Impurity Phases and Optoelectronic Properties of CuSbSe2 Thin Films Prepared by Cosputtering Process for Absorber Layer in Solar Cells
Abstract
1. Introduction
2. Experimental Details
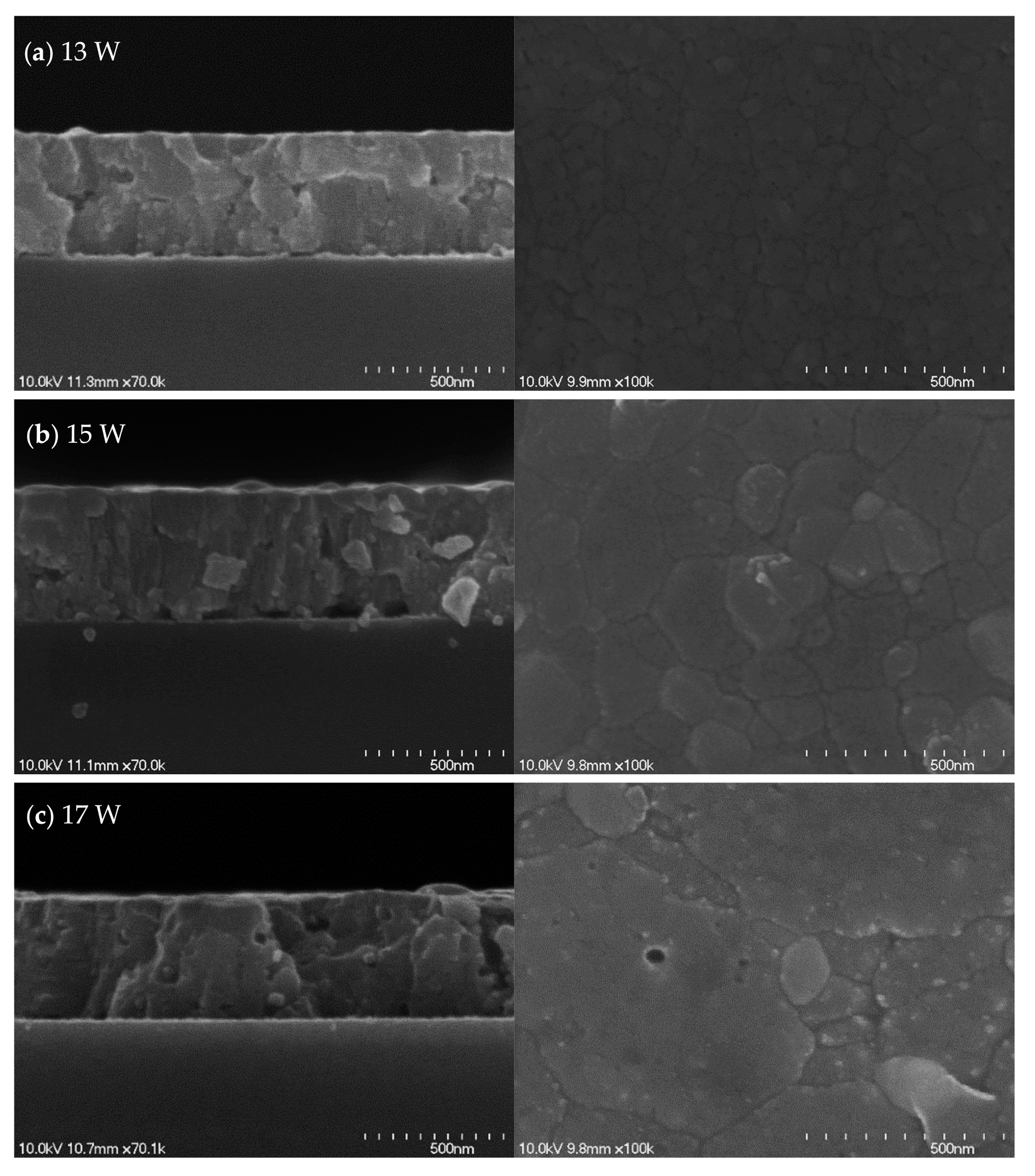
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Nakamura, M.; Yamaguchi, K.; Kimoto, Y.; Yasaki, Y.; Kato, T.; Sugimoto, H. Cd-free Cu(In,Ga)(Se,S)2 thin-film solar cell with record efficiency of 23.35%. IEEE J. Photovolt. 2019, 9, 1863–1867. [Google Scholar] [CrossRef]
- Ravindirana, M.; Praveenkumar, C. Status review and the future prospects of CZTS based solar cell—A novel approach on the device structure and material modeling for CZTS based photovoltaic device. Renew. Sustain. Energy Rev. 2018, 94, 317–329. [Google Scholar] [CrossRef]
- Schorr, S.; Gurieva, G.; Guc, M.; Dimitrievska, M.; Pérez-Rodríguez, A.; Izquierdo-Roca, V.; Schnohr, C.S.; Kim, J.; Jo, W.; Merino, J.M. Point defects, compositional fluctuations, and secondary phases in non-stoichiometric kesterites. J. Phys. Energy 2020, 2, 012002. [Google Scholar] [CrossRef]
- Wang, W.; Winkler, M.T.; Gunawan, O.; Gokmen, T.; Todorov, T.K.; Zhu, Y.; Mitzi, D.B. Device characteristics of CZTSSe thin-film solar cells with 12.6% efficiency. Adv. Energy Mater. 2014, 4, 1301465. [Google Scholar] [CrossRef]
- Yousefi, M.; Minbashi, M.; Monfared, Z.; Memarian, N.; Hajjiah, A. Improving the efficiency of CZTSSe solar cells by engineering the lattice defects in the absorber layer. Sol. Energy 2020, 208, 884–893. [Google Scholar] [CrossRef]
- Peccerillo, E.; Durose, K. Copper–antimony and copper–bismuth chalcogenides—Research opportunities and review for solar photovoltaics. MRS Energy Sustain. 2018, 5, E13. [Google Scholar] [CrossRef]
- Wada, T.; Maeda, T. Optical properties and electronic structures of CuSbS2, CuSbSe2, and CuSb(S1−xSex)2 solid solution. Phys. Status Solidi C 2017, 14, 1600196. [Google Scholar] [CrossRef]
- Kumar, M.; Persson, C. Cu(Sb,Bi)(S,Se)2 as Indium-free absorber material with high optical efficiency. Energy Procedia 2014, 44, 176–183. [Google Scholar] [CrossRef]
- Welch, A.W.; Baranowski, L.L.; Zawadzki, P.; Lany, S.; Wolden, C.A.; Zakutayev, A. CuSbSe2 photovoltaic devices with 3% efficiency. Appl. Phys. Express 2015, 8, 082301. [Google Scholar] [CrossRef]
- Xue, D.-J.; Yang, B.; Yuan, Z.-K.; Wang, G.; Liu, X.; Zhou, Y.; Hu, L.; Pan, D.; Chen, S.; Tang, J. CuSbSe2 as a potential photovoltaic absorber material: Studies from theory to experiment. Adv. Energy Mater. 2015, 5, 1501203. [Google Scholar] [CrossRef]
