Processing of Spark Plasma Sintered Fe Alloy and Enhancing Its Surface Properties by AlCrN Monolayer Coating by Cathodic Arc Plasma Physical Vapor Deposition Process
Abstract
:1. Introduction
2. Experimental
2.1. Sintering Process
2.2. Coating Process
3. Result and Discussions
3.1. Sintered Compacts Analysis
3.1.1. Density Analysis
3.1.2. Hardness Analysis
3.1.3. Tensile Test Analysis
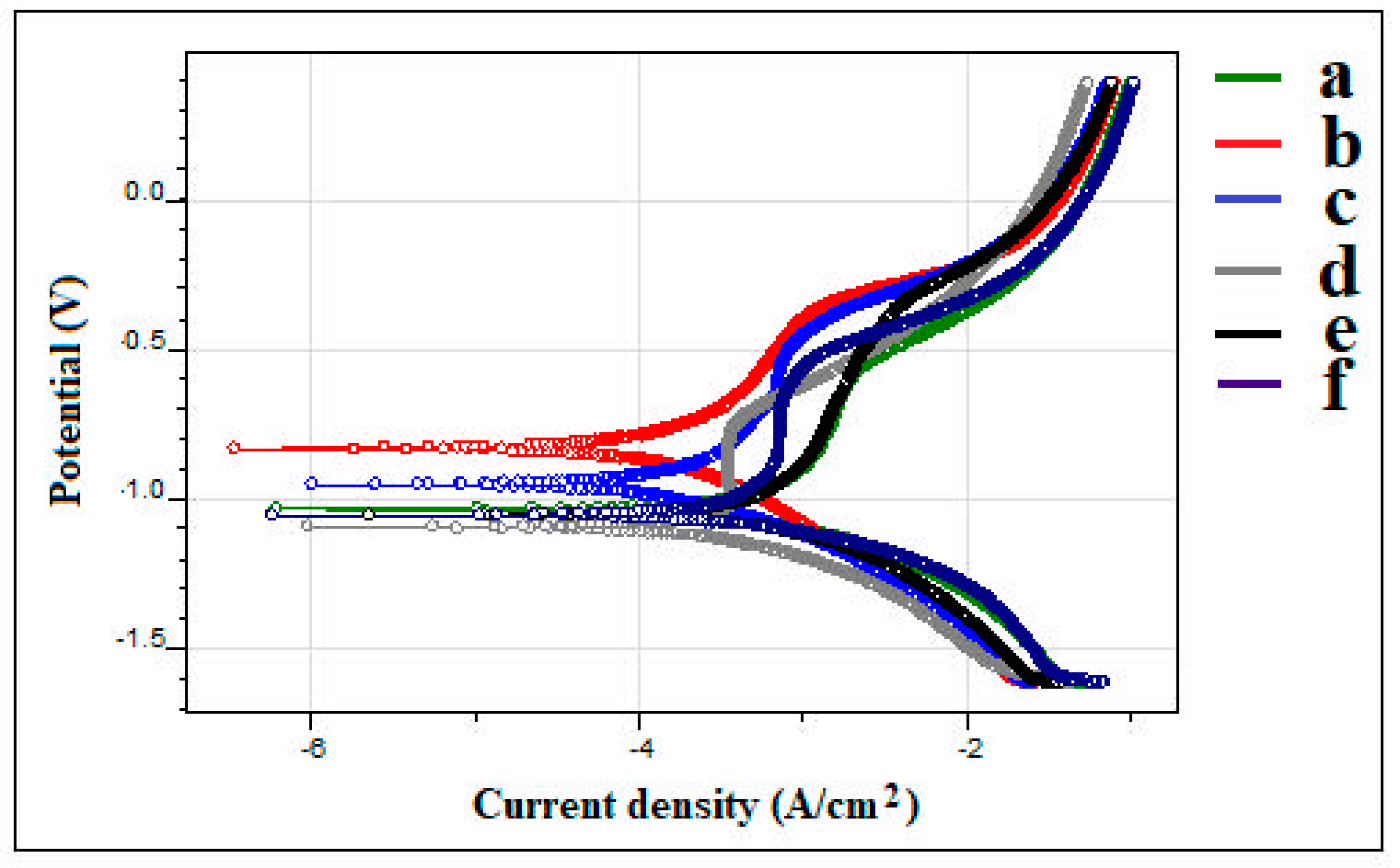
3.1.4. Corrosion Analysis
4. Coating Characterization
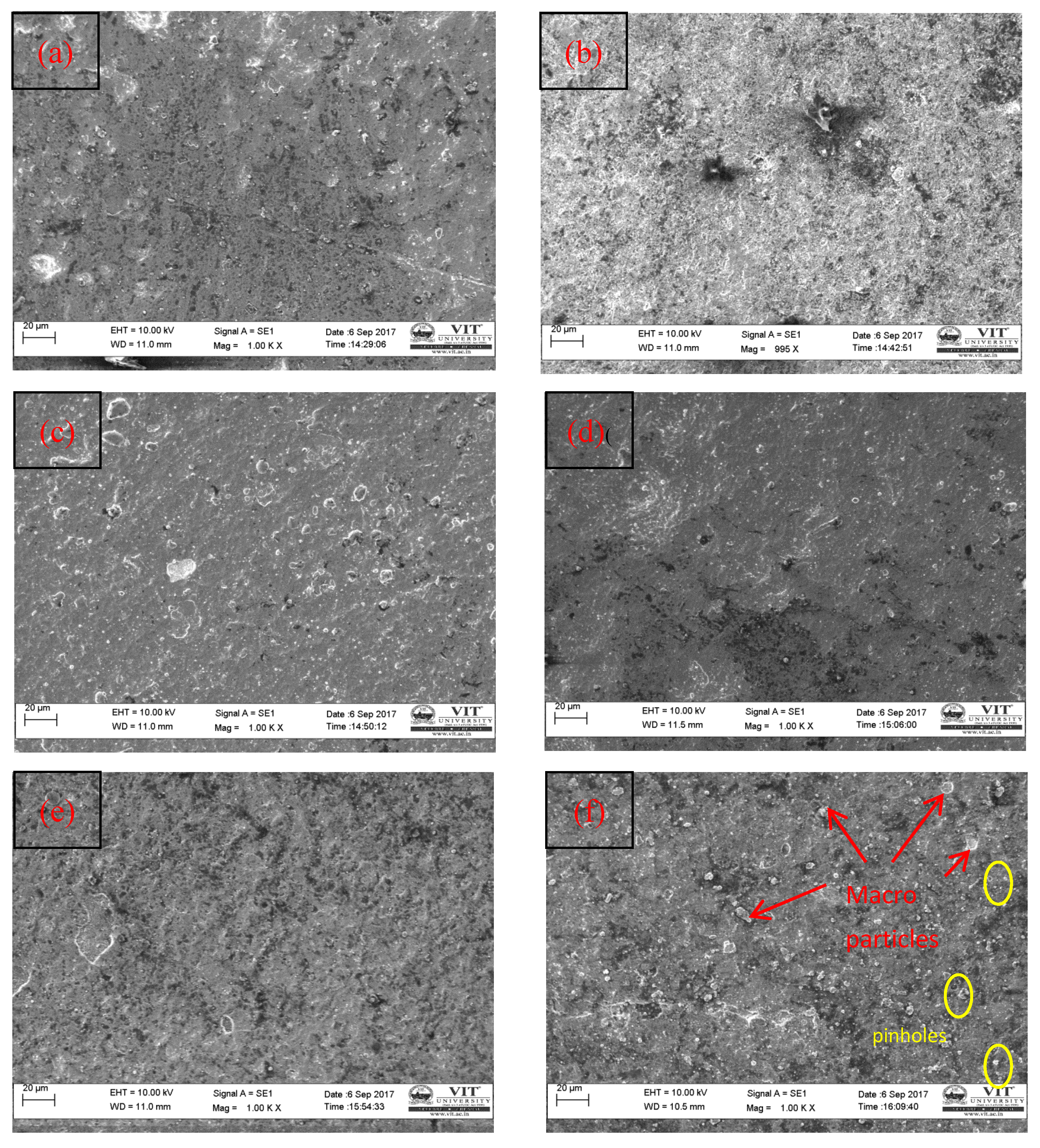
4.1. SEM Investigation
4.2. XRD Analysis
4.3. Microhardness Analysis
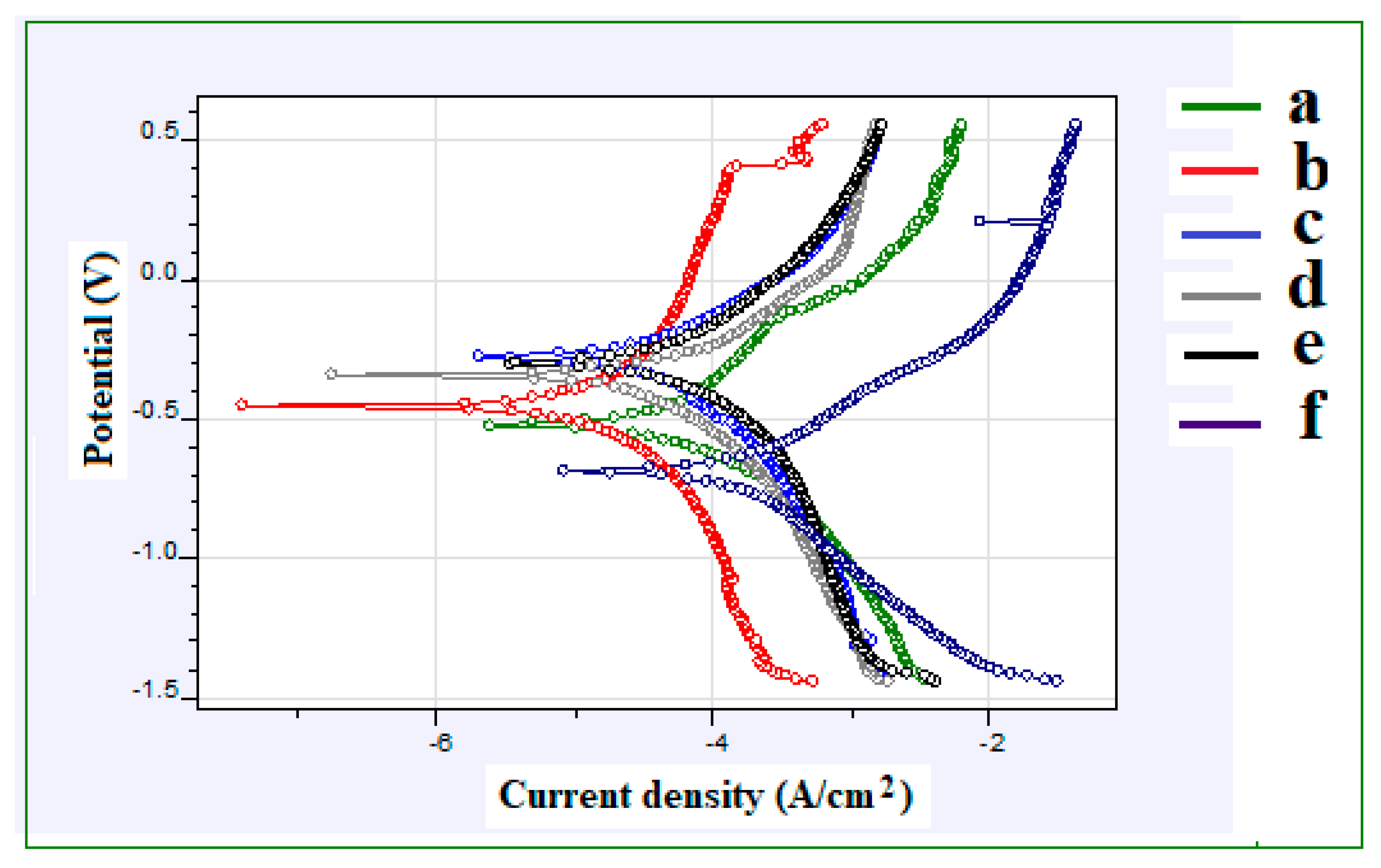
4.4. Corrosion Analysis
5. Conclusions
- A Fe–2Cu–0.8C–0.6Mo compact sample has obtained a higher relative sintering density of 97.20%, high hardness of 96 HRB, and high ultimate tensile strength of 1000 MPa compared with the other sintered compacts. The SEM fractography shows both ductile and brittle type fracture surfaces.
- The Fe–2Cu compact has obtained a lower corrosion rate of 0.657 mmpy while related to the other sintered compacts.
- The SEM images clearly show that the interlayer diffusion of the AlCrN coating with the Fe–2Cu alloy sintered compacts.
- The XRD analysis shows that the high peaks obtained for the AlN and CrN patterns and the average crystallite size from 38 to 58 nm was obtained.
- The microhardness testing shows that the Fe–2Cu–0.8C–0.6Mo coated compact sample has obtained a high hardness of 1134.85 HV.
- Fe–2Cu–0.8C–0.2Mo coated compact sample has obtained high corrosion resistance (0.08306 mmpy) compared to the other coated compact samples. Few pinholes and micro particles are formed on the coating surface of Fe–2Cu–0.8C–0.2Mo alloy compared with the other coated alloys.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Nekatibeb, F.; Annamalai, A.R.; Upadhyaya, A. Effect of copper and graphite addition on sinterability of iron. Trans. Indian Inst. Met. 2011, 64, 81–84. [Google Scholar] [CrossRef]
