Microstructure of High Temperature Oxidation Resistant Hf6B10Si31C2N50 and Hf7B10Si32C2N44 Films
Abstract
:1. Introduction
2. Materials and Methods
3. Results
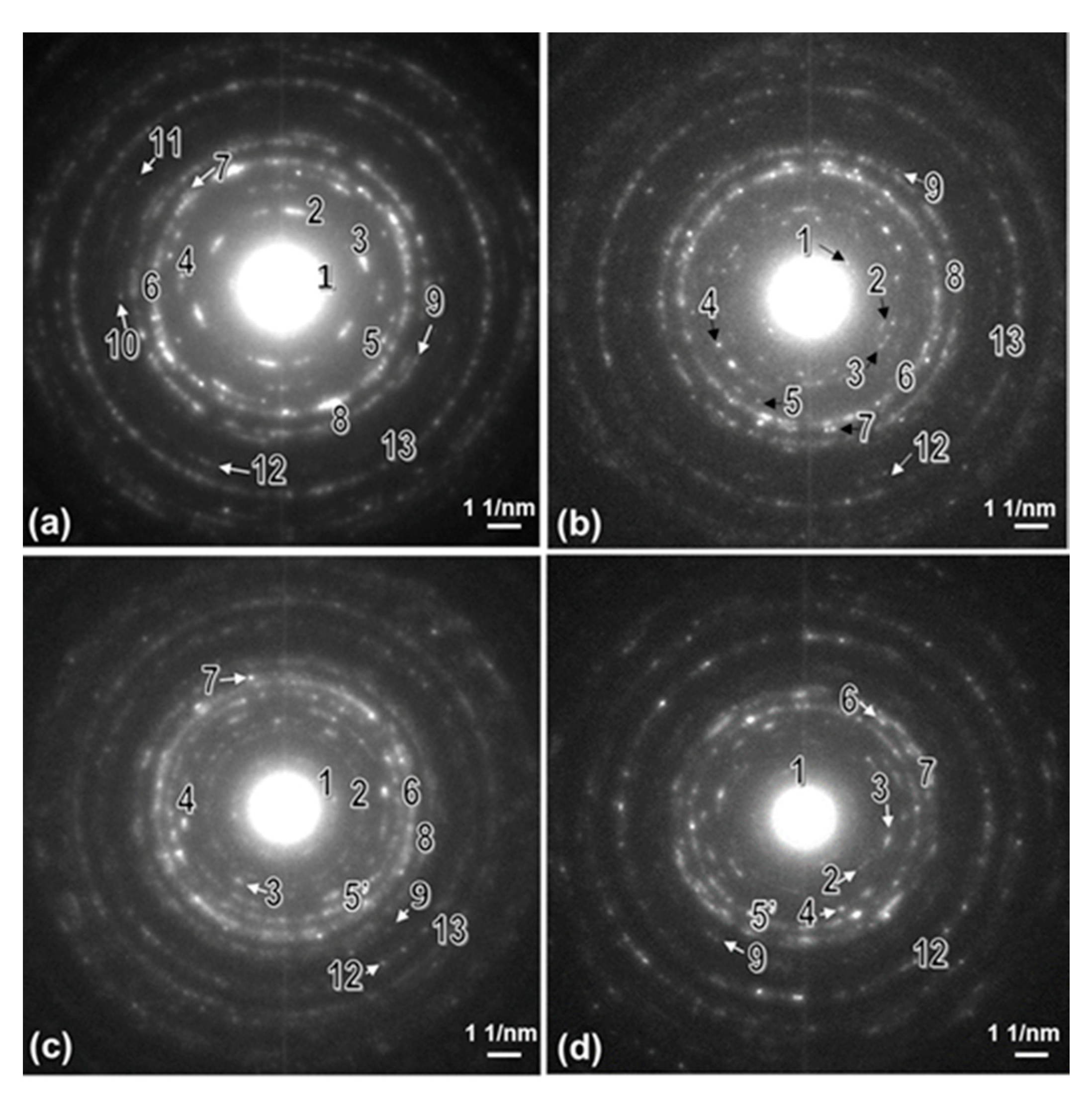
3.1. XRD Analysis
3.2. TEM Studies
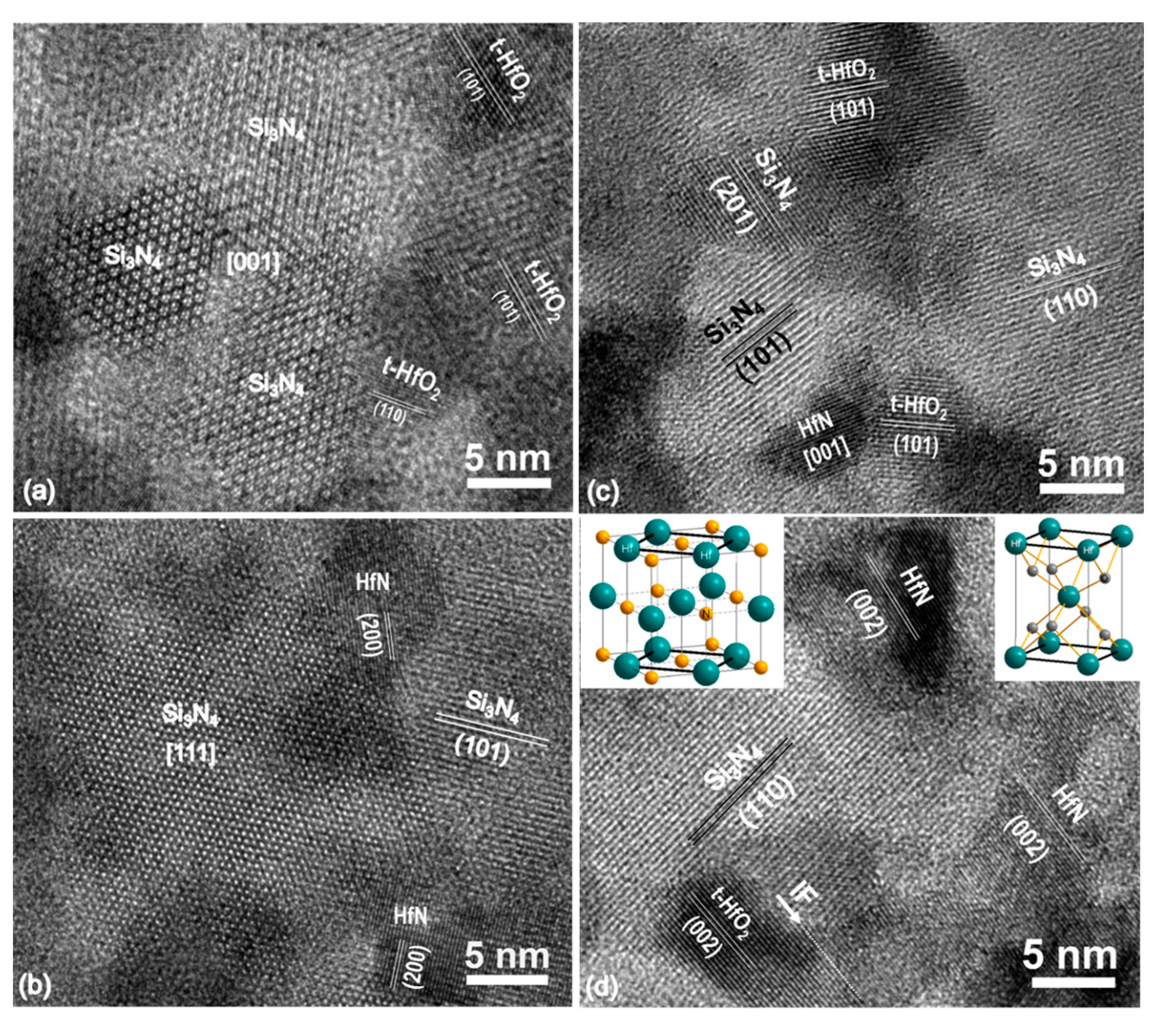
3.2.1. Microstructure of the Annealed Hf6B10Si31C2N50 Film
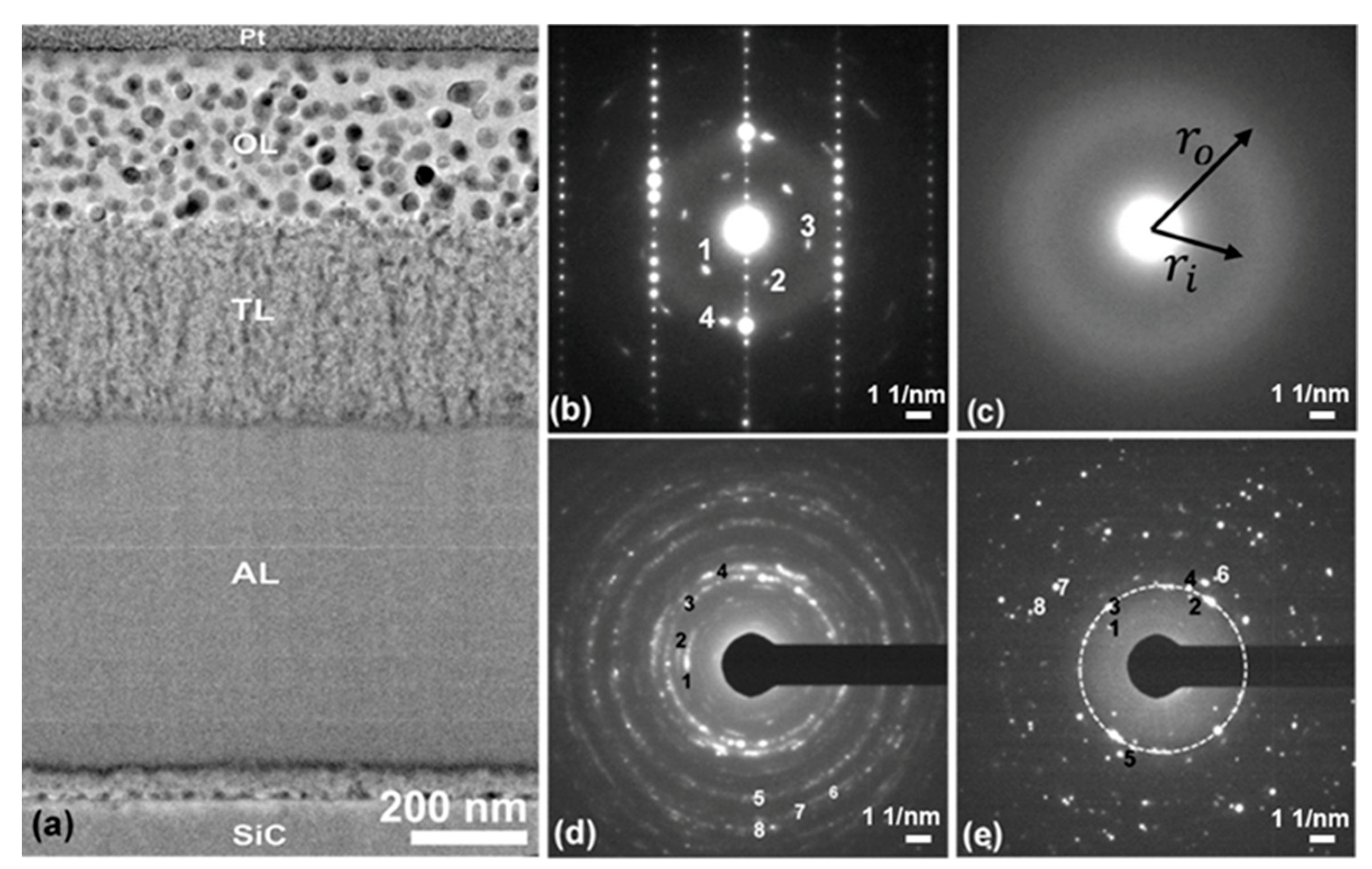
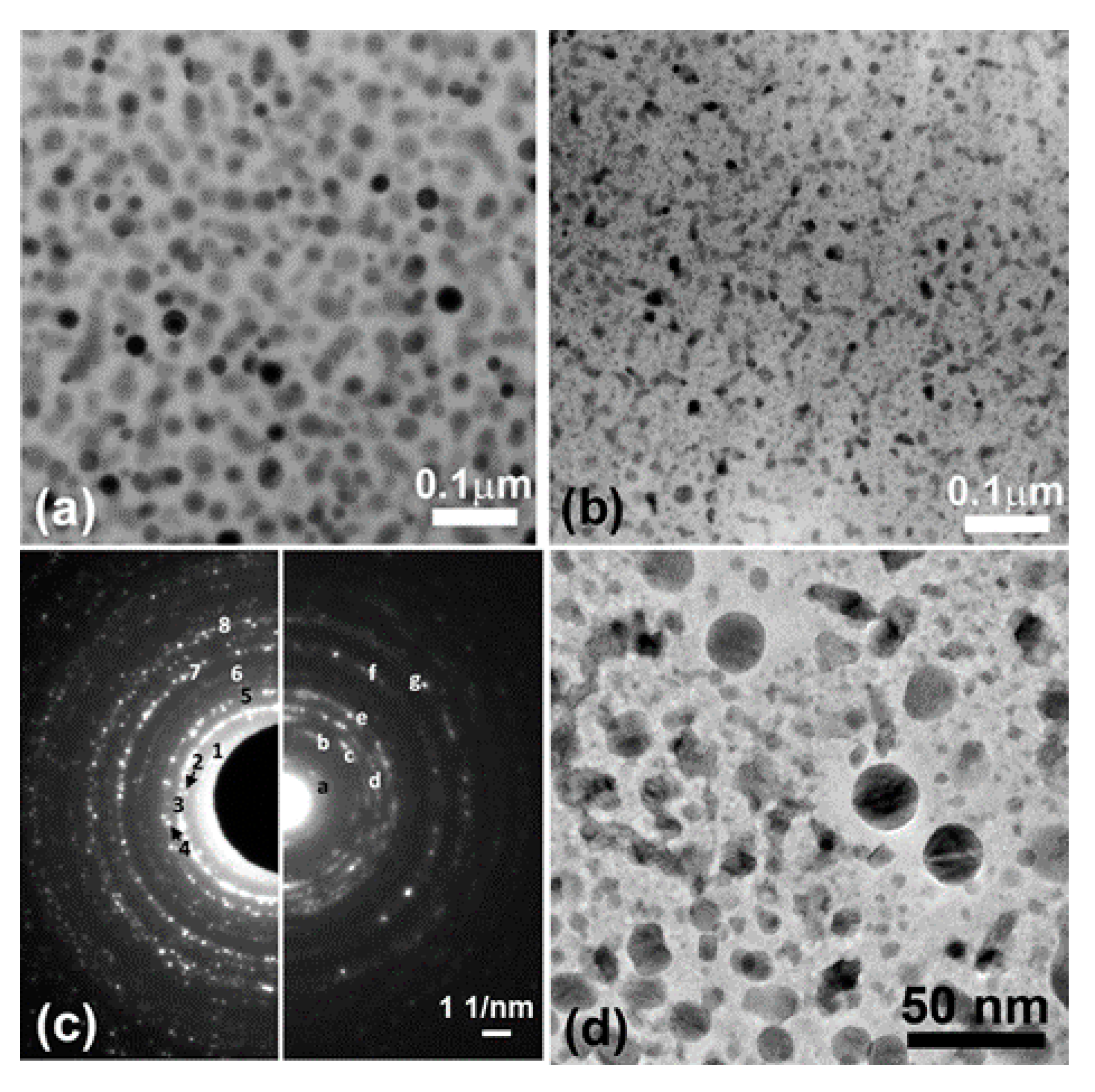
Overall Film Structure
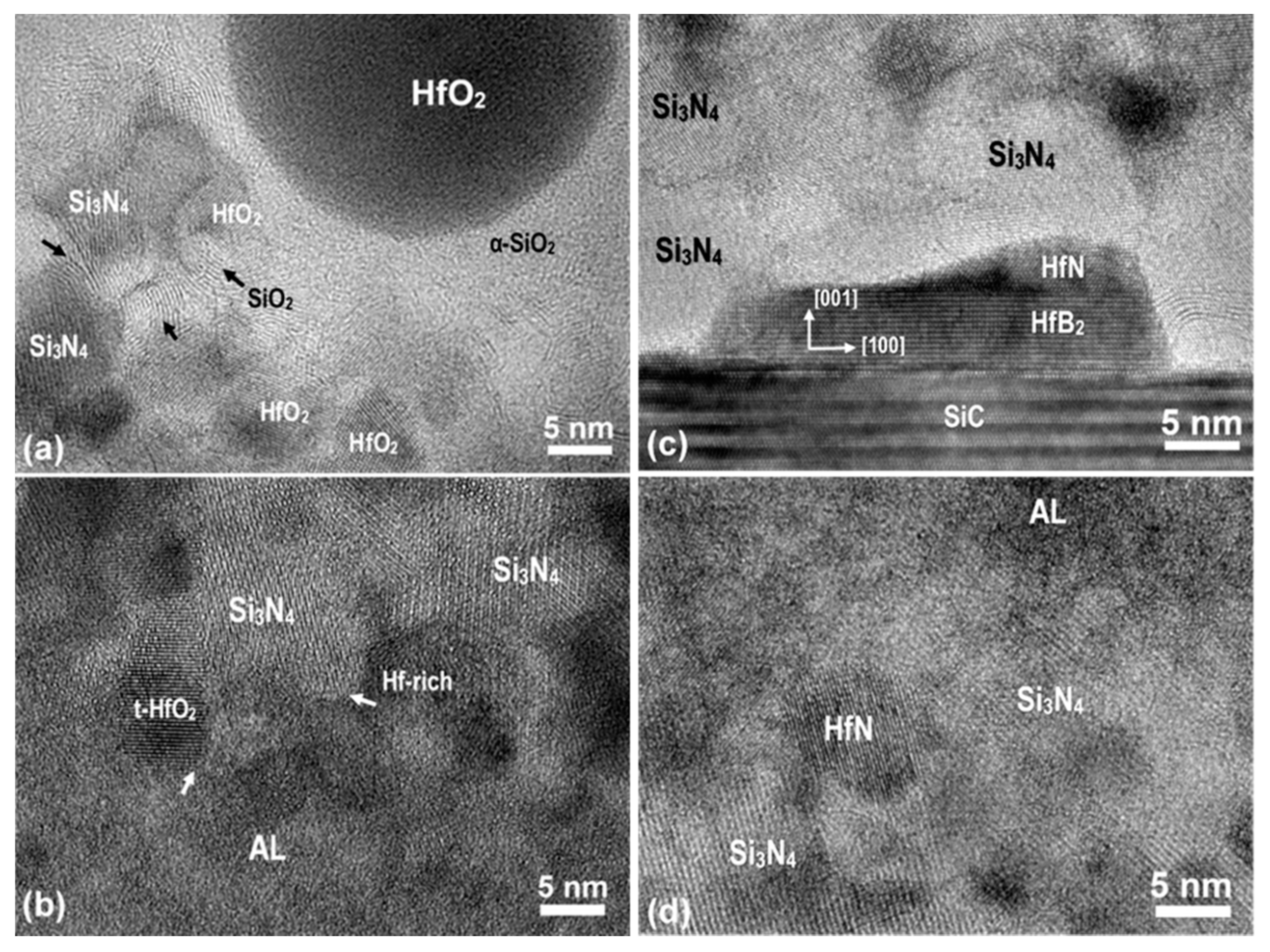
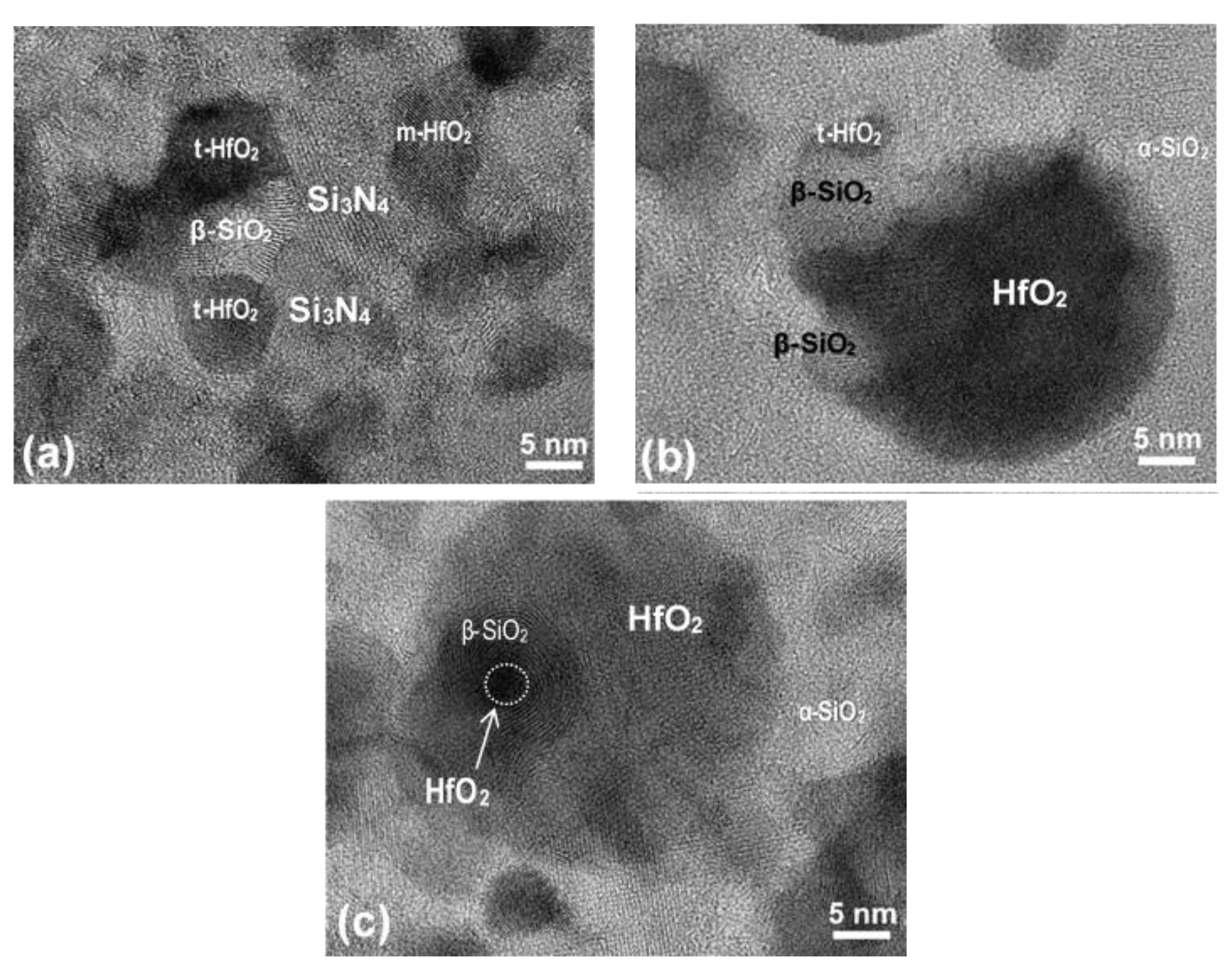
Interface Structures
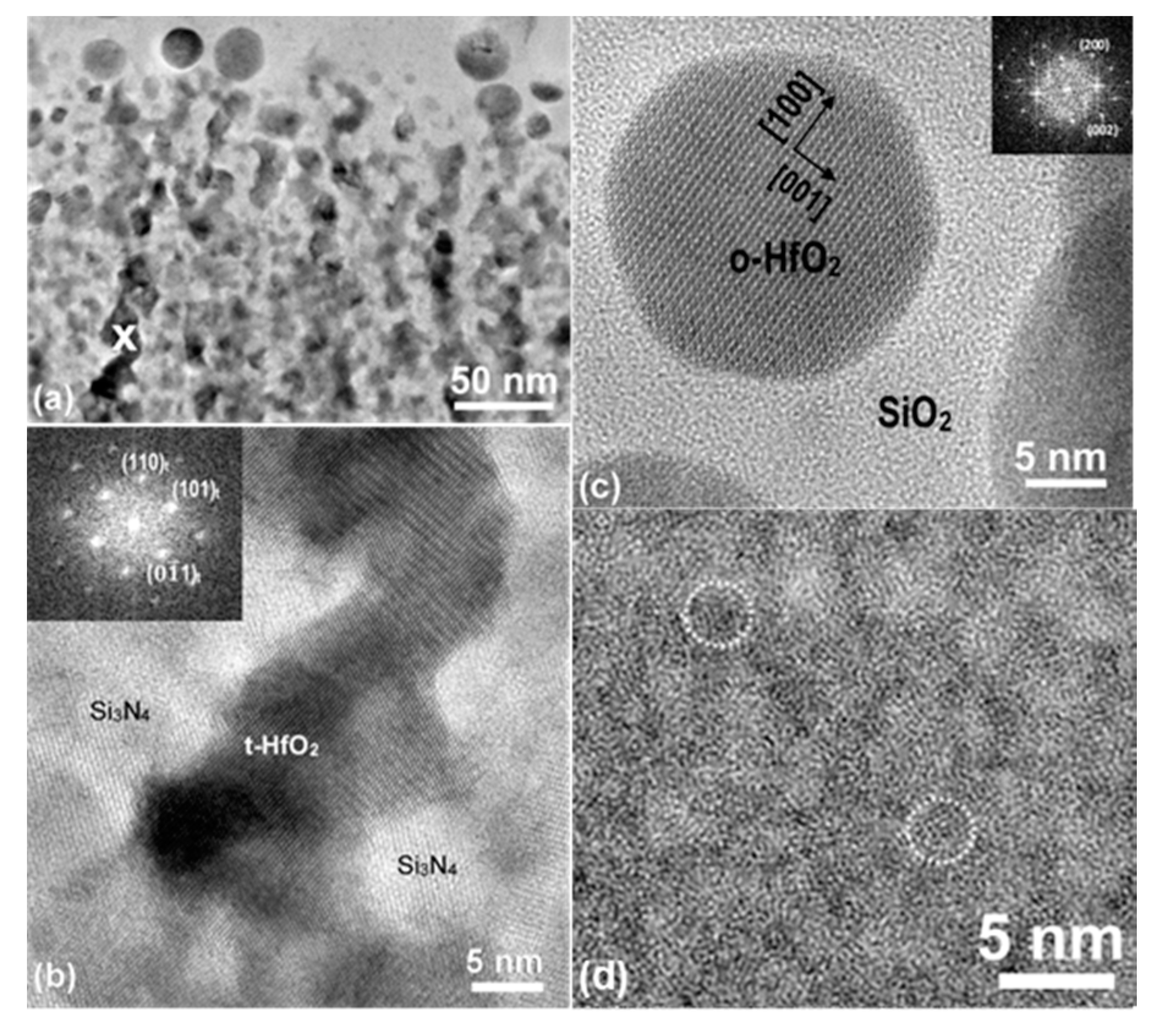
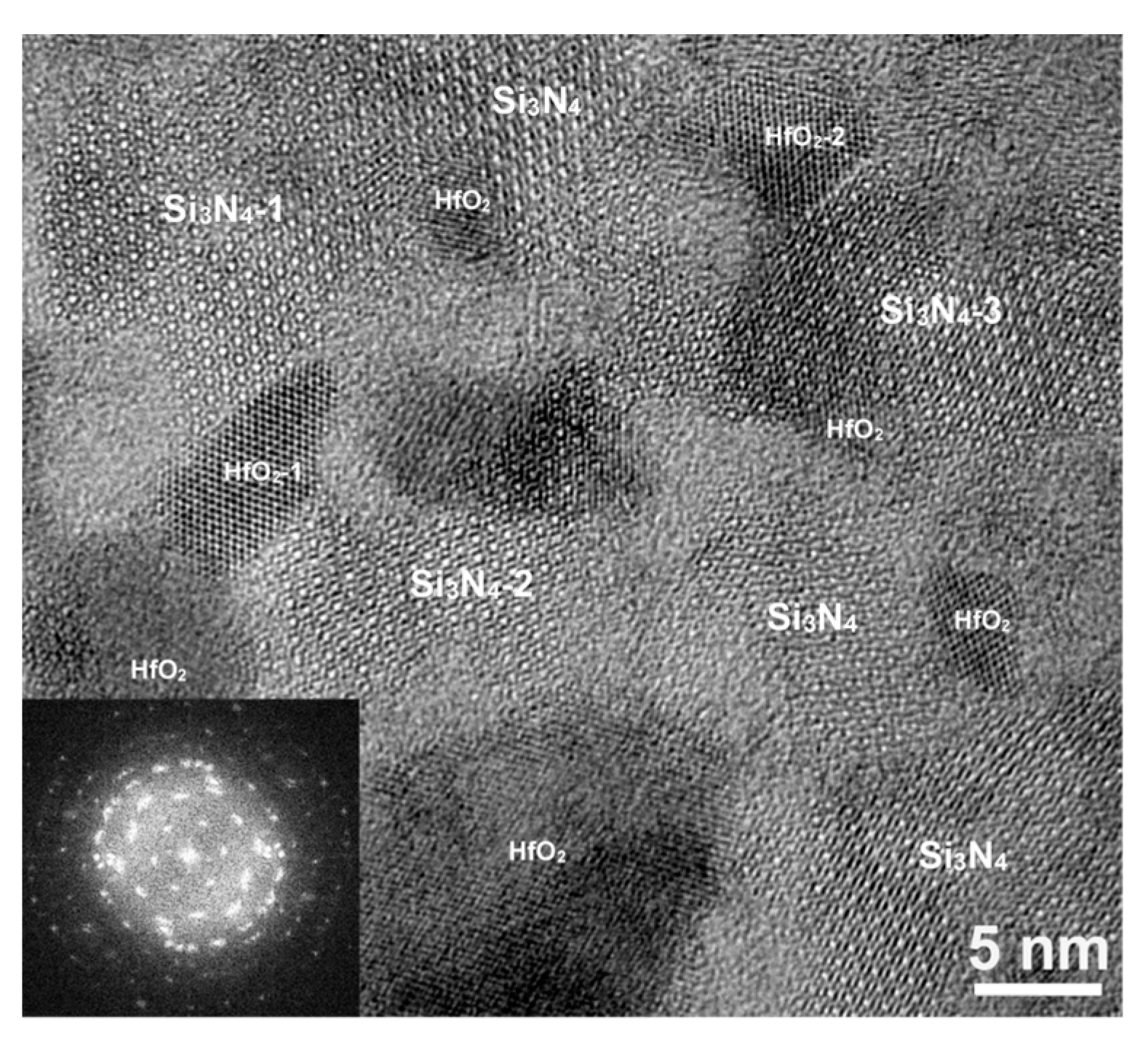
Microstructure within the Film Plane
3.2.2. Microstructure of the Annealed Hf7B10Si32C2N44 Film
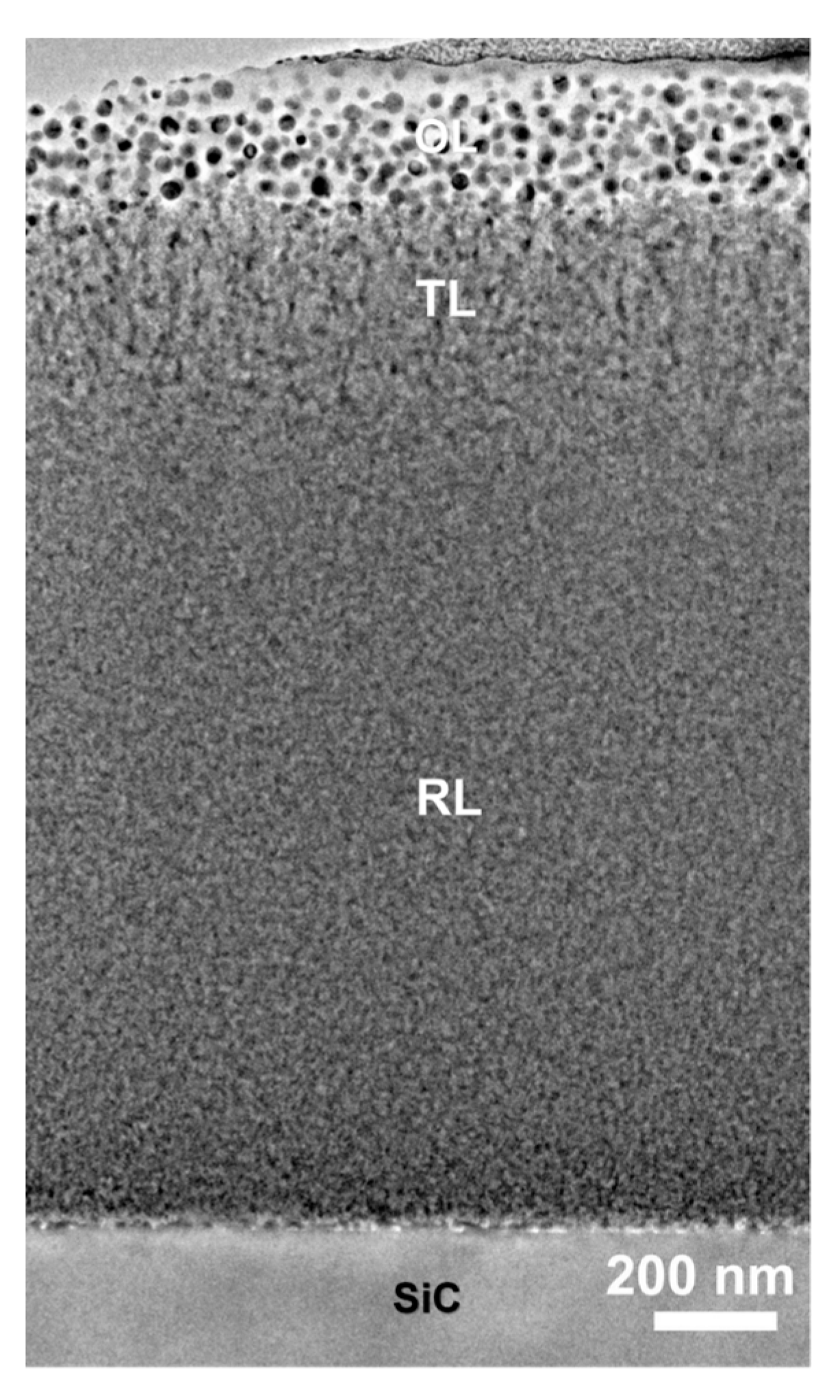
Overall Film Structure
Microstructure of the TL and RL Sections
Structure Transformations at TL/OL Interface
3.3. Microstructure Evolution and Oxidation Mechanism
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Mitterer, C. Borides in thin film technology. J. Solid State Chem. 1997, 133, 279–291. [Google Scholar] [CrossRef]