- Li, Z.; Liang, X.; Li, G.; Liu, H.; Zhang, H.; Guo, J.; Chen, J.; Shen, K.; San, X.; Yu, W.; et al. 9.2%-efficient core-shell structured antimony selenide nanorod array solar cells. Nat. Commun. 2019, 10, 125. [Google Scholar] [CrossRef] [PubMed]
- Skoug, E.J.; Morelli, D.T. Role of lone-pair electrons in producing minimum thermal conductivity in nitrogen-group chalcogenide compounds. Phys. Rev. Lett. 2011, 107, 235901. [Google Scholar] [CrossRef] [PubMed]
- Eraky, M.S.; Sanad, M.M.S.; El-Sayed, E.M.; Shenouda, A.Y.; El-Sherefy, E.-S. Phase transformation and photoelectrochemical characterization of Cu/Bi and Cu/Sb based selenide alloys as promising photoactive electrodes. AIP Adv. 2019, 9, 115115. [Google Scholar] [CrossRef]
- Wang, C.; Yang, B.; Ding, R.; Chen, W.; Kondrotas, R.; Zhao, Y.; Lu, S.; Li, Z.; Tang, J. Reactive close-spaced sublimation processed CuSbSe2 thin films and their photovoltaic application. APL Mater. 2018, 6, 084801. [Google Scholar] [CrossRef]
- Tiwari, K.J.; Vinod, V.; Subrahmanyam, A.; Malar, P. Growth and characterization of chalcostibite CuSbSe2 thin films for photovoltaic application. Appl. Surf. Sci. 2017, 418, 216–224. [Google Scholar] [CrossRef]
- Zhang, D.; Yang, J.; Jiang, Q.; Fu, L.; Xiao, Y.; Luo, Y.; Zhou, Z. Ternary CuSbSe2 chalcostibite: Facile synthesis, electronic-structure and thermoelectric performance enhancement. J. Mater. Chem. A 2016, 4, 4188–4193. [Google Scholar] [CrossRef]
- Ismailova, E.N.; Mashadieva, L.F.; Bakhtiyarly, I.B.; Babanly, M.B. Phase equilibria in the Cu2Se-SnSe-CuSbSe2 system. Russ. J. Inorg. Chem. 2019, 64, 801–809. [Google Scholar] [CrossRef]
- Yang, B.; Wang, C.; Yuan, Z.; Chen, S.; He, Y.; Song, H.; Ding, R.; Zhao, Y.; Tang, J. Hydrazine solution processed CuSbSe2: Temperature dependent phase and crystal orientation evolution. Sol. Energy Mater. Sol. Cells 2017, 168, 112–118. [Google Scholar] [CrossRef]
- Yan, H.; Xiao, R.; Pei, Y.; Yang, K.; Li, B. Structural, electrical and optical characteristics of CuSbSe2 films prepared by pulsed laser deposition and magnetron sputtering processes. J. Mater. Sci. Mater. Electron. 2020, 31, 644–651. [Google Scholar] [CrossRef]
- Oh, S.; Park, Y.S.; Ko, P.J.; Kim, N.-H. Effects of rapid thermal treatment on properties of magnetron-sputtered NiO thin films for supercapacitor applications. J. Nanosci. Nanotechnol. 2018, 18, 6213–6219. [Google Scholar] [CrossRef]
