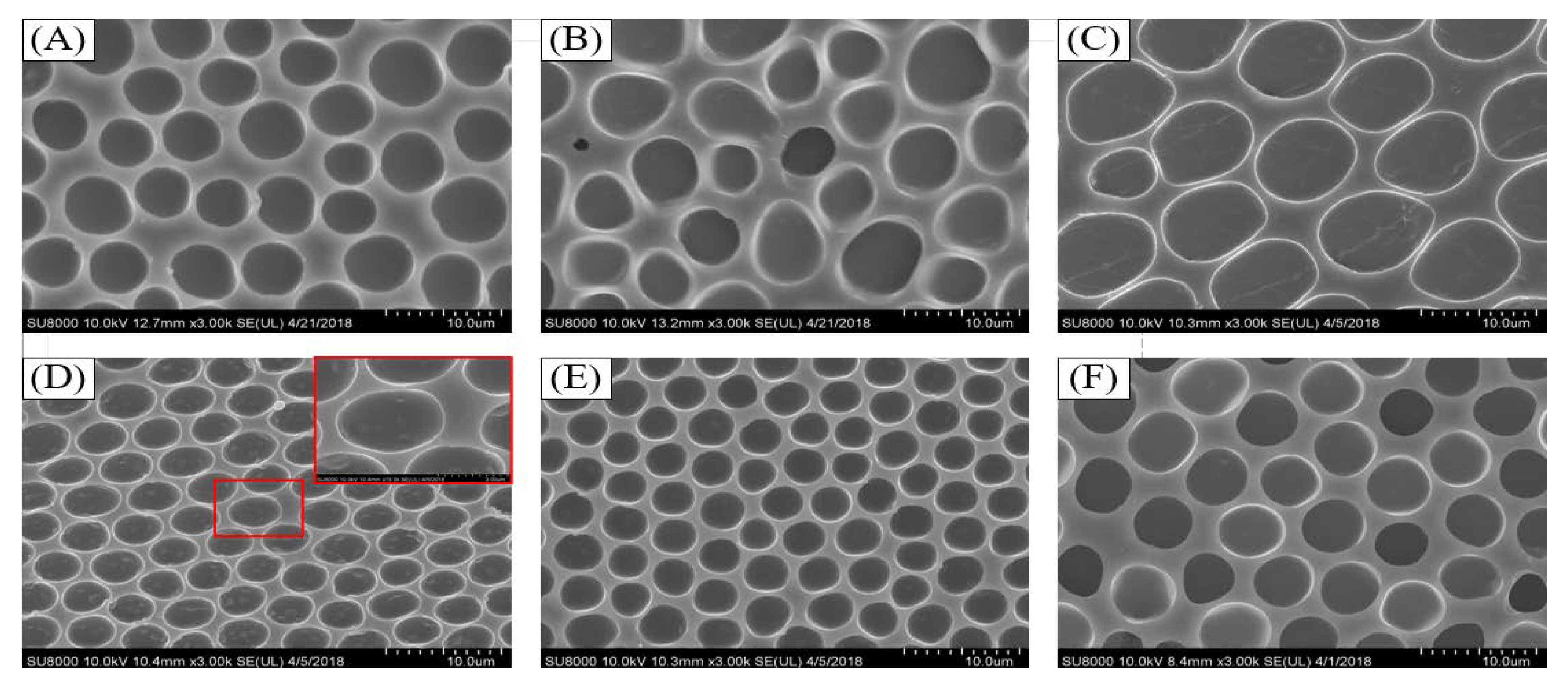
3.3. Influence of Weight Ratio of the PS/SBS
3.4. Influence of the Concentration
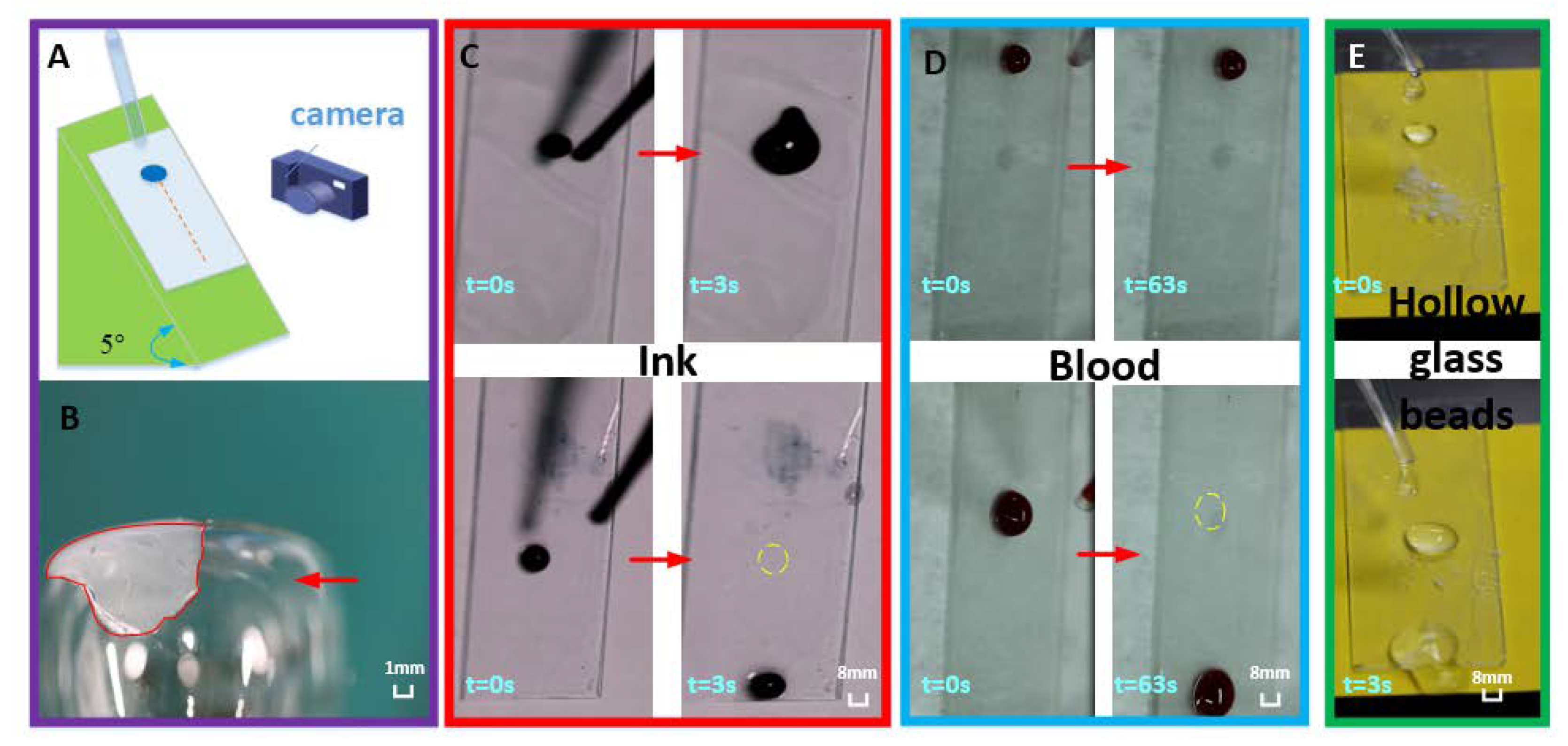
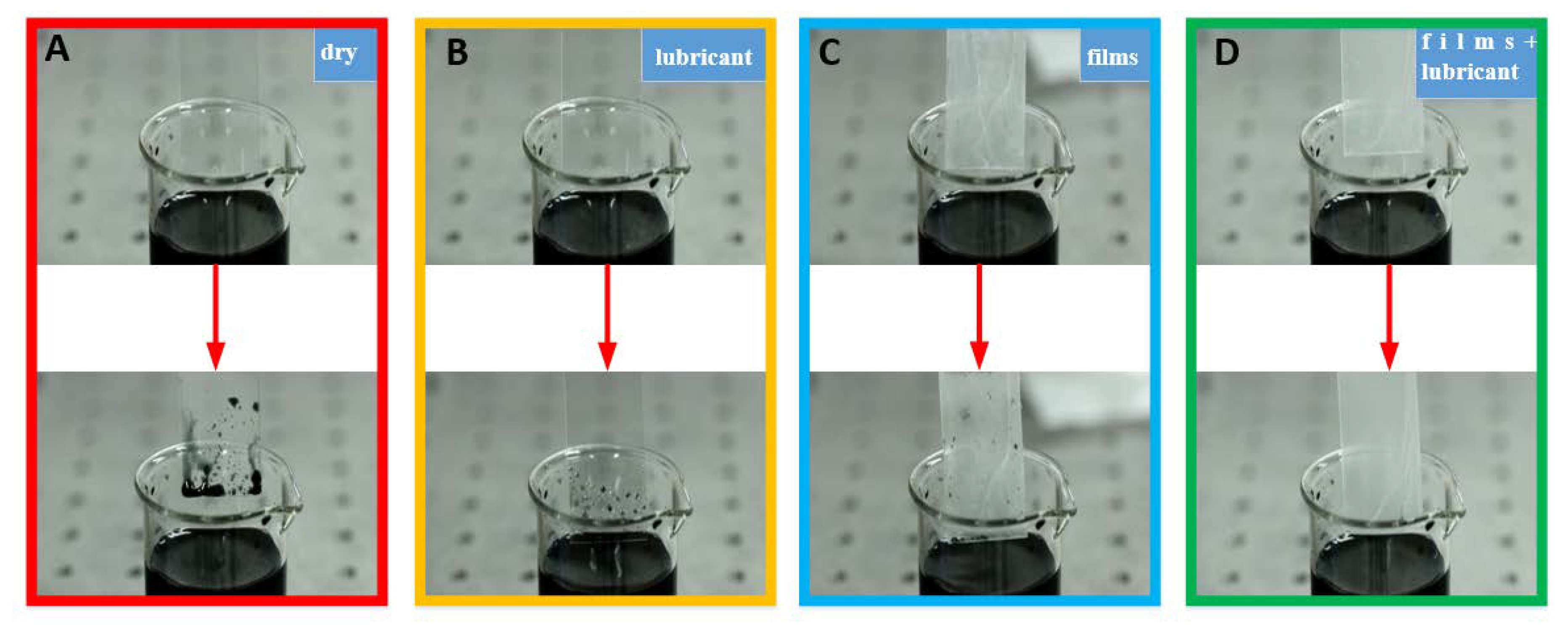
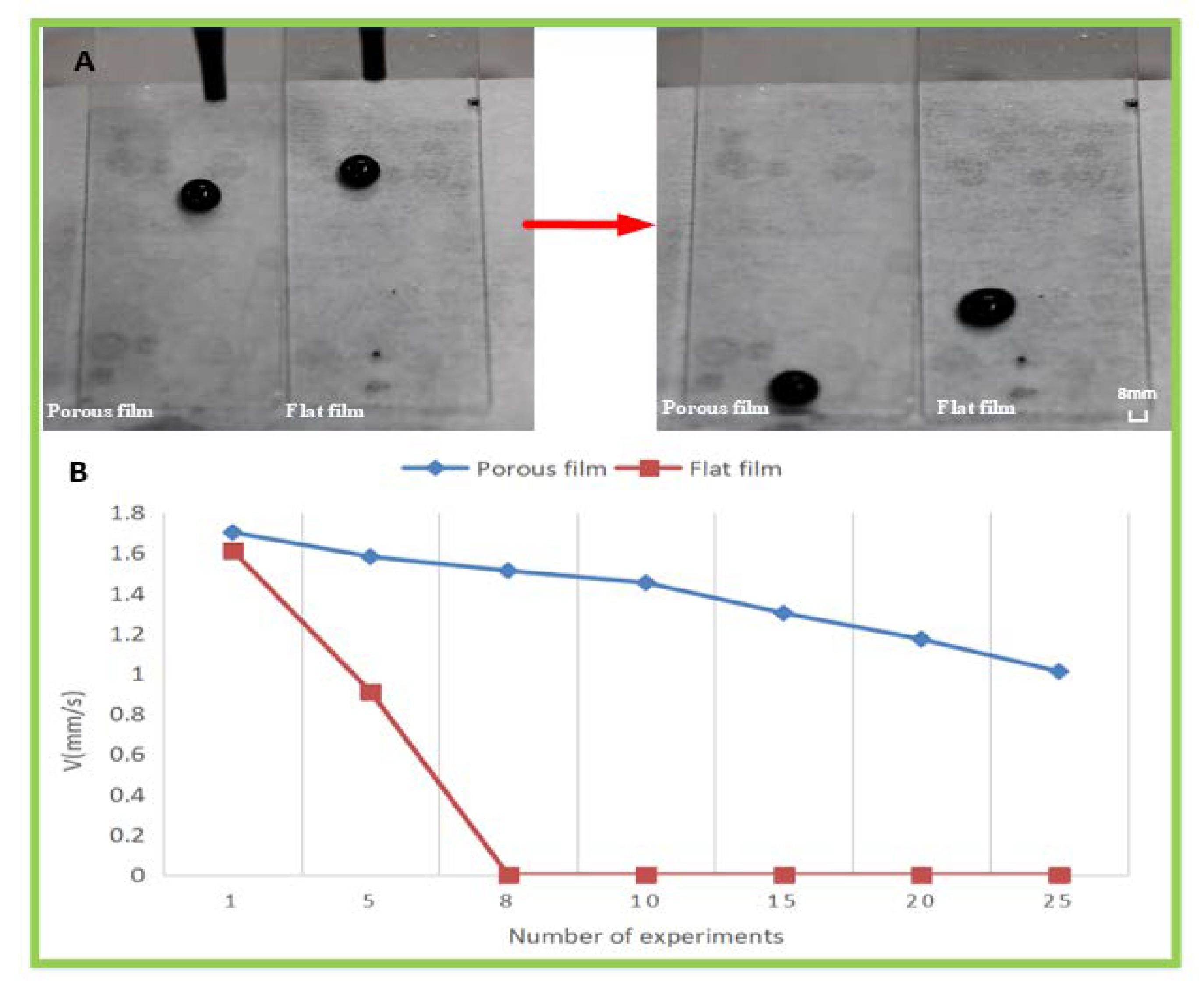
3.5. Self-Cleaning Test
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Wijnhoven, J.E.; Vos, W.L. Preparation of Photonic Crystals Made of Air Spheres in Titania. Science 1998, 281, 802–804. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lewandowski, K.; Murer, P.; Frantisek, S.; Jean, M. The Design of Chiral Separation Media Using Monodisperse Functionalized Macroporous Beads: Effects of Polymer Matrix, Tether, and Linkage Chemistry. Anal. Chem. 1998, 70, 1629–1638. [Google Scholar] [CrossRef] [PubMed]
- Yan, F.; Goedel, W.A. A Simple and Effective Method for the Preparation of Porous Membranes with Three-Dimensionally Arranged Pores. Adv. Mater. 2004, 16, 911–915. [Google Scholar] [CrossRef]
- Shastri, V.P.; Martin, I.; Langer, R. Macroporous polymer foams by hydrocarbon templating. Proc. Natl. Acad. Sci. USA 2000, 97, 1970–1975. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xuan, S.; Zhang, Z.; Chen, Z.; Chen, Y. Porous Structure Fabrication Using a Stereolithography-Based Sugar Foaming Method. J. Manuf. Sci. Eng. 2017, 139, 031015. [Google Scholar]