- Opeka, M.M.; Talmy, I.G.; Wuchina, E.J.; Zaykoski, J.A.; Causey, S.J. Mechanical, thermal, and oxidation properties of refractory hafnium and zirconium compounds. J. Eur. Ceram. Soc. 1999, 19, 2405–2414. [Google Scholar] [CrossRef] [Green Version]
- Levine, S.R.; Opila, E.J.; Halbig, M.C.; Kiser, J.D.; Singh, M.; Salem, J.A. Evaluation of ultra-high temperature ceramics for aeropropulsion use. J. Eur. Ceram. Soc. 2002, 22, 2752–2756. [Google Scholar] [CrossRef]
- Opeka, M.M.; Talmy, I.G.; Zaykoski, J.A. Oxidation-based materials selection for 2000 °C + hypersonic aerosurfaces: Theoretical considerations and historical experience. J. Mater. Sci. 2004, 9, 5887–5904. [Google Scholar] [CrossRef]
- Fahrenholtz, W.G.; Hilmas, G.E.; Talmy, I.G.; Zaykoski, J.A. Refractory diborides of zirconium and hafnium. J. Am. Ceram. Soc. 2007, 90, 1347–1364. [Google Scholar] [CrossRef]
- Wuchina, E.; Opila, E.; Opeka, M.; Fahrenholtz, W.; Talmy, I. UHTCs: Ultra-high temperature ceramic materials for extreme environment applications. Electrochem. Soc. Interface 2007, 16, 30–36. [Google Scholar]