- Welch, A.W.; Baranowski, L.L.; Peng, H.; Hempel, H.; Eichberger, R.; Unold, T.; Lany, S.; Wolden, C.; Zakutayev, A. Trade-offs in thin film solar cells with layered chalcostibite photovoltaic absorbers. Adv. Energy Mater. 2017, 7, 1601935. [Google Scholar] [CrossRef]
- Cang, Q.; Guo, H.; Jia, X.; Ning, H.; Ma, C.; Zhang, J.; Yuan, N.; Ding, J. Enhancement in the efficiency of Sb2Se3 solar cells by adding low lattice mismatch CuSbSe2 hole transport layer. Sol. Energy 2020, 199, 19–25. [Google Scholar] [CrossRef]
- Gilić, M.; Petrović, M.; Kostić, R.; Stojanović, D.; Barudžija, T.; Mitrić, M.; Romčević, N.; Ralević, U.; Trajić, J.; Romčević, M.; et al. Structural and optical properties of CuSe2 nanocrystals formed in thin solid Cu–Se film. Infrared Phys. Technol. 2016, 76, 276–284. [Google Scholar] [CrossRef]
- Yoo, M.H.; Ko, P.J.; Kim, N.-H.; Lee, H.-Y. Cu(In,Ga)Se2 thin films annealed using a continuous wave Nd:YAG laser (λo = 532 nm): Effects of laser-annealing time. J. Korean Phys. Soc. 2017, 71, 1038–1047. [Google Scholar] [CrossRef]
- Moon, E.-A.; Jun, Y.-K.; Kim, N.-H.; Lee, W.-S. Heavily-doped ZnO:Al thin films prepared by using magnetron co-sputtering: Optical and electrical properties. J. Korean Phys. Soc. 2016, 69, 220–225. [Google Scholar] [CrossRef]
- Sun, X.H.; Pan, Y.P.; Dong, L.; Zhao, M.L.; Wan, R.X.; Gu, H.Q.; Li, D.J. Modulation period of Ag deposition on co-sputtered TiN-Ag leading to different microstructures: Implication on mechanical properties and living cells growth. Surf. Coat. Technol. 2017, 326, 382–387. [Google Scholar] [CrossRef]
- Joung, Y.-H.; Kang, H.I.; Kim, J.H.; Lee, H.-S.; Lee, J.; Choi, W.S. SiC formation for a solar cell passivation layer using an RF magnetron co-sputtering system. Nanoscale Res. Lett. 2012, 7, 22. [Google Scholar] [CrossRef]
- Wen, X.; Chen, C.; Lu, S.; Li, K.; Kondrotas, R.; Zhao, Y.; Chen, W.; Gao, L.; Wang, C.; Zhang, J.; et al. Vapor transport deposition of antimony selenide thin film solar cells with 7.6% efficiency. Nat. Commun. 2018, 9, 2179. [Google Scholar] [CrossRef]
- Dabaa, B.; Srouji, F.; Brgol, M. Electrical, structural and optical properties of Cu3SbSe4 with high thermoelectric performance. Nano Sci. Nano Technol. Indian J. 2018, 12, 124. Available online: https://www.tsijournals.com/articles/electrical-structural-and-optical-properties-of-cu3sbse4-with-high-thermoelectric-performance-13738.html (accessed on 1 November 2020).