- Hadas, S.; Raja, A. Annamalai and Anish Upadhyaya, Effect of compaction pressure and tempering on densification, microstructural evolution, mechanical properties, wear, and machinability response of sinter hardened Ancorsteel 4300-0.6Gr. Mater. Technol. 2011, 26, 263–270. [Google Scholar] [CrossRef]
- Annamalai, A.R.; Upadhyaya, A.; Agarwal, D. Effect of heating mode on sintering of ferrous compacts through powder metallurgy route. Int. Heat Treat. Surf. Eng. 2011, 5, 155–160. [Google Scholar] [CrossRef]
- Upadhyaya, G.S. Sintered Metallic and Ceramic Materials Preparation, Properties and Applications; John Wiley & Sons Inc.: New York, NY, USA, 1999. [Google Scholar]
- Causton, R.J.; Davala, A.; Miller, T.J. Improved efficiency by use of sinter-hardened P/M automotive components. SAE Tech. Pap. 2000. [Google Scholar] [CrossRef] [Green Version]
- Annamalai, A.R.; Hadas, S.; Upadhyaya, A. Effect of compaction pressure on sinter hardening and tempered response on SH737-2CU-0.8C steels. Int. J. Mater. Sci. 2012, 7, 21–33. [Google Scholar]
- Annamalai, A.R.; Nekatibeb, F.; Upadhyaya, A.; Agarwal, D.K. Effect of heating mode on sinterability of carbonyl iron compacts. Mater. Res. Innov. 2013, 17, 10–16. [Google Scholar] [CrossRef]
- Annamalai, R.; Upadhyaya, A.; Agrawal, D. An investigation on microwave sintering of Fe, Fe–Cu, and Fe–Cu–C alloys. Bull. Mater. Sci. 2013, 36, 447–456. [Google Scholar] [CrossRef]
- Shon, Y.; Joshi, S.S.; Katakam, S.; ShankerRajamure, R.; Dahotre, N.B. Laser additive synthesis of high entropy alloy coating on aluminum: Corrosion behavior. Mater. Lett. 2015, 142, 122–125. [Google Scholar] [CrossRef]