- Yabu, H.; Shimomura, M. Mesoscale pincushions, microrings, and microdots prepared by heating and peeling of self-organized honeycomb-patterned films deposited on a solid substrate. Langmuir ACS J. Surf. Colloids 2006, 22, 4992–4997. [Google Scholar] [CrossRef] [PubMed]
- Alexandra, M.; Marta, F.; Juan, R. Towards hierarchically ordered functional porous polymeric surfaces prepared by the breath figures approach. Prog. Polym. Sci. 2014, 39, 510–554. [Google Scholar]
- Alexander, B.; Yao, L.; Kristen, C.; Reina, H. Hierarchical nanoparticle assemblies formed by decorating breath figures. Nat. Mater. 2004, 3, 302–306. [Google Scholar]
- Hiroshi, Y. Fabrication of honeycomb films by the breath figure technique and their applications. Sci. Technol. Adv. Mater. 2018, 19, 802–822. [Google Scholar]
- Rahmawan, Y.; Xu, L.B.; Yang, S. Self-assembly of nanostructures towards transparent, superhydrophobic surfaces. J. Mater. Chem. A 2013, 1, 2955–2969. [Google Scholar] [CrossRef]
- Yabu, H.; Shimomura, M. Single-Step Fabrication of Transparent Superhydrophobic Porous Polymer Films. Chem. Mater. 2005, 17, 5231–5234. [Google Scholar] [CrossRef]
- Liu, Y.; Xu, Q.F.; Lyons, A. Durable, optically transparent, superhydrophobic polymer films. Appl. Surf. Sci. 2019, 470, 187–195. [Google Scholar] [CrossRef]
