Interfacial Microstructure of FeCoNiCrAl0.1 High Entropy Alloy and Pure Copper Prepared by Explosive Welding
Abstract
:1. Introduction
2. Materials and Method
3. Results and Discussion
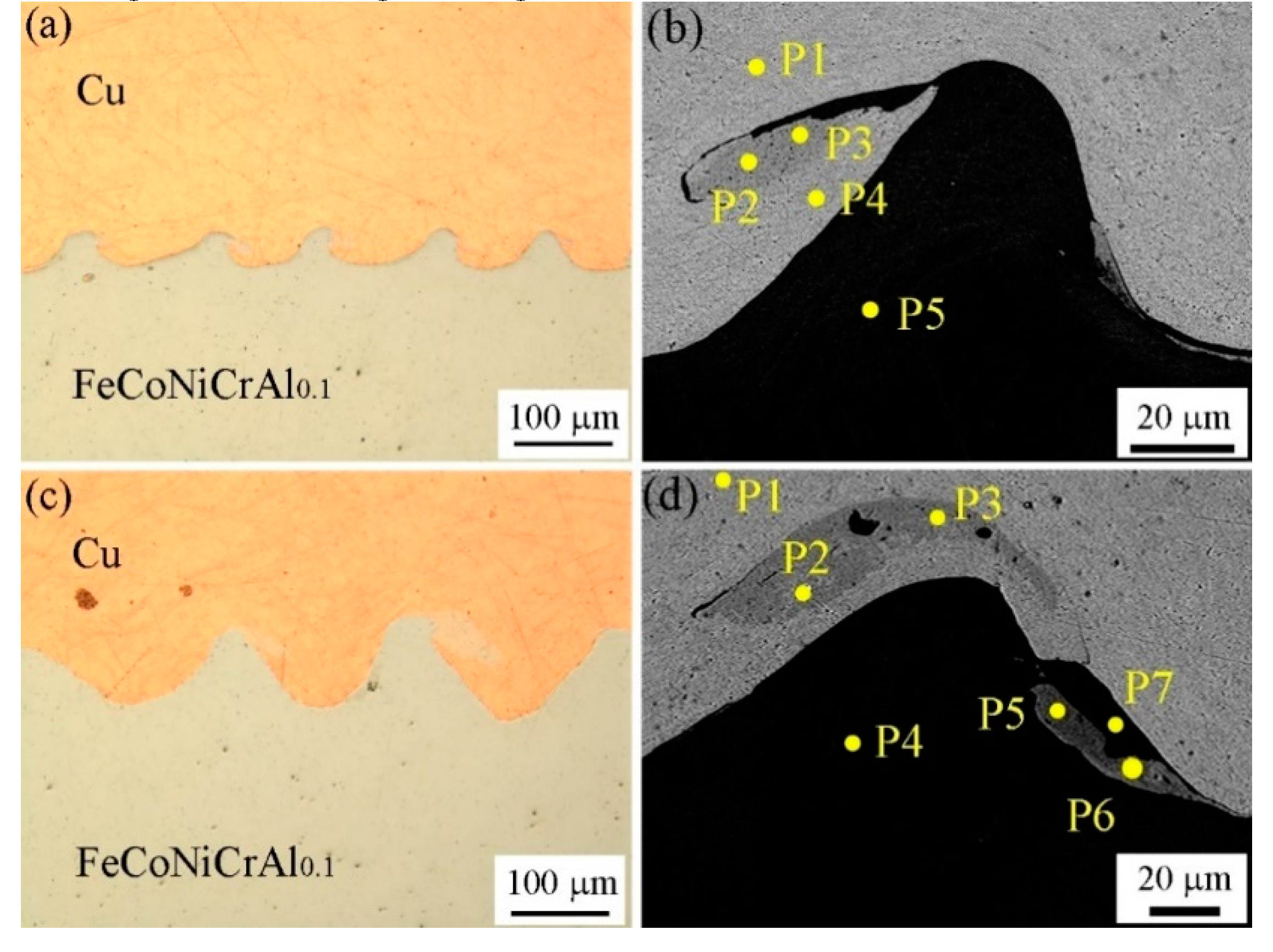
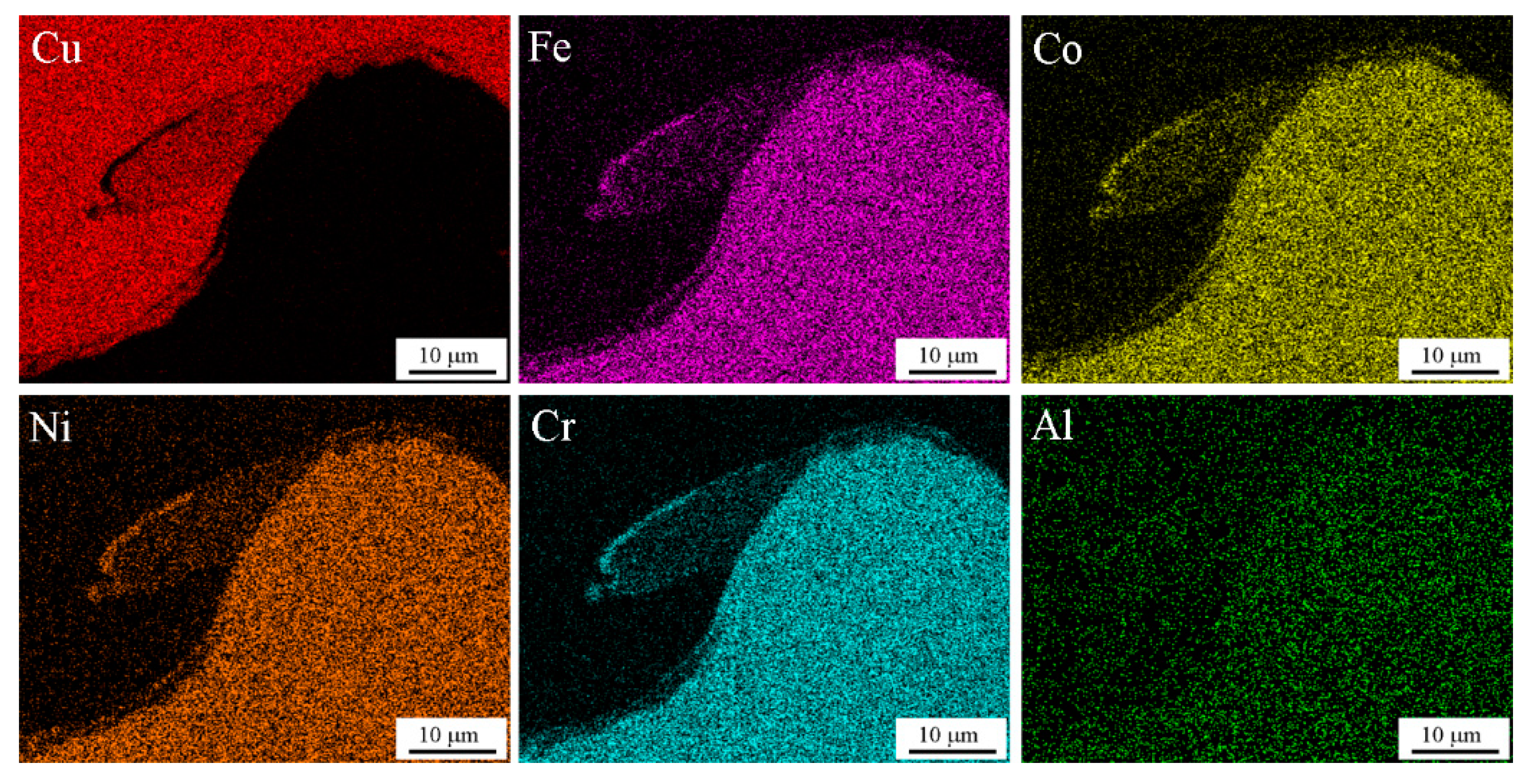
3.1. Interface Morphology
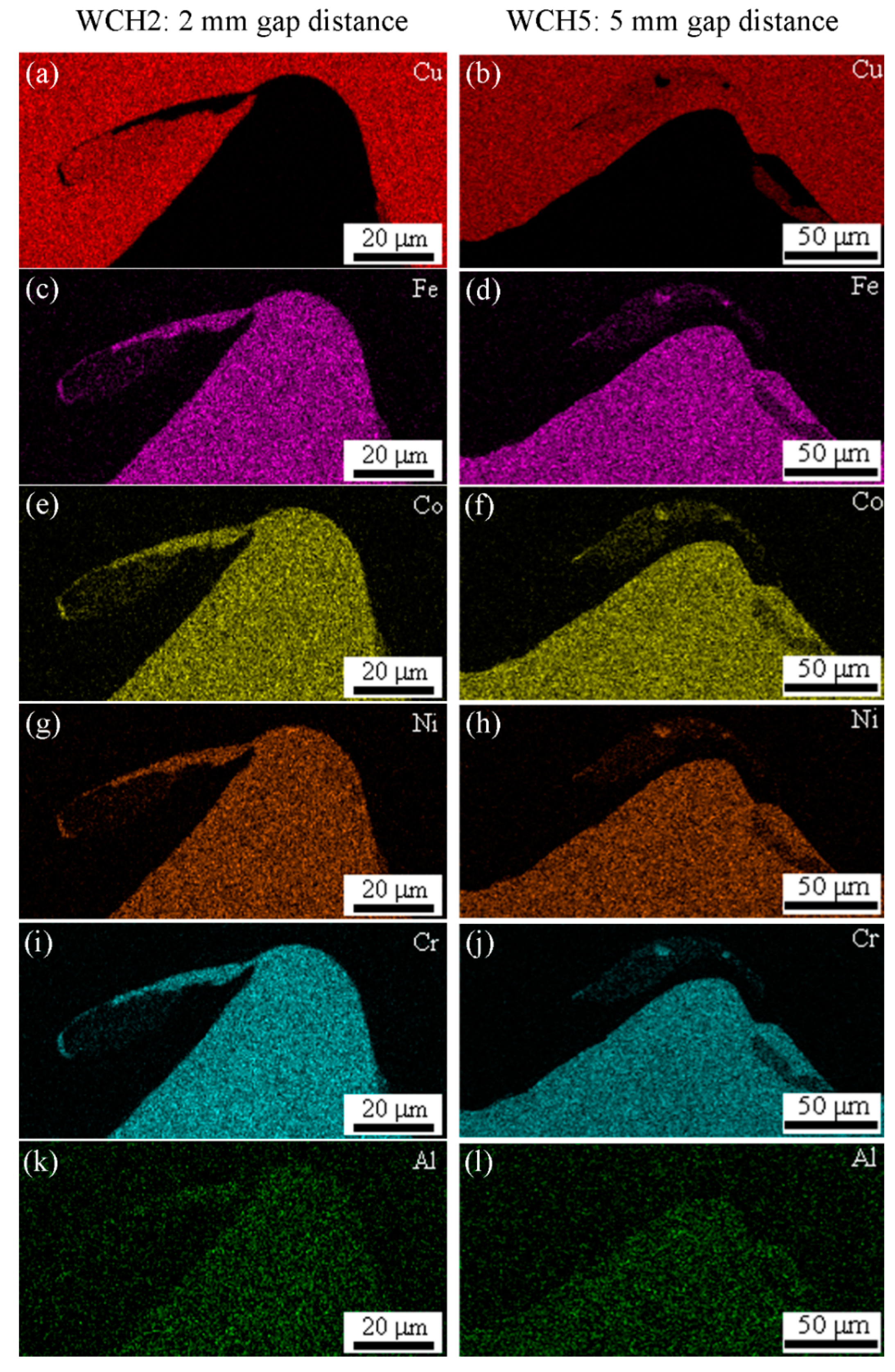
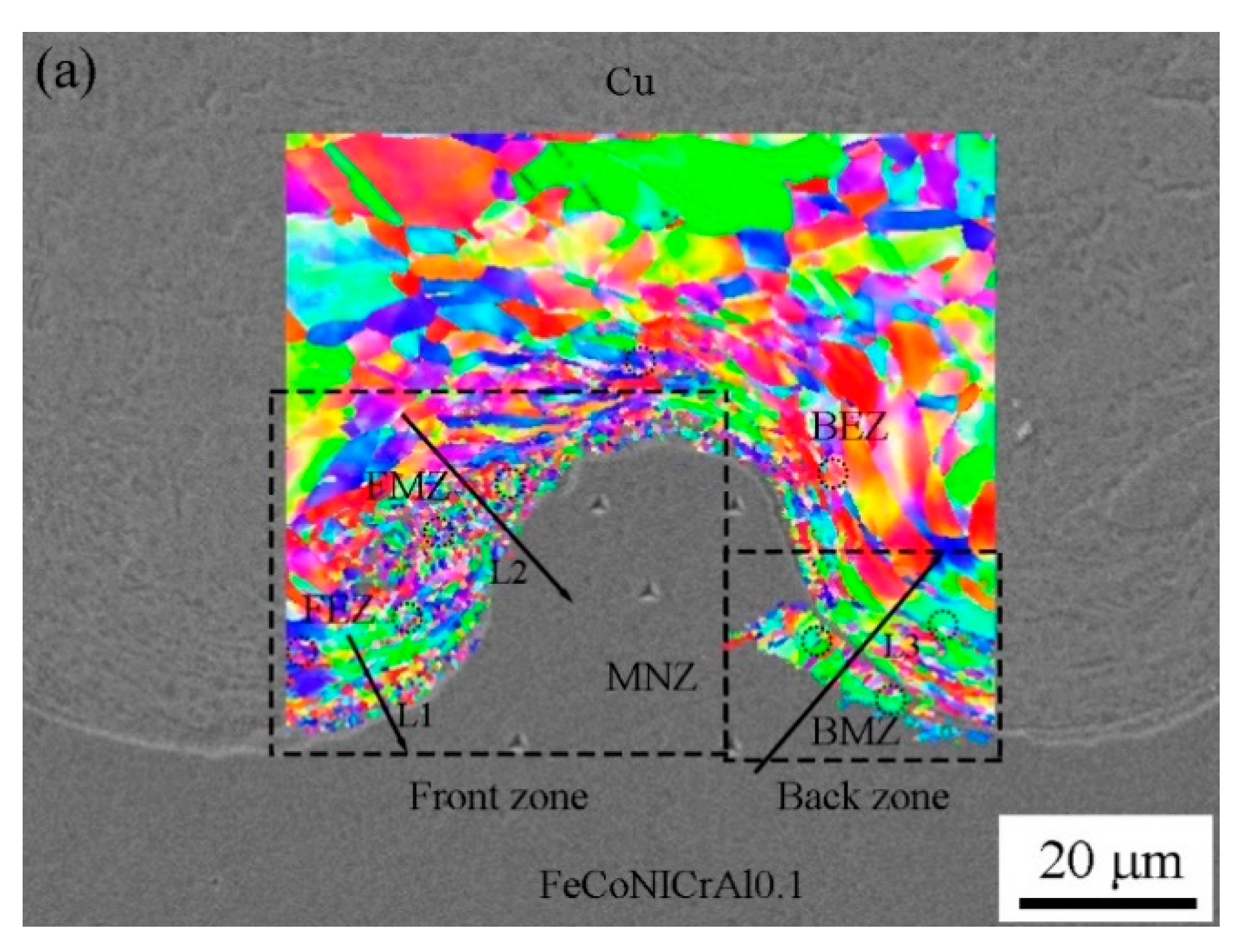
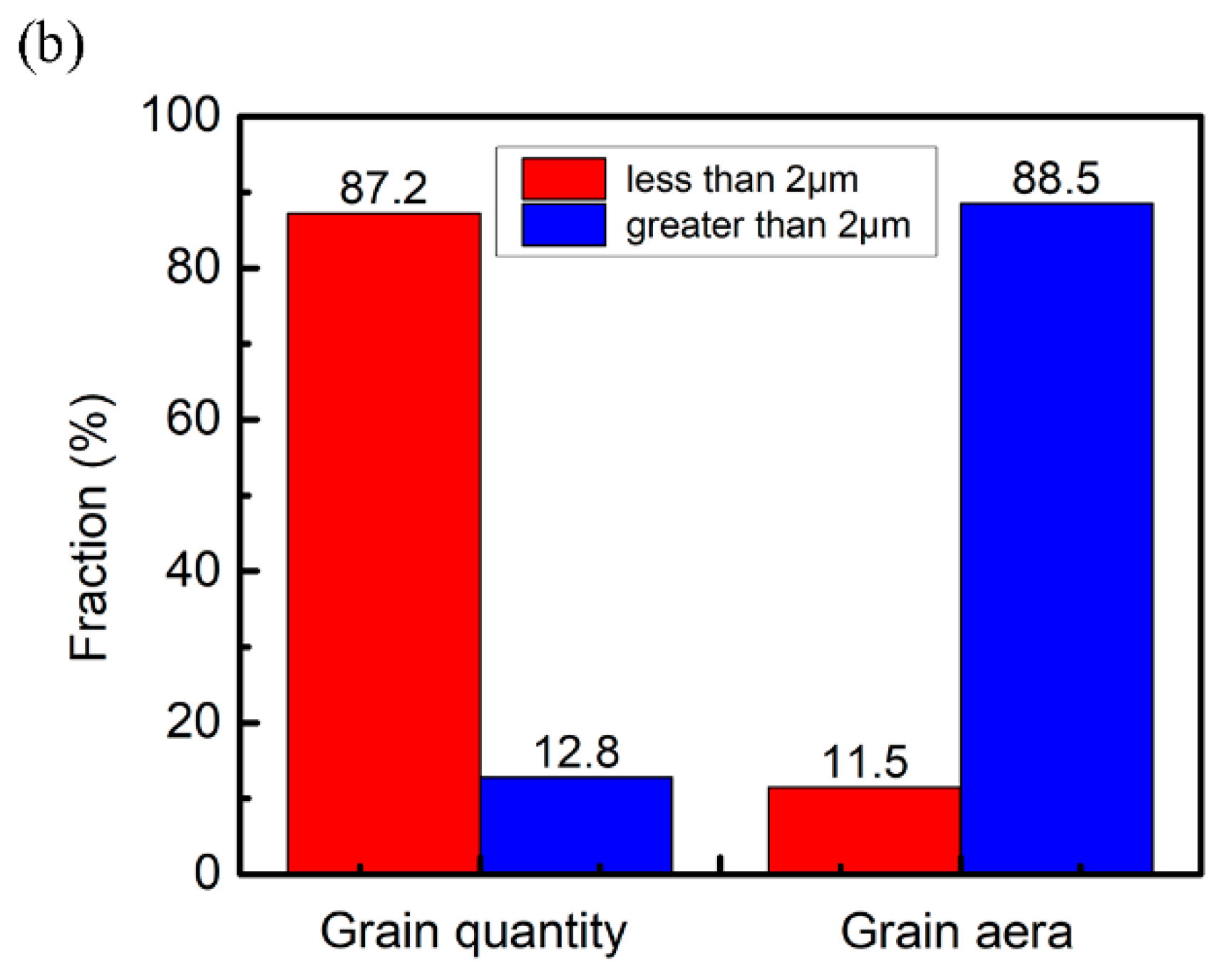