- Li, Q.H.; Yue, T.M.; Guo, Z.N.; Lin, X. Microstructure and corrosion properties of AlCoCrFeNi high entropy alloy coatings deposited on AISI 1045 steel by the electro spark process. Met. Mater. Trans. 2012, 44, 1767–1778. [Google Scholar] [CrossRef]
- An, Z.; Jia, H.; Wu, Y.; Rack, P.D.; Patchen, A.D.; Liu, Y.; Ren, Y.; Li, N.; Liaw, P.K. Solid-solution CrCoCuFeNihigh-entropy alloy thin films synthesized by sputter deposition. Mater. Res. Lett. 2015, 3, 203–209. [Google Scholar] [CrossRef] [Green Version]
- Carboneras, M.; Hernández, L.S.; del Valle, J.A.; García-Alonso, M.C.; Escudero, M.L. Corrosion protection of different environmentally friendly coatings on powder metallurgy magnesium. J. Alloys Compd. 2010, 496, 442–448. [Google Scholar] [CrossRef] [Green Version]
- Pezzato, L.; Dabalà, M.; Gross, S.; Brunelli, K. Effect of microstructure and porosity of alsi10mg alloy produced by selective laser melting on the corrosion properties of plasma electrolytic oxidation coatings. Surf. Coat. Technol. 2020, 404, 126477. [Google Scholar] [CrossRef]
- Fernee, H.; Nairn, J.; Atrens, A. Precipitation hardening of Cu-Fe-Cr alloys. J. Mater. Sci. 2011, 36, 2711–2719. [Google Scholar] [CrossRef]
- Demetrio, K.; Klein, A.; Schaeffer, L.; Consoni, D.R.; Martinelli, A.E.; Bendo, T. Sinterability and microstructure evolution during sintering of ferrous powder mixtures. Mater. Res. 2013, 65, 1030–1038. [Google Scholar] [CrossRef] [Green Version]
- Baczek, E.; Konstantly, J.; Romanski, A.; Podsiadlo, M.; Cyboron, J. Processing and characterization of Fe–Mn–Cu–Sn–C alloys prepared by ball milling and spark plasma sintering. J. Mater. Eng. Perform. 2018, 27, 1475–1483. [Google Scholar] [CrossRef] [Green Version]
- German, R.M.; Suri, P.; Park, S.J. Liquid phase sintering. J. Mater. Sci. 2019, 44, 1–39. [Google Scholar] [CrossRef] [Green Version]
- Zhang, S.; Sun, D.; Fu, Y.Q.; Du, H.J. Recent advances of superhard nanocomposite coatings: A review. Surf. Coat. Technol. 2003, 167, 113. [Google Scholar] [CrossRef]