- Chang, K.C.; Chen, Y.K.; Chen, H. Fabrication of superhydrophobic silica-based surfaces with high transmittance by using polypropylene and tetraeyhoxysilane precursors. J. Appl. Polym. Sci. 2008, 107, 1530–1538. [Google Scholar] [CrossRef]
- Maedeh, R.; Mohammad, R.V.; Asghar, K. Preparation of silane-functionalized silica films via two-step dip coating sol-gel and evaluation of their superhydrophobic properties. Appl. Surf. Sci. 2014, 317, 147–153. [Google Scholar]
- Imhof, A.; Pine, D.J. Ordered macroporous materials by emulsion templating. Nature 1997, 389, 948–951. [Google Scholar] [CrossRef]
- Xu, L.; Karunakaran, R.G.; Guo, J.; Yang, S. Transparent, superhydrophobic surfaces from one-step spin coating of hydrophobic nanoparticles. ACS Appl. Mater. Interfaces 2012, 4, 1118–1125. [Google Scholar] [CrossRef]
- Li, Y.; Men, X.; Zhu, X.; Ge, B.; Chu, F.; Zhang, Z. One-step spraying to fabricate nonfluorinated superhydrophobic coatings with high transparency. J. Mater. Sci. 2016, 51, 2411–2419. [Google Scholar] [CrossRef]
- Kim, F.; Kwan, S.; Akana, J.; Yang, P. Langmuir−Blodgett Nanorod Assembly. J. Am. Chem. Soc. 2001, 123, 4360–4361. [Google Scholar] [CrossRef]
- Vogel, N.; Goerres, S.; Landfester, K.; Weiss, C. A Convenient Method to Produce Close- and Non-close-Packed Monolayers using Direct Assembly at the Air-Water Interface and Subsequent Plasma-Induced Size Reduction. Macromol. Chem. Phys. 2011, 212, 1719–1734. [Google Scholar] [CrossRef]