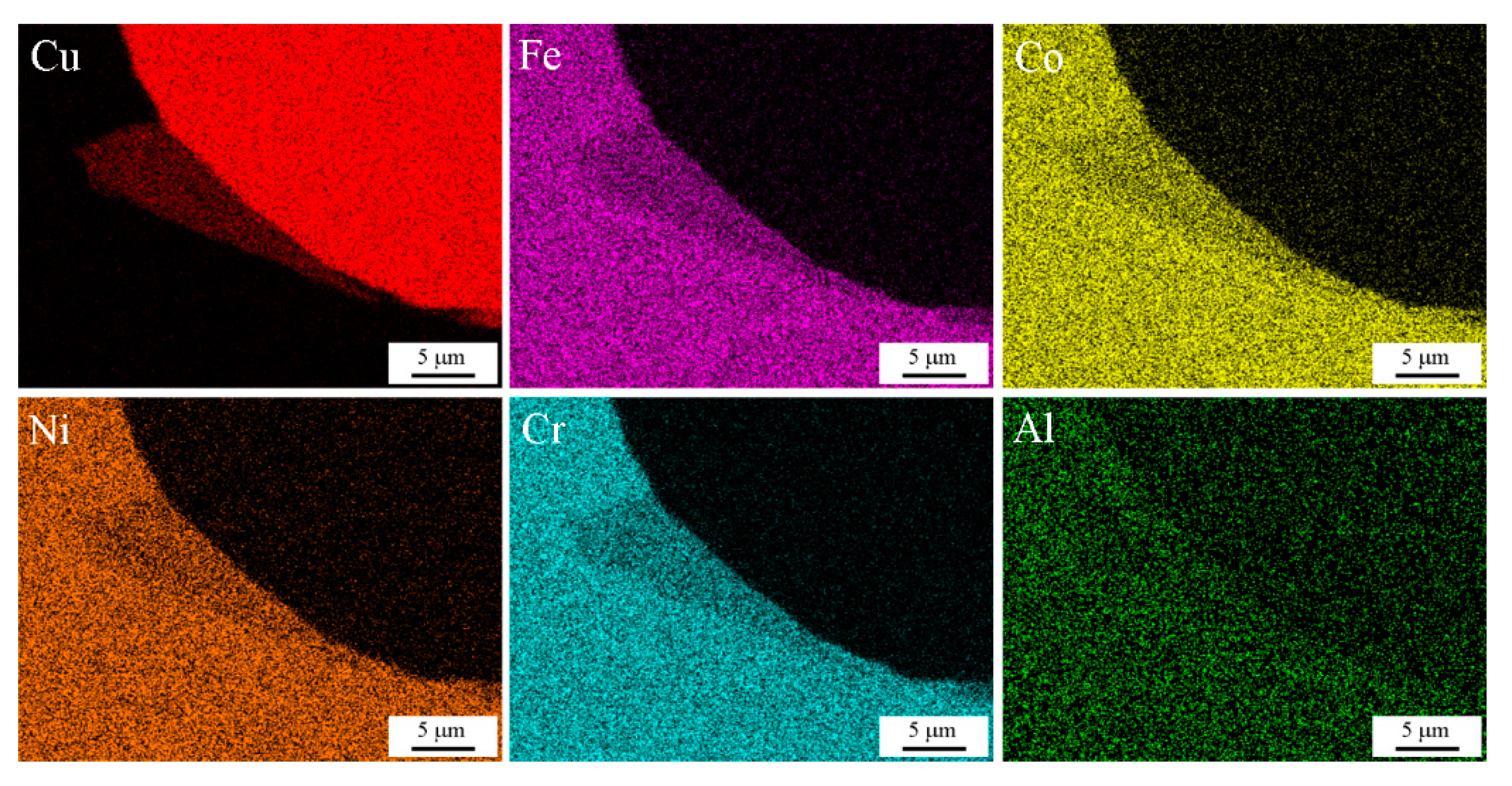
3.2. Microstructure Evolution
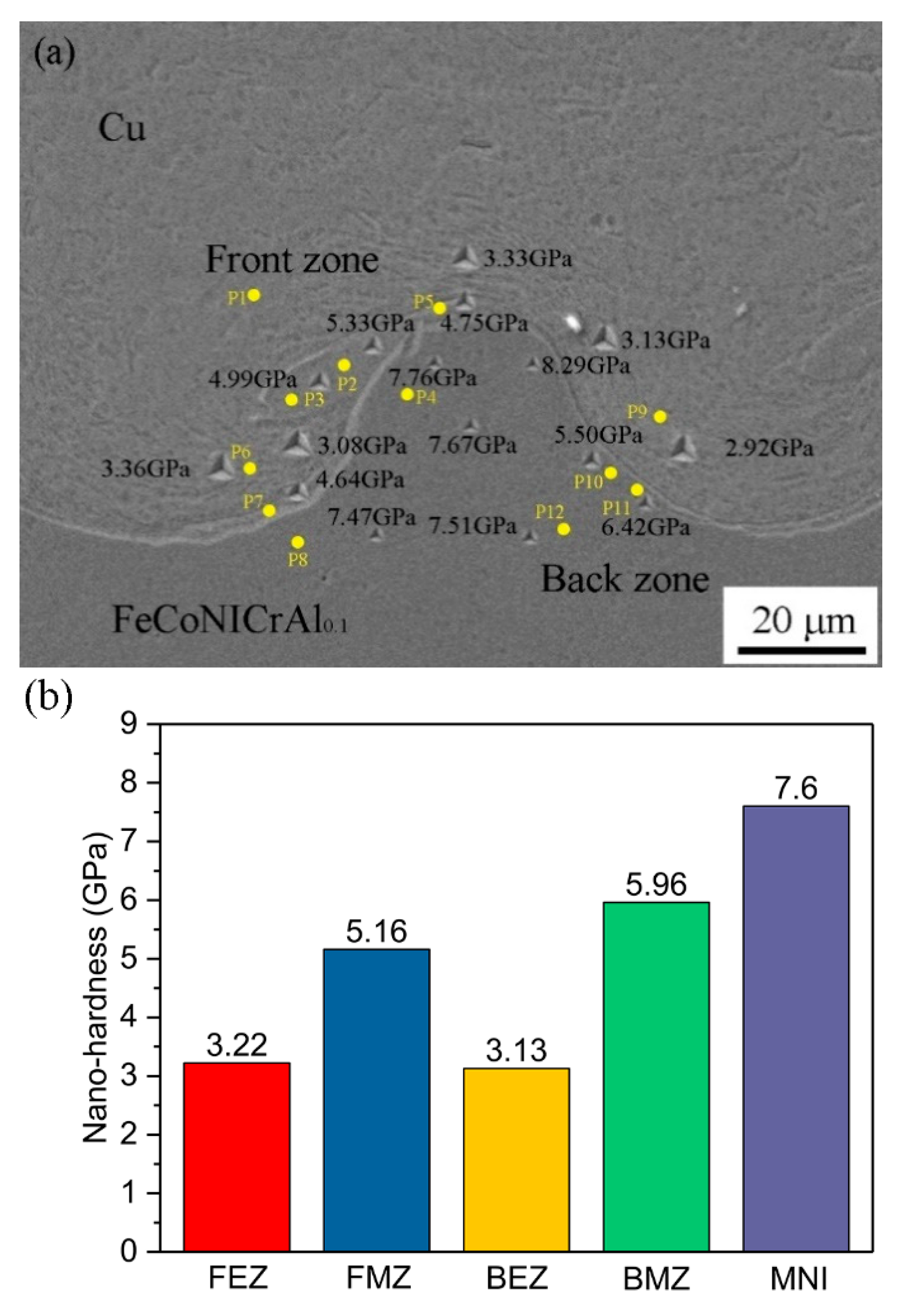
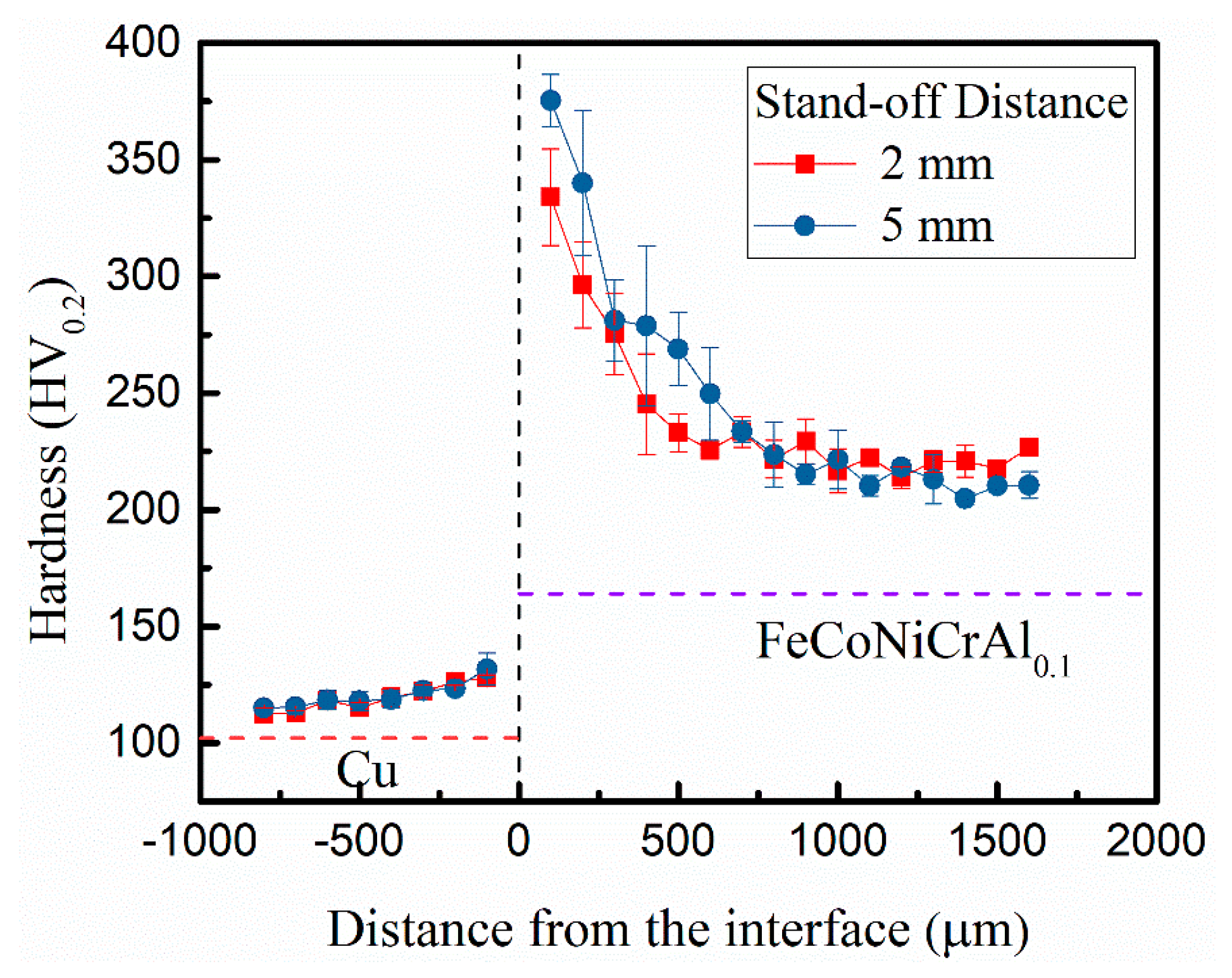
3.3. Hardness Distribution
4. Conclusions
- EXW was successfully employed to produce bimetallic composites containing HEAs and commercial copper alloy. Different wavy bonding interfaces could be obtained by EXW controlling the welding parameters. In addition, a defect-free explosive welding joint could be obtained with suitable welding parameters.
- The mixed region formed at the interface had a chemical composition in which the compositions of the two plates were mixed. At lower impact velocity, the vortex zone exhibited a uniform composition, with a particular mixing ratio depending on the location at the interface. However, the mixed region of HEA and dissimilar metals still exhibited a multi-element mixture and maintained crystal structure.
- Due to the action of the jet, the microstructure adjacent to the interface exhibited considerable grain deformation, which was elongated in the detonation direction. Due to the excellent heat-conducting property of copper, the cooling rates in the vortex regions at the front and rear of the waves were different, and fine and columnar grains are formed, respectively.
- The hardness was lower away from the interface and gradually increased near the interface due to microstructural evolution and plastic deformation. As the gap distance increased, the collision speed increased, causing the wavy interface to be increased and the hardness to also be increased due to more energy to be consumed at the interface after the collision between plates.