- ASTM E8/E8M-16ae1. Standard Test Methods for Tension Testing of Metallic Materials; ASTM International: West Conshohocken, PA, USA, 2016. [Google Scholar]
- Barshilia, H.C.; Deepthi, B.; Selvakumar, N.; Jain, A.; Rajam, K.S. Nanolayered multilayer coatings of CrN/CrAlN prepared by reactive DC magnetron sputtering. Appl. Surf. Sci. 2007, 253, 5076. [Google Scholar] [CrossRef]
- Muthuchamy, A.; Annamalai, A.R.; Karthikeyan, M.; Thakur, A.; Nagaraju, N.; Agrawal, D.K. Microstructural evolution of iron-based alloys produced by spark plasma sintering method. Phys. Met. Metallogr. 2018, 119, 678–684. [Google Scholar] [CrossRef]
- Tahir, A.M.; Amberg, G.; Hedström, P.; Bergman, O.; Frisk, K. Cu redistribution during sintering of Fe–2Cu and Fe–2Cu–0.5C compacts. Powder Metall. 2014, 57, 373–379. [Google Scholar] [CrossRef]
- ASTM G59-97. Standard Test Method for Conducting Potentiodynamic Polarization Resistance Measurements; American Society for Testing and Materials: West Conshohocken, PA, USA, 2014. [Google Scholar]
- Danninger, H. Sintering of Mo alloyed P/M steels prepared from elemental powders. Powder Met. Int. 1992, 24, 163–167. [Google Scholar]
- Chen, W.; Cheng, J.; Chen, P.; Zhang, J.; Wei, B. Preparation and performance of sintered Fe–2Cu–2Mo–0.8 C materials containing different forms of molybdenum powder. Materials 2019, 12, 417. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raja Annamalai, A.; Upadhyaya, A.; Agrawal, D.K. Effect of heating mode and electrochemical response on austenitic and ferritic stainless steels. Can. Metall. Q. 2015, 54, 142–148. [Google Scholar] [CrossRef]
- Shi, Y.; Yang, B.; Liaw, P.K. Corrosion-resistant high-entropy alloys: A review. Metals 2017, 7, 43. [Google Scholar] [CrossRef] [Green Version]