- Oh, S.; Yang, M.; Bouffard, J.; Hong, S.; Park, S. Air-Liquid Interfacial Self-Assembly of Non-Amphiphilic Poly(3-hexylthiophene) Homopolymers. ACS Appl. Mater. Interfaces 2017, 9, 12865–12871. [Google Scholar] [CrossRef]
- Chokprasombat, K.; Sirisathitkul, C.; Ratphonsan, P. Liquid–air interface self-assembly: A facile method to fabricate long-range nanoparticle monolayers. Surf. Sci. 2014, 621, 162–167. [Google Scholar] [CrossRef]
- Wang, L.; Sun, Y.; Che, G.; Li, Z. Self-assembled silver nanoparticle films at an air–liquid interface and their applications in SERS and electrochemistry. Appl. Surf. Sci. 2011, 257, 7150–7155. [Google Scholar] [CrossRef]
- Kadota, S.; Aoki, K.; Nagano, S.; Seki, T. Photocontrolled microphase separation of block copolymers in two dimensions. J. Am. Chem. Soc. 2005, 127, 8266–8267. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Feng, C.W.; Pang, X.C.; Jung, J.; Mihaela, C.; Prakash, S.; Han, R.; Ning, F.; Lin, Z. Self-assembly of a conjugated triblock copolymer at the air-water interface. Soft Matter 2013, 9, 8050–8056. [Google Scholar] [CrossRef]
- Zhang, X.M.; Li, L.; Zhang, Y. Study on the Surface Structure and Properties of PDMS/PMMA Antifouling Coatings. Phys. Procedia 2013, 50, 328–336. [Google Scholar] [CrossRef] [Green Version]