- Monteverde, F.; Bellosi, A.; Scatteia, L. Processing and properties of ultra-high temperature ceramics for space applications. Mater. Sci. Eng. A 2008, 485, 415–421. [Google Scholar] [CrossRef]
- Ghosh, D.; Subhash, G. Recent Progress in Zr(Hf)B2 Based Ultrahigh Temperature Ceramics in Handbook of Advanced Ceramics, 2nd ed.; Materials, Applications, Processing, and Properties; Elsevier: Amsterdam, The Netherlands, 2013; pp. 267–269. [Google Scholar]
- Zapata-Solvas, E.; Jayaseelan, D.D.; Lin, H.T.; Brown, P.; Lee, W.E. Mechanical properties of ZrB2- and HfB2- based ultra-high temperature ceramics fabricated by spark plasma sintering. J. Eur. Ceram. Soc. 2013, 33, 1373–1386. [Google Scholar] [CrossRef]
- Fahrenholtz, W.G.; Hilmas, G.E. Ultra-high temperature ceramics: Materials for extreme environment. Scr. Mater. 2017, 129, 94–99. [Google Scholar] [CrossRef] [Green Version]
- Menteverde, F. Progress in fabrication of ultra-high temperature ceramics: “In situ” synthesis, microstructure and properties of a reactive hot-pressed HfB2-SiC composite. Compos. Sci. Technol. 2005, 65, 1869–1879. [Google Scholar] [CrossRef]