- Braic, V.; Balaceanu, M.; Braic, M.; Vladescu, A.; Panseri, S.; Russo, A. Characterization of multi-principal-element (TiZrNbHfTa)N and (TiZrNbHfTa)C coatings for biomedical applications. J. Mech. Behav. Biomed. Mater. 2012, 1, 197–205. [Google Scholar] [CrossRef]
- Wan, X.S.; Zhao, S.S.; Yang, Y.; Gong, J.; Sun, C. Effects of nitrogen pressure and pulse bias voltage on the properties of Cr-N coatings deposited by arc ion plating. Surf. Coat. Technol. 2010, 204, 800–1810. [Google Scholar] [CrossRef]
- Musil, J.; Kunc, F.; Zeman, H.; Polakova, H. Relationships between Hardness, YoungÕs and elastic recovery in hard nanocomposite coatings. Surf. Coat. Technol. 2002, 154, 304–313. [Google Scholar] [CrossRef]
- Dobrzanski, L.A.; Zukowska, L.W. Properties of the multicomponent and gradient PVD coatings. Mater. Sci. Pol. Acad. Sci. 2017, 28, 621–624. [Google Scholar]
- Veprek, S.; Maritza, J.G.; Veprek–Hejiman, M. Industrial applications of superhard nanocomposite coatings. Surf. Coat. Technol. 2008, 202, 5063–5073. [Google Scholar] [CrossRef]
- Kumar, T.S.; Jebaraj, A.V. Metallurgical characterization of CrN and AlCrN physical vapor deposition coatings on aluminum alloy AA 6061. Mater. Today: Proc. 2020, 22, 1479–1488. [Google Scholar]
- Tański, T.; Dobrzańska-Danikiewicz, A.D.; Labisz, K.; Matysiak, W. Long-term development perspectives of selected groups of engineering materials used in the automotive industry. Arch. Met. Mater. 2014, 59, 1717–1728. [Google Scholar] [CrossRef] [Green Version]
- Żaba, K.; Nowosielski, M.; Kita, P.; Kwiatkowski, M.; Tokarski, T.; Puchlerska, S. Effect of heat treatment on the corrosion resistance of aluminized steel strips. Arch. Met. Mater. 2015, 60, 1825–1831. [Google Scholar] [CrossRef]
- Kumar, T.S.; Prabu, S.B.; Manivasagam, G. Metallurgical characteristics of TiAlN/AlCrN coating synthesized by the PVD process on a cutting insert. J. Mater. Eng. Perform. 2014, 23, 2877–2884. [Google Scholar] [CrossRef]