Author Contributions
Funding
Conflicts of Interest
References
- Zhang, Y.; Zuo, T.T.; Tang, Z.; Gao, M.C.; Dahmen, K.A.; Liaw, P.K.; Lu, Z.P. Microstructures and properties of high-entropy alloys. Prog. Mater. Sci. 2014, 61, 1–93. [Google Scholar] [CrossRef]
- George, E.P.; Raabe, D.; Ritchie, R.O. High-entropy alloys. Nat. Rev. Mater. 2019, 4, 515–534. [Google Scholar] [CrossRef]
- Guo, J.; Tang, C.; Rothwell, G.; Li, L.; Wang, Y.-C.; Yang, Q.; Ren, X. Welding of High Entropy Alloys—A Review. Entropy 2019, 21, 431. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hemphill, M.A.; Yuan, T.; Wang, G.Y.; Yeh, J.W.; Tsai, C.W.; Chuang, A.; Liaw, P.K. Fatigue behavior of Al0.5CoCrCuFeNi high entropy alloys. Acta Mater. 2012, 60, 5723–5734. [Google Scholar] [CrossRef]
- Pancikiewicz, K.; Swierczynska, A.; Hucko, P.; Tumidajewicz, M. Laser Dissimilar Welding of AISI 430F and AISI 304 Stainless Steels. Materials 2020, 13, 4540. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.H.; Shi, Z.F.; Deng, C.; Liu, Y.; Li, X. The Effect of Laser Offset Welding on Microstructure and Mechanical Properties of 301L to TA2 with and without Cu Intermediate Layer. Metals 2020, 10, 1138. [Google Scholar] [CrossRef]
- Cui, Y.; Liu, D.; Zhang, Y.; Deng, G.P.; Fan, M.Y.; Chen, D.; Sun, L.X.; Zhang, Z.W. The Microstructure and Mechanical Properties of TA1-Low Alloy Steel Composite Plate Manufactured by Explosive Welding. Metals 2020, 10, 663. [Google Scholar] [CrossRef]
- Arab, A.; Guo, Y.S.; Zhou, Q.; Chen, P.W. Joining AlCoCrFeNi high entropy alloys and Al-6061 by explosive welding method. Vacuum 2020, 174, 109221. [Google Scholar] [CrossRef]
- Wu, Z.; David, S.A.; Feng, Z.; Bei, H. Weldability of a high entropy CrMnFeCoNi alloy. Scr. Mater. 2016, 124, 81–85. [Google Scholar] [CrossRef] [Green Version]
- Zhu, Z.; Sun, Y.; Ng, F.; Goh, M.; Liaw, P.; Fujii, H.; Nguyen, Q.; Xu, Y.; Shek, C.; Nai, S. Friction-stir welding of a ductile high entropy alloy: Microstructural evolution and weld strength. Mater. Sci. Eng. A 2018, 711, 524–532. [Google Scholar] [CrossRef]
- Nam, H.; Park, C.; Moon, J.; Na, Y.; Kim, H.; Kang, N. Laser weldability of cast and rolled high-entropy alloys for cryogenic applications. Mater. Sci. Eng. A 2019, 742, 224–230. [Google Scholar] [CrossRef]
- Sokkalingam, R.; Mishra, S.; Cheethirala, S.R.; Muthupandi, V.; Sivaprasad, K. Enhanced Relative Slip Distance in Gas-Tungsten-Arc-Welded Al 0.5 CoCrFeNi High-Entropy Alloy. Metall. Mater. Trans. A 2017, 48, 3630–3634. [Google Scholar] [CrossRef]
- Chu, Q.; Zhang, M.; Li, J.; Yan, C. Experimental and numerical investigation of microstructure and mechanical behavior of titanium/steel interfaces prepared by explosive welding. Mater. Sci. Eng. A 2017, 689, 323–331. [Google Scholar] [CrossRef]
- Paul, H.; Miszczyk, M.; Chulist, R.; Prażmowski, M.; Morgiel, J.; Gałka, A.; Faryna, M.; Brisset, F. Microstructure and phase constitution in the bonding zone of explosively welded tantalum and stainless steel sheets. Mater. Des. 2018, 153, 177–189. [Google Scholar] [CrossRef]
- Findik, F. Recent developments in explosive welding. Mater. Des. 2011, 32, 1081–1093. [Google Scholar] [CrossRef]