- Monteverde, F. The thermal stability in air of hot-pressed diboride matrix composites for uses at ultra-high temperatures. Cor. Sci. 2005, 47, 2020–2033. [Google Scholar] [CrossRef]
- Rezaie, A.; Fahrenholtz, W.G.; Hilmas, G.E. Evaluation of structure during oxidation of zirconium diboride-silicon carbide in air up to 1500 °C. J. Eur. Ceram. Soc. 2007, 27, 2495–2501. [Google Scholar] [CrossRef]
- Menteverde, F.; Bellosi, A. The resistance to oxidation of an HfB2-SiC composite. J. Eur. Ceram. Soc. 2008, 25, 1025–1031. [Google Scholar] [CrossRef]
- Carney, C.M. Oxidation resistance of hafnium diboride-silicon carbide from 1400 to 2000 °C. J. Mater. Sci. 2009, 44, 5673–5681. [Google Scholar] [CrossRef]
- Carney, C.M.; Mogilvesky, P.; Parthasarathy, T.A. Oxidation behavior of zirconium diboride silicon carbide produced by the spark plasma sintering method. J. Am. Ceram. Soc. 2009, 92, 2046–2052. [Google Scholar] [CrossRef]
- Mallik, M.; Ray, K.K.; Mitra, R. Oxidation behavior of hot pressed ZrB2-SiC and HfB2-SiC composites. J. Eur. Ceram. Soc. 2011, 31, 199–215. [Google Scholar] [CrossRef]
- Kohout, J.; Vlček, J.; Houška, J.; Mareš, P.; Čerstvý, R.; Zeman, P.; Zhang, M.H.; Jiang, J.C.; Meletis, E.I.; Zuzjaková, Š. Hard multifunctional Hf–B–Si–C films prepared by pulsed magnetron sputtering. Surf. Coat. Technol. 2014, 257, 301–307. [Google Scholar] [CrossRef]
- Zhang, M.H.; Jiang, J.C.; Meletis, E.I.; Mares, P.; Houška, J.; Vlček, J. Effect of the Si content on the microstructure of hard, multifunctional Hf-B-Si-C films prepared by pulsed magnetron sputtering. Appl. Surf. Sci. 2015, 357, 1343–1354. [Google Scholar] [CrossRef]
- Čapek, J.; Hřeben, S.; Zeman, P.; Vlček, J.; Čerstvý, R.; Houška, J. Effect of the gas mixture composition on high-temperature behavior of magnetron sputtered Si-B-CN coatings. Surf. Coat. Technol. 2008, 203, 466–469. [Google Scholar] [CrossRef]