- Willmann, H.; Mayrhofer, P.H.; Hultman, L.; Mitterer, C. Thermal stability and age hardenings of supersaturated AlCrN hard coatings. Int. Heat Treat. Surf. Eng. 2017, 1, 75–79. [Google Scholar] [CrossRef]
- Feng, Y.; Zhang, L.; Ke, R.; Wan, Q.; Zhi, Z.; Lu, H. Thermal stability and oxidation behavior of AlTiN, AlCrN, and AlCrSiWN coatings. Int. J. Refract. Met. Hard Mater. 2014, 43, 241–249. [Google Scholar] [CrossRef]
- Kumar, T.S.; Prabu, S.B.; Madhavan, S.; Padmanabhan, K.A. Thermal stability of TiAlN/AlCrN and AlCrN/TiAlN coatings synthesized by the cathodic arc vapor deposition process on a tungsten carbide tool. Trans. Indian Inst. Met. 2018, 71, 665–676. [Google Scholar] [CrossRef]
- Chawla, V.; Chawla, A.; Mehta, Y.; Puri, D.; Prakash, S.; Sidhu, B.S. Investigation of properties and corrosion behavior of hard TiAlN and AlCrN PVD thin coatings in the 3 wt.% NaCl solution. J. Aust. Ceram. Soc. 2011, 47, 48–55. [Google Scholar]

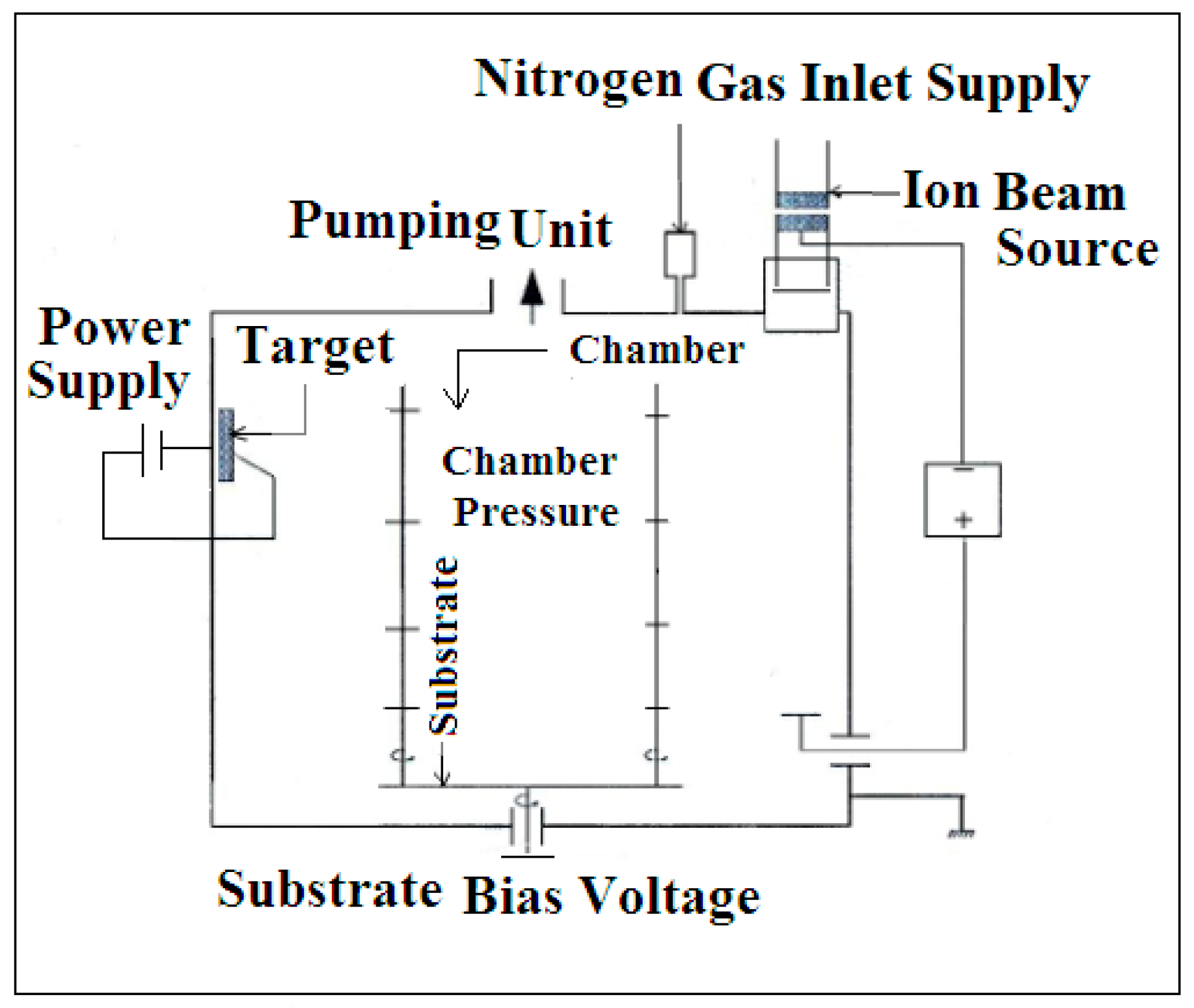

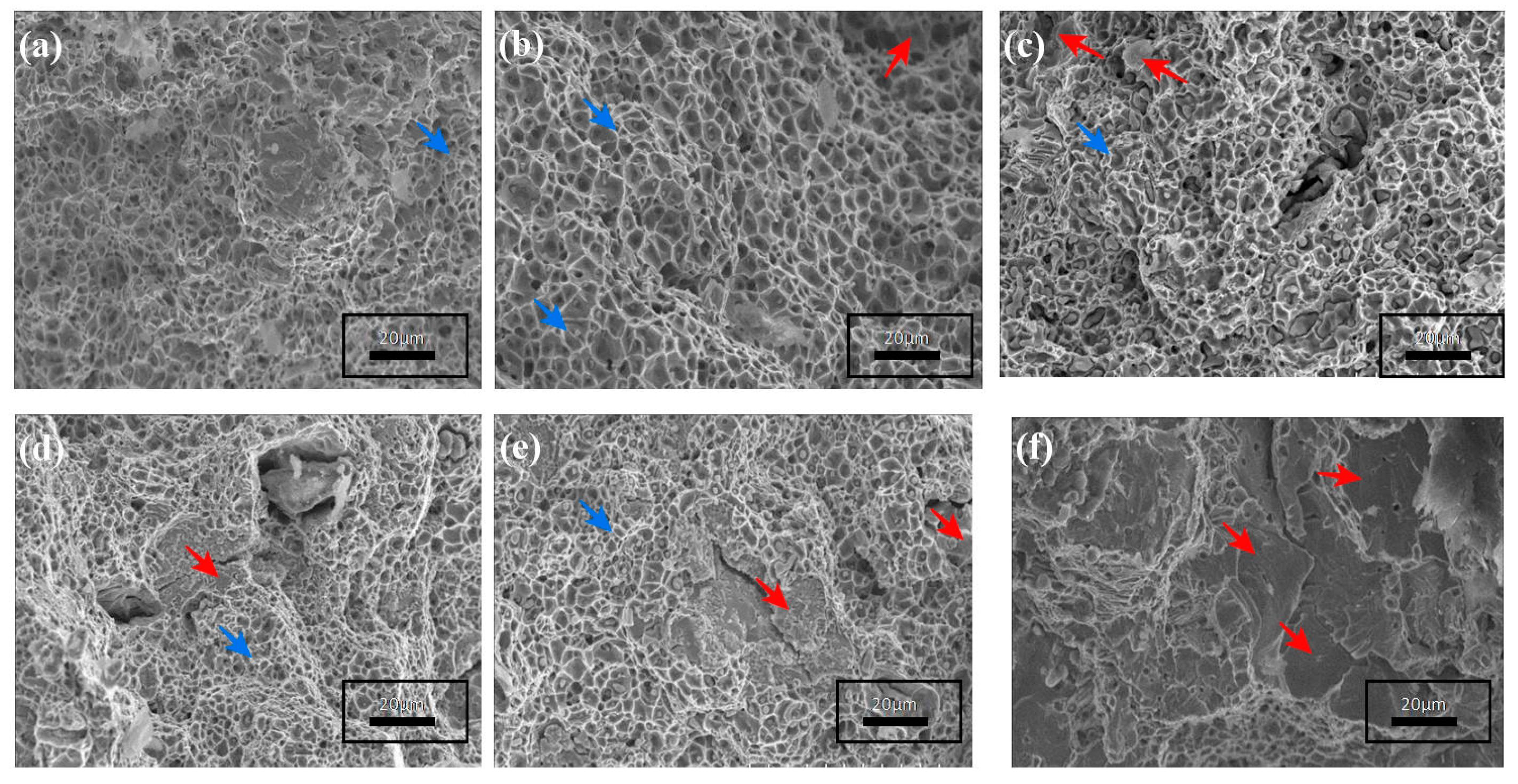
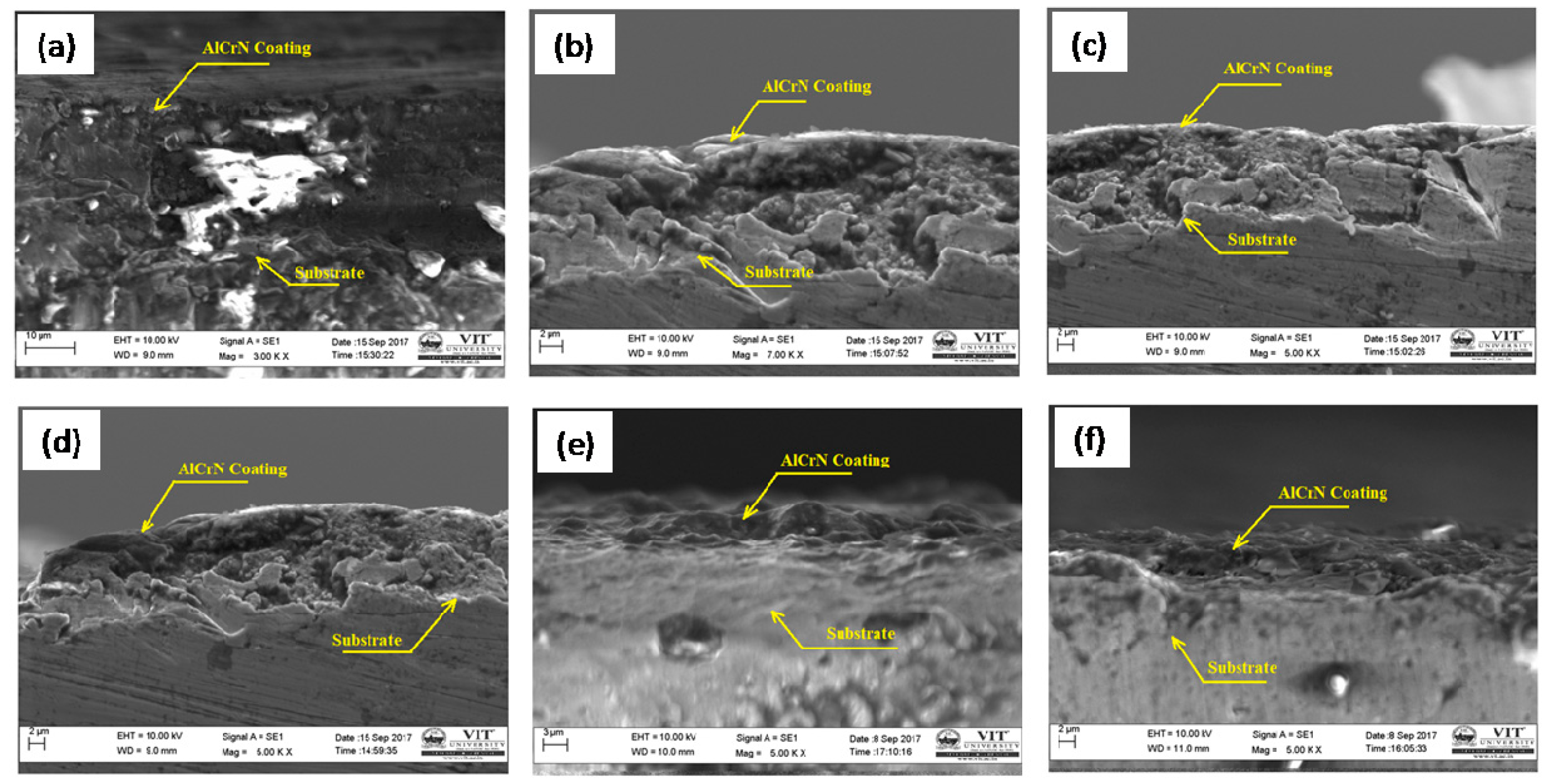

| Characteristics | Fe | Cu | Graphite | Mo | |
|---|---|---|---|---|---|
| Apparent density, (g/cm3) | 3.10 | 2.03 | 0.17 | 2.59 | |
| Tap density, (g/cm3) | 3.50 | 3.28 | 0.33 | 2.5 | |
| Flow rate, (s/50 g) | 29.29 | Non flowing | Non flowing | Non flowing | |
| Particle size, (μm) | D10 | 32.68 | 4.17 | 3.90 | 6.80 |
| D50 | 90.12 | 9.5 | 8.84 | 23.56 | |
| D90 | 179.80 | 20.16 | 17.77 | 58.08 | |
| Theoretical density, (g/cm3) | 7.86 | 6.92 | 2.32 | 10.2 | |
| Surface area (m2/g) | 0.668 | 0.246 | 0.842 | 0.576 | |
| Process Factors | Standards with Units |
|---|---|
| AlCrN monolayer coating thickness | 5 ± 1 µm |
| Substrate temperature | 400 ± 10 °C |
| Power supply for targets | 5 kW |
| Coating period | 160 min |
| Nitrogen rate of flow | 1250 sccm |