- Tyagi, K.; Gahtori, B.; Bathula, S.; Srivastava, A.K.; Shukla, A.K.; Auluck, S.; Dhar, A. Thermoelectric properties of Cu3SbSe3 with intrinsically ultralow lattice thermal conductivity. J. Mater. Chem. A 2014, 2, 15829–15835. [Google Scholar] [CrossRef]
- Wei, T.-R.; Wu, C.-F.; Sun, W.; Pan, Y.; Li, J.-F. Is Cu3SbSe3 a promising thermoelectric material? RSC Adv. 2015, 5, 42848–42854. [Google Scholar] [CrossRef]
- Ko, T.-Y.; Shellaiah, M.; Sun, K.W. Thermal and thermoelectric transport in highly resistive single Sb2Se3 nanowires and nanowire bundles. Sci. Rep. 2016, 6, 35086. [Google Scholar] [CrossRef]
- Chen, S.; Qiao, X.; Zheng, Z.; Cathelinaud, M.; Ma, H.; Fan, X.; Zhang, X. Enhanced electrical conductivity and photoconductive properties of Sn-doped Sb2Se3 crystals. J. Mater. Chem. C 2018, 6, 6465–6470. [Google Scholar] [CrossRef]
- Liu, Y.-Q.; Zhang, M.; Wang, F.-X.; Pan, G.-B. Facile microwave-assisted synthesis of uniform Sb2Se3 nanowires for high performance photodetectors. J. Mater. Chem. A 2014, 2, 240–244. [Google Scholar]
- Pattini, F.; Rampino, S.; Mezzadri, F.; Calestani, D.; Spaggiari, G.; Sidoli, M.; Delmonte, D.; Sala, A.; Gilioli, E.; Mazzer, M. Role of the substrates in the ribbon orientation of Sb2Se3 films grown by low-temperature pulsed electron deposition. Sol. Energy Mater. Sol. Cells 2020, 218, 110724. [Google Scholar] [CrossRef]
- Ghosh, S.; Moreira, M.V.B.; Fantini, C.; Gonzalez, J.C. Growth and optical properties of nanocrystalline Sb2Se3 thin-films for the application in solar-cells. Sol. Energy 2020, 211, 613–621. [Google Scholar] [CrossRef]
- Hamrouni, R.; Segmane, N.E.H.; Abdelkader, D.; Amara, A.; Drici, A.; Bououdina, M.; Akkari, F.C.; Khemiri, N.; Bechiri, L.; Kanzari, M.; et al. Linear and non linear optical properties of Sb2Se3 thin films elaborated from nano-crystalline mechanically alloyed powder. Appl. Phys. A 2018, 124, 861. [Google Scholar] [CrossRef]
- Hsiang, H.-I.; Yang, C.-T.; Tu, J.-H. Characterization of CuSbSe2 crystallites synthesized using a hot injection method. RSC Adv. 2016, 6, 99297–99305. [Google Scholar] [CrossRef]
- Riha, S.C.; Johnson, D.C.; Prieto, A.L. Cu2Se nanoparticles with tunable electronic properties due to a controlled solid-state phase transition driven by copper oxidation and cationic conduction. J. Am. Chem. Soc. 2011, 133, 1383–1390. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, J.; Gu, E.; Cao, T.; Su, Z.; Jiang, L.; Yan, C.; Hao, X.; Liu, F.; Liu, Y. Colloidal synthesis and characterisation of Cu3SbSe3 nanocrystals. J. Mater. Chem. A 2014, 2, 6363–6367. [Google Scholar] [CrossRef]
- Biesinger, M.C. Advanced analysis of copper X-ray photoelectron spectra. Surf. Interface Anal. 2017, 49, 1325–1334. [Google Scholar] [CrossRef]
- Nyholm, R.; Martensson, N.; Lebugle, A.; Axelsson, U. Auger and Coster-Kronig broadening effects in the 2p and 3p photoelectron spectra from the metals 22Ti-30Zn. J. Phys. F Met. Phys. 1981, 11, 1727–1733. [Google Scholar] [CrossRef]
- Qiao, J.; Zhao, Y.; Jin, Q.; Tan, J.; Kang, S.; Qiu, J.; Tai, K. Tailoring nanoporous structures in Bi2Te3 thin films for improved thermoelectric performance. ACS Appl. Mater. Interfaces 2019, 11, 38075–38083. [Google Scholar] [CrossRef]
- Krishnan, B.; Shaji, S.; Ornelas, R.E. Progress in development of copper antimony sulfide thin films as an alternative material for solar energy harvesting. J. Mater. Sci. Mater. Electron. 2015, 26, 4770–4781. [Google Scholar] [CrossRef]