- Cativo, M.; Helen, M.; Kim, D.K.; Riggleman, R.A.; Yager, K.; Nonnenmann, S. Air-liquid interfacial self-assembly of conjugated block copolymers into ordered nanowire arrays. ACS Nano 2014, 8, 12755–12762. [Google Scholar] [CrossRef]
- Baker, S.M.; Leach, K.A.; Devereaux, C.E.; Gragson, D.E. Controlled Patterning of Diblock Copolymers by Monolayer Langmuir−Blodgett Deposition. Macromolecules 2000, 33, 5432–5436. [Google Scholar] [CrossRef]
- Jin, Y.P.; Advincula, R.C. Nanostructuring polymers, colloids, and nanomaterials at the air–water interface through Langmuir and Langmuir–Blodgett techniques. Soft Matter 2011, 7, 9829–9843. [Google Scholar]
- Adhikari, R.; Huy, T.A.; Buschnakowski, M.; Michler, G.; Knoll, K. Asymmetric PS-block-(PS-co-PB)-block-PS block copolymers: Morphology formation and deformation behavior. New J. Phys. 2004, 6, 28–47. [Google Scholar] [CrossRef]
- Vilaplana, F.; Osorio-Galindo, M.; Iborra-Clar, A.; Alcaina-Miranda, M.; Ribes-Greus, A. Swelling behavior of PDMS–PMHS pervaporation membranes in ethyl acetate–water mixtures. J. Appl. Polym. Sci. 2004, 93, 1384–1393. [Google Scholar] [CrossRef]
- Cheyne, R.B.; Moffitt, M.G. Novel two-dimensional “ring and chain” morphologies in Langmuir-Blodgett monolayers of PS-b-PEO block copolymers: Effect of spreading solution concentration on self-assembly at the air-water interface. Langmuir 2005, 21, 5453–5460. [Google Scholar] [CrossRef] [PubMed]