| Compartment pressure | 3.4 × 10−2 mbar |
| Voltage/current | 30 Volts/160 amps |
| Feed rate | 60 mg/min for each target |
| Distance between target and substrate | 200 mm |
| Composition | Sintered Density % (ρs) |
|---|---|
| Fe | 90.18 ± 1.21 |
| Fe–2Cu | 93.85 ± 2.60 |
| Fe–2Cu–0.8C | 94.29 ± 1.96 |
| Fe–2Cu–0.8C–0.2Mo | 95.63 ± 2.254 |
| Fe–2Cu–0.8C–0.4Mo | 96.83 ± 2.14 |
| Fe–2Cu–0.8C–0.6Mo | 97.20 ± 2.89 |
| Composition | Hardness (HRB) |
|---|---|
| Spark Plasma Sintered Compacts | |
| Fe | 45 ± 3.43 |
| Fe–2Cu | 47 ± 2.1 |
| Fe–2Cu–0.8C | 79 ± 5.21 |
| Fe–2Cu–0.8C–0.2Mo | 88 ± 2.6 |
| Fe–2Cu–0.8C–0.4Mo | 95 ± 1.59 |
| Fe–2Cu–0.8C–0.6Mo | 96 ± 3.2 |
| Sample | UTS (MPa) | Yield Strength (MPa) | Elongation % | Reduction in Area % |
|---|---|---|---|---|
| Fe | 444 ± 12 | 266 ± 5 | 6.3 | 1.3 |
| Fe–2Cu | 497 ± 8 | 298 ± 7 | 9.4 | 0.8 |
| Fe–2Cu–0.8C | 772 ± 6 | 463 ± 4 | 5.3 | 0.4 |
| Fe–2Cu–0.8C–0.2Mo | 900 ± 6 | 546 ± 7 | 5.1 | 0.6 |
| Fe–2Cu–0.8C–0.4Mo | 918 ± 3 | 553 ± 4 | 4.9 | 0.4 |
| Fe–2Cu–0.8C–0.6Mo | 1000 ± 5 | 600 ± 4 | 3.2 | 0.6 |
| Samples | Ecorr (V) | icorr (A/cm2) | Rp (Ohms) | CR (mmpy) |
|---|---|---|---|---|
| Fe | −1.0585 | 0.000825 | 94.27 | 2.7 |
| Fe–2Cu | −0.8387 | 0.0002011 | 482.4 | 0.6579 |
| Fe–2Cu–0.8C | −0.9593 | 0.0002266 | 339 | 0.7415 |
| Fe–2Cu–0.8C–0.2Mo | −1.0981 | 0.0003012 | 252.3 | 0.9856 |
| Fe–2Cu–0.8C–0.4Mo | −1.0603 | 0.0005948 | 124.1 | 1.946 |
| Fe–2Cu–0.8C–0.6Mo | −1.0713 | 0.0004539 | 104.6 | 1.485 |
| Substrate | Coating | Elements | |||||
| Nitrogen (N) | Aluminum (Al) | Chromium (Cr) | |||||
| AlCrN | Weight% (100) | Atomic% (100) | Weight% (100) | Atomic% (100) | Weight% (100) | Atomic% (100) | |
| Fe | 29.34 | 52.36 | 30.7 | 28.44 | 39.95 | 19.20 | |
| Fe–2Cu | 28.40 | 51.03 | 31.87 | 29.73 | 39.73 | 19.23 | |
| Fe–2Cu–0.8C | 30.60 | 53.71 | 30.74 | 28.01 | 38.66 | 18.28 | |