- Kalaš, J.; Vernhes, R.; Hřeben, S.; Vlček, J.; Klemberg-Sapieha, J.E.; Martinu, L. High temperature stability of the mechanical and optical properties of Si–B–C–N films prepared by magnetron sputtering. Thin Solid Films 2009, 518, 174–179. [Google Scholar] [CrossRef]
- Zeman, P.; Čapek, J.; Čerstvý, R.; Vlček, J. Thermal stability of magnetron sputtered Si–B–C–N materials at temperatures up to 1700 °C. Thin Solid Films 2010, 519, 306–311. [Google Scholar] [CrossRef]
- He, J.; Zhang, M.H.; Jiang, J.C.; Vlček, J.; Zeman, P.; Steidl, P.; Meletis, E.I. Microstructure characterization of high-temperature, oxidation-resistant Si–B–C–N films. Thin Solid Films 2013, 542, 167–173. [Google Scholar] [CrossRef]
- Vlček, J.; Calta, P.; Steidl, P.; Zeman, P.; Čerstvý, R.; Houška, J.; Kohout, J. Pulsed reactive magnetron sputtering of high-temperature Si–B–C–N films with high optical transparency. Surf. Coat. Technol. 2013, 226, 34–39. [Google Scholar] [CrossRef]
- Zeman, P.; Zuzjaková, Š.; Mareš, P.; Čerstvý, R.; Zhang, M.H.; Jiang, J.C.; Meletis, E.I.; Vlček, J. Superior high-temperature oxidation resistance of magnetron sputtered Hf–B–Si–C–N film. Ceram. Int. 2016, 42, 4853–4859. [Google Scholar] [CrossRef] [Green Version]