- Chen, C.; Bobela, D.C.; Yang, Y.; Lu, S.; Zeng, K.; Ge, C.; Yang, B.; Gao, L.; Zhao, Y.; Beard, M.C.; et al. Characterization of basic physical properties of Sb2Se3 and its relevance for photovoltaics. Front. Optoelectron. 2017, 10, 18–30. [Google Scholar] [CrossRef]
- Shinde, U.P. Hall coefficient, mobility and carrier concentration as a function of composition and thickness of Zn-Te thin films. Adv. Appl. Sci. Res. 2015, 4, 215–220. [Google Scholar]
- Li, Z.-Q.; Ni, M.; Feng, X.-D. Simulation of the Sb2Se3 solar cell with a hole transport layer. Mater. Res. Express 2020, 7, 016416. [Google Scholar] [CrossRef]
- Firdausa, C.M.; Rizam, M.S.B.S.; Rusop, M.; Hidayah, S.R. Characterization of ZnO and ZnO: TiO2 thin films prepared by sol-gel spray-spin coating technique. Procedia Eng. 2012, 41, 1367–1373. [Google Scholar] [CrossRef]
- Aduda, B.O.; Ravirajan, P.; Choy, K.L.; Nelson, J. Effect of morphology on electron drift mobility in porous TiO2. Int. J. Photoenergy 2004, 6, 141–147. [Google Scholar] [CrossRef]
- Park, C.I.; Kim, N.-H. Hydrogenation in 808-nm diode laser annealing of CdTe thin films: Structural, optical, and electrical properties. Sci. Adv. Mater. 2016, 8, 1813–1818. [Google Scholar] [CrossRef]
- Tauc, J. Optical properties and electronic structure of amorphous Ge and Si. Mater. Res. Bull. 1968, 3, 37–46. [Google Scholar] [CrossRef]
- Rampino, S.; Pattini, F.; Bronzoni, M.; Mazzer, M.; Sidoli, M.; Spaggiari, G.; Gilioli, E. CuSbSe2 thin film solar cells with ~4% conversion efficiency grown by low-temperature pulsed electron deposition. Sol. Energy Mater. Sol. Cells 2018, 185, 86–96. [Google Scholar] [CrossRef]
- Sudha, A.P.; Henry, J.; Mohanraj, K.; Sivakumar, G. Synthesis and characterization of monovalent, divalent and trivalent cation doping of Cu2Se thin films using chemical bath deposition method. Jordan J. Phys. 2018, 11, 125–130. [Google Scholar]
- Rath, T.; MacLachlan, A.J.; Brown, M.D.; Haque, S.A. Structural, optical and charge generation properties of chalcostibite and tetrahedrite copper antimony sulfide thin films prepared from metal xanthates. J. Mater. Chem. A 2015, 3, 24155–24162. [Google Scholar] [CrossRef] [PubMed]
- Lakhdar, M.H.; Smida, Y.B.; Amlouk, M. Synthesis, optical characterization and DFT calculations of electronic structure of Sb2O3 films obtained by thermal oxidation of Sb2S3. J. Alloys Compd. 2016, 681, 197–204. [Google Scholar] [CrossRef]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, S.; Kim, N.-H. Impurity Phases and Optoelectronic Properties of CuSbSe2 Thin Films Prepared by Cosputtering Process for Absorber Layer in Solar Cells. Coatings 2020, 10, 1209. https://doi.org/10.3390/coatings10121209
Kim S, Kim N-H. Impurity Phases and Optoelectronic Properties of CuSbSe2 Thin Films Prepared by Cosputtering Process for Absorber Layer in Solar Cells. Coatings. 2020; 10(12):1209. https://doi.org/10.3390/coatings10121209
Chicago/Turabian StyleKim, Sara, and Nam-Hoon Kim. 2020. "Impurity Phases and Optoelectronic Properties of CuSbSe2 Thin Films Prepared by Cosputtering Process for Absorber Layer in Solar Cells" Coatings 10, no. 12: 1209. https://doi.org/10.3390/coatings10121209
APA StyleKim, S., & Kim, N.-H. (2020). Impurity Phases and Optoelectronic Properties of CuSbSe2 Thin Films Prepared by Cosputtering Process for Absorber Layer in Solar Cells. Coatings, 10(12), 1209. https://doi.org/10.3390/coatings10121209