| Fe–2Cu–0.8C–0.2Mo | 30.34 | 53.71 | 29.59 | 27.19 | 40.07 | 19.11 | |
| Fe–2Cu–0.8C–0.4Mo | 27.73 | 50.13 | 30.00 | 28.67 | 42.77 | 21.21 | |
| Fe–2Cu–0.8C–0.6Mo | 27.53 | 50.06 | 31.80 | 30.02 | 40.67 | 19.92 | |
| Coating | Substrate | Hardness (HV) |
|---|---|---|
| AlCrN | Fe | 171.55 ± 5.6 |
| Fe–2Cu | 191.10 ± 6.1 | |
| Fe–2Cu–0.8C | 336.65 ± 5.65 | |
| Fe–2Cu–0.8C–0.2Mo | 452.40 ± 8.93 | |
| Fe–2Cu–0.8C–0.4Mo | 517.30 ± 9.63 | |
| Fe–2Cu–0.8C–0.6Mo | 1134.85 ± 11.3 |
| Coating | Samples | Ecorr (V) | icorr (A/cm2) | Rp (Ohms) | CR (mmpy) |
|---|---|---|---|---|---|
| AlCrN | Fe | −0.4858 | 0.00005992 | 1593 | 0.196 |
| Fe–2Cu | −0.3394 | 0.00003602 | 7785 | 0.1179 | |
| Fe–2Cu–0.8C | −0.2712 | 0.00003317 | 2258 | 0.1085 | |
| Fe–2Cu–0.8C–0.2Mo | −0.3649 | 0.00002539 | 2177 | 0.08306 | |
| Fe–2Cu–0.8C–0.4Mo | −0.2952 | 0.00003155 | 1898 | 0.1032 | |
| Fe–2Cu–0.8C–0.6Mo | −0.6896 | 0.00008557 | 526.2 | 0.280 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kumar, T.S.; Annamalai, A.R.; Srikanth, M.; Jen, C.-P. Processing of Spark Plasma Sintered Fe Alloy and Enhancing Its Surface Properties by AlCrN Monolayer Coating by Cathodic Arc Plasma Physical Vapor Deposition Process. Coatings 2020, 10, 1166. https://doi.org/10.3390/coatings10121166
Kumar TS, Annamalai AR, Srikanth M, Jen C-P. Processing of Spark Plasma Sintered Fe Alloy and Enhancing Its Surface Properties by AlCrN Monolayer Coating by Cathodic Arc Plasma Physical Vapor Deposition Process. Coatings. 2020; 10(12):1166. https://doi.org/10.3390/coatings10121166
Chicago/Turabian StyleKumar, T. Sampath, A. Raja Annamalai, Muthe Srikanth, and Chun-Ping Jen. 2020. "Processing of Spark Plasma Sintered Fe Alloy and Enhancing Its Surface Properties by AlCrN Monolayer Coating by Cathodic Arc Plasma Physical Vapor Deposition Process" Coatings 10, no. 12: 1166. https://doi.org/10.3390/coatings10121166