- Šímová, V.; Vlček, J.; Zuzjaková, S.; Houška, J.; Shen, Y.; Jiang, J.C.; Meletis, E.I.; Peřina, V. Magnetron sputtered Hf–B–Si–C–N films with controlled electrical conductivity and optical transparency, and with ultrahigh oxidation resistance. Thin Solid Film. 2018, 653, 333–340. [Google Scholar] [CrossRef]
- Zhang, M.H.; Jiang, J.C.; Zeman, P.; Zuzjaková, Š.; Vlček, J.; Meletis, E.I. Study of the High-temperature oxidation resistance mechanism of magnetron sputtered Hf7B23Si17C4N45 film. J. Vac. Sci. Technol. A 2018, 36, 021505. [Google Scholar] [CrossRef]
- Shen, Y.; Jiang, J.C.; Zeman, P.; Šímová, V.; Vlček, J.; Meletis, E.I. Microstructure evolution in amorphous Hf–B–Si–C–N high temperature resistant coatings after annealing to 1500 °C in air. Sci. Rep. 2019, 9, 3603. [Google Scholar] [CrossRef] [Green Version]
- International Centre for Diffraction Data. PDF-4 Database Sets; International Centre for Diffraction Data: Newtown Square, PA, USA, 2020. [Google Scholar]