- Zhu, Z.; Ng, F.L.; Qiao, J.; Liaw, P.K.; Chen, H.-C.; Nai, S.M.L.; Wei, J.; Bi, G.J. Interplay between microstructure and deformation behavior of a laser-welded CoCrFeNi high entropy alloy. Mater. Res. Express. 2019, 6, 046514V. [Google Scholar] [CrossRef]
- Zhang, T.; Wang, W.; Zhang, W.; Wei, Y.; Cao, X.; Yan, Z.; Zhou, J. Microstructure evolution and mechanical properties of an AA6061/AZ31B alloy plate fabricated by explosive welding. J. Alloy. Compd. 2018, 735, 1759–1768. [Google Scholar] [CrossRef]
- Mendes, R.; Ribeiro, J.; Plaksin, I.; Campos, J.; Tavares, B. Differences between the detonation behavior of emulsion explosives sensitized with glass or with polymeric micro-balloons. J. Phys. Conf. Ser. 2014, 500, 052030. [Google Scholar] [CrossRef]
- Li, X.; Ma, H.; Shen, Z. Research on explosive welding of aluminum alloy to steel with dovetail grooves. Mater. Des. 2015, 87, 815–824. [Google Scholar] [CrossRef]
- Athar, M.H.; Tolaminejad, B. Weldability window and the effect of interface morphology on the properties of Al/Cu/Al laminated composites fabricated by explosive welding. Mater. Des. 2015, 86, 516–525. [Google Scholar] [CrossRef]
- Yang, M.; Ma, H.; Shen, Z. Study on self-restrained explosive welding with high energy efficiency. Int. J. Adv. Manuf. Technol. 2018, 99, 3123–3132. [Google Scholar] [CrossRef]
- Deribas, A.; Zakharenko, I. Surface effects with oblique collisions between metallic plates. Combust. Explos. Shock Waves 1974, 10, 358–367. [Google Scholar] [CrossRef]
- Carpenter, S.; Wittman, R. Explosion welding. Annu. Rev. Mater. Sci. 1975, 5, 177–199. [Google Scholar] [CrossRef]
- Cowan, G.; Bergmann, O.; Holtzman, A. Mechanism of bond zone wave formation in explosion-clad metals. Metall. Mater. Trans. B 1971, 2, 3145–3155. [Google Scholar] [CrossRef]
- Abrahamson, G.R. Permanent periodic surface deformations due to a traveling jet. J. Appl. Mech. 1961, 28, 519–528. [Google Scholar] [CrossRef]
- Cooper, P.W. Explosives Engineering; John Wiley & Sons: Hoboken, NJ, USA, 2018. [Google Scholar]
- Flis, W.J. A Lagrangian approach to modeling the acceleration of metal by explosives. In Proceedings of the 17th Southeastern Conference on Theoretical and Applied Mechanics, Hot Springs National Park, AR, USA, 10–12 April 1994. [Google Scholar]
- Skuza, W.; Paul, H.; Berent, K.; Prazmowski, M.; Bobrowski, P. In Microstructure and Mechanical Properties of Ti/Cu Clads Manufactured by Explosive Bonding at Different Stand-Off Distances. Key Eng. Mater. 2016, 716, 464–471. [Google Scholar] [CrossRef]
- Liang, H.L.; Luo, N.; Shen, T.; Sun, X.; Fan, X.R.; Cao, Y. Experimental and numerical simulation study of Zr-based BMG/Al composites manufactured by underwater explosive welding. J. Mater. Res. Technol. 2020, 9, 1539–1548. [Google Scholar] [CrossRef]
- Bataev, I.; Tanaka, S.; Zhou, Q.; Lazurenko, D.; Junior, A.J.; Bataev, A.; Hokamoto, K.; Mori, A.; Chen, P. Towards better understanding of explosive welding by combination of numerical simulation and experimental study. Mater. Des. 2019, 169, 107649. [Google Scholar] [CrossRef]
- Zhang, H.; Jiao, K.X.; Zhang, J.L.; Liu, J. Comparisons of the microstructures and micro-mechanical properties of copper/steel explosive-bonded wave interfaces. Mater. Sci. Eng. A 2019, 756, 430–441. [Google Scholar] [CrossRef]