- And, C.A.D.; Baker, S.M. Surface Features in Langmuir−Blodgett Monolayers of Predominantly Hydrophobic Poly(styrene)−Poly(ethylene oxide) Diblock Copolymer. Macromolecules 2002, 35, 1921–1927. [Google Scholar]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Deng, J.; Wang, Y.; Zhan, X.; Zhang, D.; Chen, H. Self-Assembly of Self-Cleaning Polystyrene/Styrene-Butadiene-Styrene Films with Well-Ordered Micro-Structures. Coatings 2020, 10, 1133. https://doi.org/10.3390/coatings10111133
Liu Y, Deng J, Wang Y, Zhan X, Zhang D, Chen H. Self-Assembly of Self-Cleaning Polystyrene/Styrene-Butadiene-Styrene Films with Well-Ordered Micro-Structures. Coatings. 2020; 10(11):1133. https://doi.org/10.3390/coatings10111133
Chicago/Turabian StyleLiu, Yang, Jianchao Deng, Yamei Wang, Xiaoyang Zhan, Deyuan Zhang, and Huawei Chen. 2020. "Self-Assembly of Self-Cleaning Polystyrene/Styrene-Butadiene-Styrene Films with Well-Ordered Micro-Structures" Coatings 10, no. 11: 1133. https://doi.org/10.3390/coatings10111133
APA StyleLiu, Y., Deng, J., Wang, Y., Zhan, X., Zhang, D., & Chen, H. (2020). Self-Assembly of Self-Cleaning Polystyrene/Styrene-Butadiene-Styrene Films with Well-Ordered Micro-Structures. Coatings, 10(11), 1133. https://doi.org/10.3390/coatings10111133