- Zeman, P.; Zuzjaková, Š.; Čerstvý, R.; Houška, J.; Shen, Y.; Todt, J.; Jiang, J.; Daniel, R.; Keckes, J.; Meletis, E.I.; et al. Extraordinary high-temperature behavior of electrically conductive Hf7B23Si22C6N40 ceramic film. Surf. Coat. Technol. 2020, 391, 125686. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shen, Y.; Jiang, J.; Zeman, P.; Kotrlová, M.; Šímová, V.; Vlček, J.; Meletis, E.I. Microstructure of High Temperature Oxidation Resistant Hf6B10Si31C2N50 and Hf7B10Si32C2N44 Films. Coatings 2020, 10, 1170. https://doi.org/10.3390/coatings10121170
Shen Y, Jiang J, Zeman P, Kotrlová M, Šímová V, Vlček J, Meletis EI. Microstructure of High Temperature Oxidation Resistant Hf6B10Si31C2N50 and Hf7B10Si32C2N44 Films. Coatings. 2020; 10(12):1170. https://doi.org/10.3390/coatings10121170
Chicago/Turabian StyleShen, Yi, Jiechao Jiang, Petr Zeman, Michaela Kotrlová, Veronika Šímová, Jaroslav Vlček, and Efstathios I. Meletis. 2020. "Microstructure of High Temperature Oxidation Resistant Hf6B10Si31C2N50 and Hf7B10Si32C2N44 Films" Coatings 10, no. 12: 1170. https://doi.org/10.3390/coatings10121170