- Bataev, I.; Lazurenko, D.; Tanaka, S.; Hokamoto, K.; Bataev, A.; Guo, Y.; Jorge, A., Jr. High cooling rates and metastable phases at the interfaces of explosively welded materials. Acta Mater. 2017, 135, 277–289. [Google Scholar] [CrossRef]
- Ning, J.; Zhang, L.-J.; Xie, M.-X.; Yang, H.-X.; Yin, X.-Q.; Zhang, J.-X. Microstructure and property inhomogeneity investigations of bonded Zr/Ti/steel trimetallic sheet fabricated by explosive welding. J. Alloy. Compd. 2017, 698, 835–851. [Google Scholar] [CrossRef]
- Yeh, J.W.; Chen, S.K.; Lin, S.J.; Gan, J.Y.; Chin, T.S.; Shun, T.T.; Tsau, C.H.; Chang, S.Y. Nanostructured high-entropy alloys with multiple principal elements: Novel alloy design concepts and outcomes. Adv. Eng. Mater. 2004, 6, 299–303. [Google Scholar] [CrossRef]
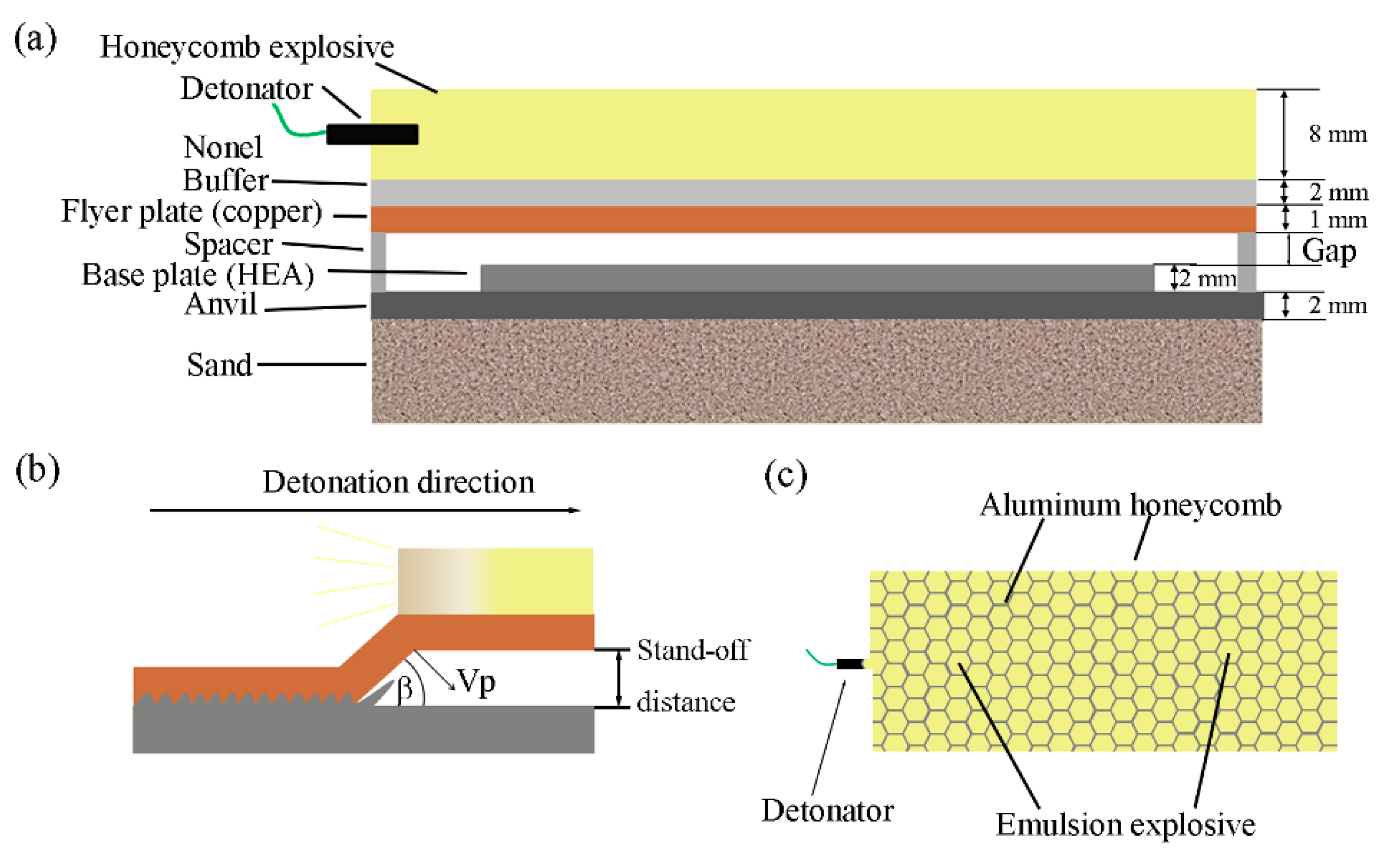
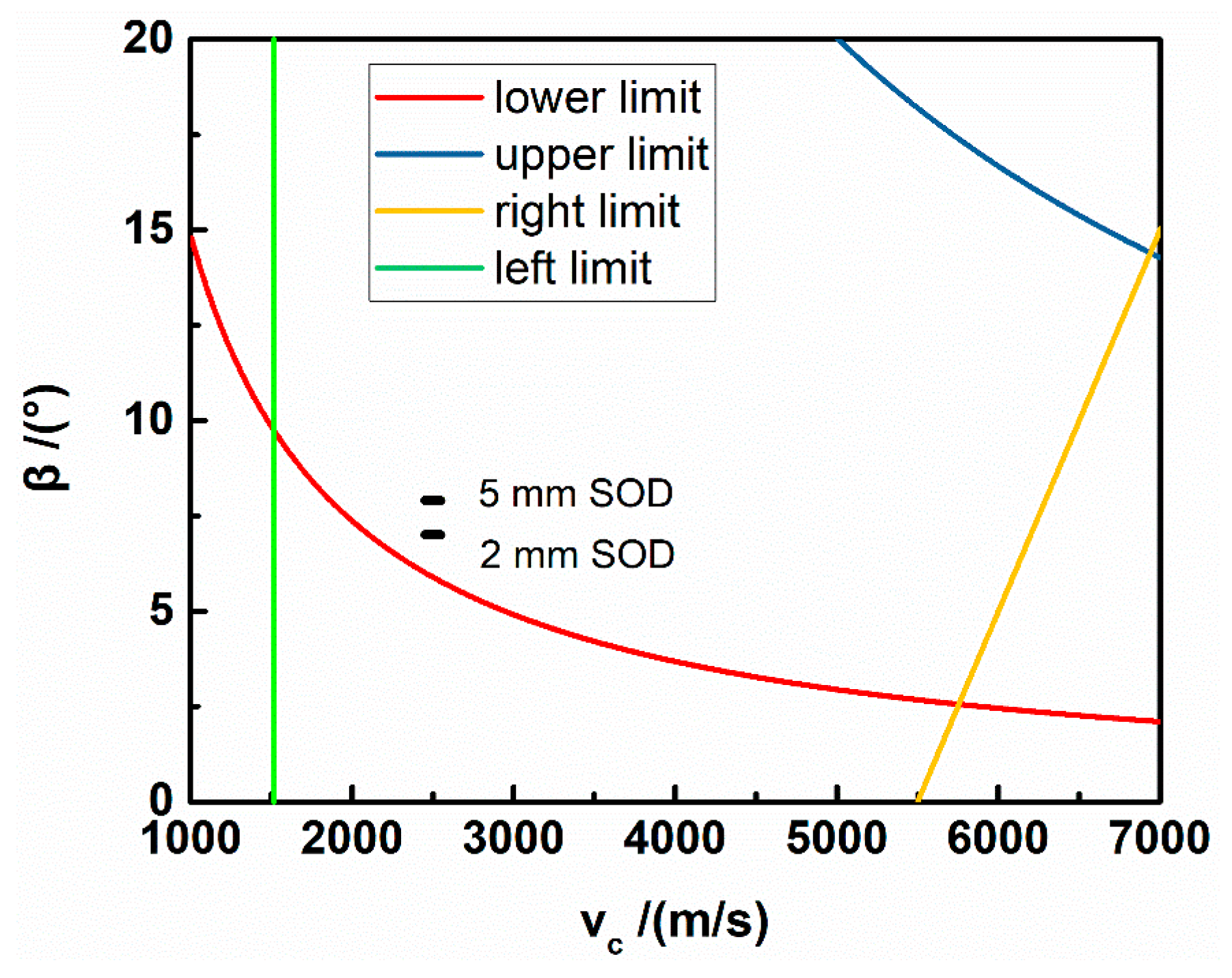


| Component | NH4NO3 | NaNO3 | H2O | C18H38 | C24H44O6 | C12H26 |
|---|---|---|---|---|---|---|
| Mass fraction | 75% | 10% | 8% | 4% | 2% | 1% |
| Point Number/ Chemical Element | Cu | Fe | Co | Ni | Cr | Al |
|---|---|---|---|---|---|---|
| 1 | 100 | – | – | – | – | – |
| 2 | 88.2 | 3.0 | 3.0 | 2.6 | 2.7 | 0.5 |
| 3 | 81.7 | 4.7 | 4.6 | 4.5 | 4.4 | 0.2 |
| 4 | 100 | – | – | – | – | – |
| 5 | – | 23.7 | 23.2 | 24.3 | 25.9 | 2.9 |
| Point Number/ Chemical Element | Cu | Fe | Co | Ni | Cr | Al |
|---|---|---|---|---|---|---|
| 1 | 100 | – | – | – | – | – |
| 2 | 79.9 | 4.7 | 4.9 | 5.1 | 4.8 | 0.6 |
| 3 | 70.5 | 7.1 | 6.8 | 7.5 | 7.4 | 0.7 |
| 4 | – | 24.1 | 24.2 | 24.4 | 25.1 | 2.2 |
| 5 | 44.6 | 13.6 | 13.6 | 13.6 | 13.2 | 1.4 |
| 6 | 70.6 | 6.9 | 7.5 | 7.0 | 7.4 | 0.6 |
| 7 | – | 25.2 | 24.9 | 23.7 | 23.7 | 2.5 |
| Point Number/ Chemical Element | Cu | Fe | Co | Ni | Cr | Al |
|---|---|---|---|---|---|---|
| 1 | 100 | – | – | – | – | – |
| 2 | 76.79 | 5.48 | 5.44 | 5.67 | 5.73 | 0.88 |
| 3 | 76.49 | 5.61 | 5.54 | 5.63 | 6.08 | 0.66 |
| 4 | – | 24.63 | 23.84 | 23.38 | 25.50 | 2.65 |
| 5 | 76.49 | 5.61 | 5.54 | 5.63 | 6.08 | 0.66 |
| 6 | 100 | – | – | – | – | – |
| 7 | 11.22 | 21.37 | 21.20 | 21.37 | 22.64 | 2.20 |
| 8 | – | 24.49 | 24.39 | 23.29 | 25.17 | 2.66 |
| 9 | 100 | – | – | – | – | – |
| 10 | 25.14 | 18.21 | 17.96 | 18.15 | 18.56 | 2.07 |
| 11 | 27.82 | 17.44 | 17.34 | 17.40 | 18.06 | 1.94 |
| 12 | – | 25.37 | 25.47 | 23.35 | 23.76 | 2.05 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tian, Q.; Liang, H.; Zhao, Y.; Ma, H.; Shen, Z.; Sun, Y.; Yang, M. Interfacial Microstructure of FeCoNiCrAl0.1 High Entropy Alloy and Pure Copper Prepared by Explosive Welding. Coatings 2020, 10, 1197. https://doi.org/10.3390/coatings10121197
Tian Q, Liang H, Zhao Y, Ma H, Shen Z, Sun Y, Yang M. Interfacial Microstructure of FeCoNiCrAl0.1 High Entropy Alloy and Pure Copper Prepared by Explosive Welding. Coatings. 2020; 10(12):1197. https://doi.org/10.3390/coatings10121197
Chicago/Turabian StyleTian, Qichao, Hanliang Liang, Yang Zhao, Honghao Ma, Zhaowu Shen, Yuling Sun, and Ming Yang. 2020. "Interfacial Microstructure of FeCoNiCrAl0.1 High Entropy Alloy and Pure Copper Prepared by Explosive Welding" Coatings 10, no. 12: 1197. https://doi.org/10.3390/coatings10121197




