Improved Mechanical Properties and Corrosion Resistance of Mg-Based Bulk Metallic Glass Composite by Coating with Zr-Based Metallic Glass Thin Film
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Sputtering Target
2.2. Sample Preparation of Mg-Based BMGC
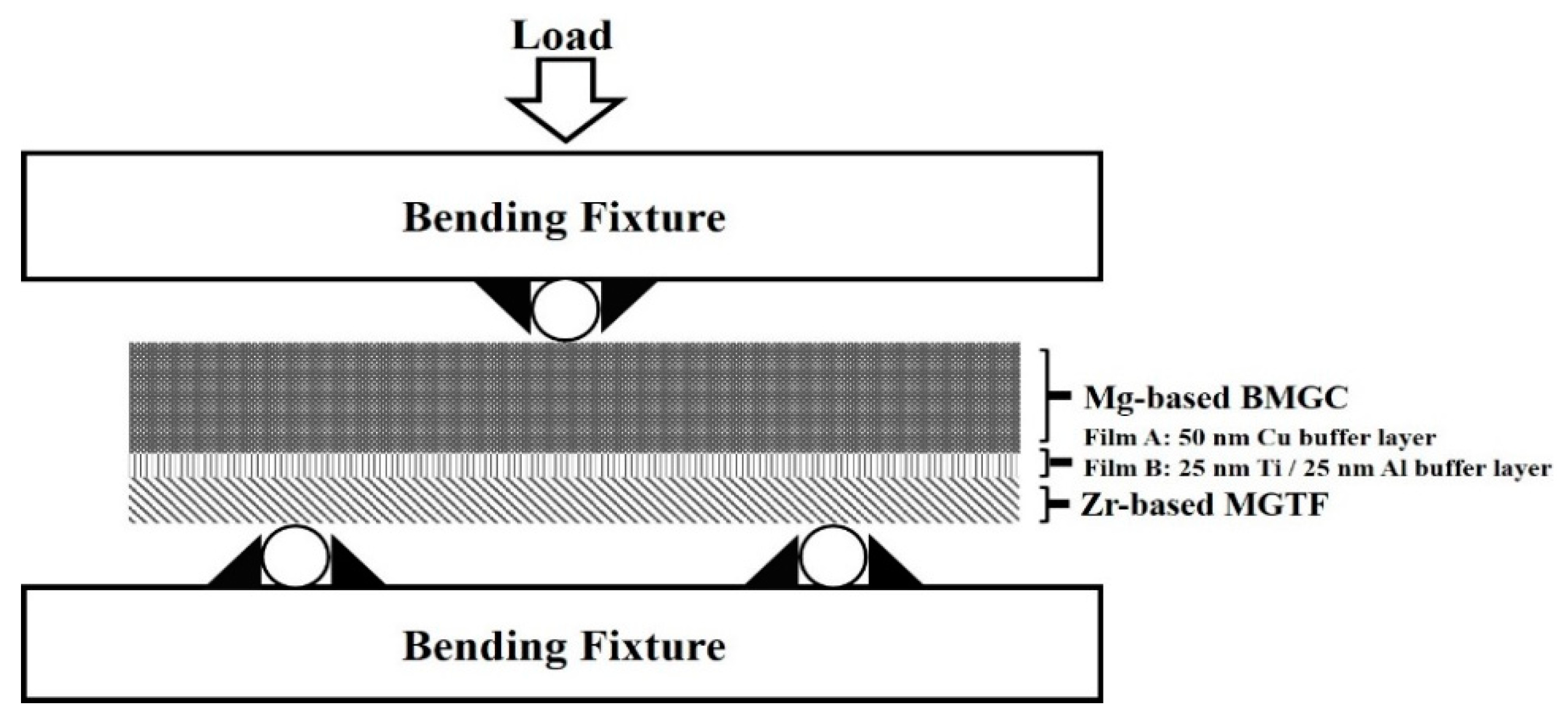
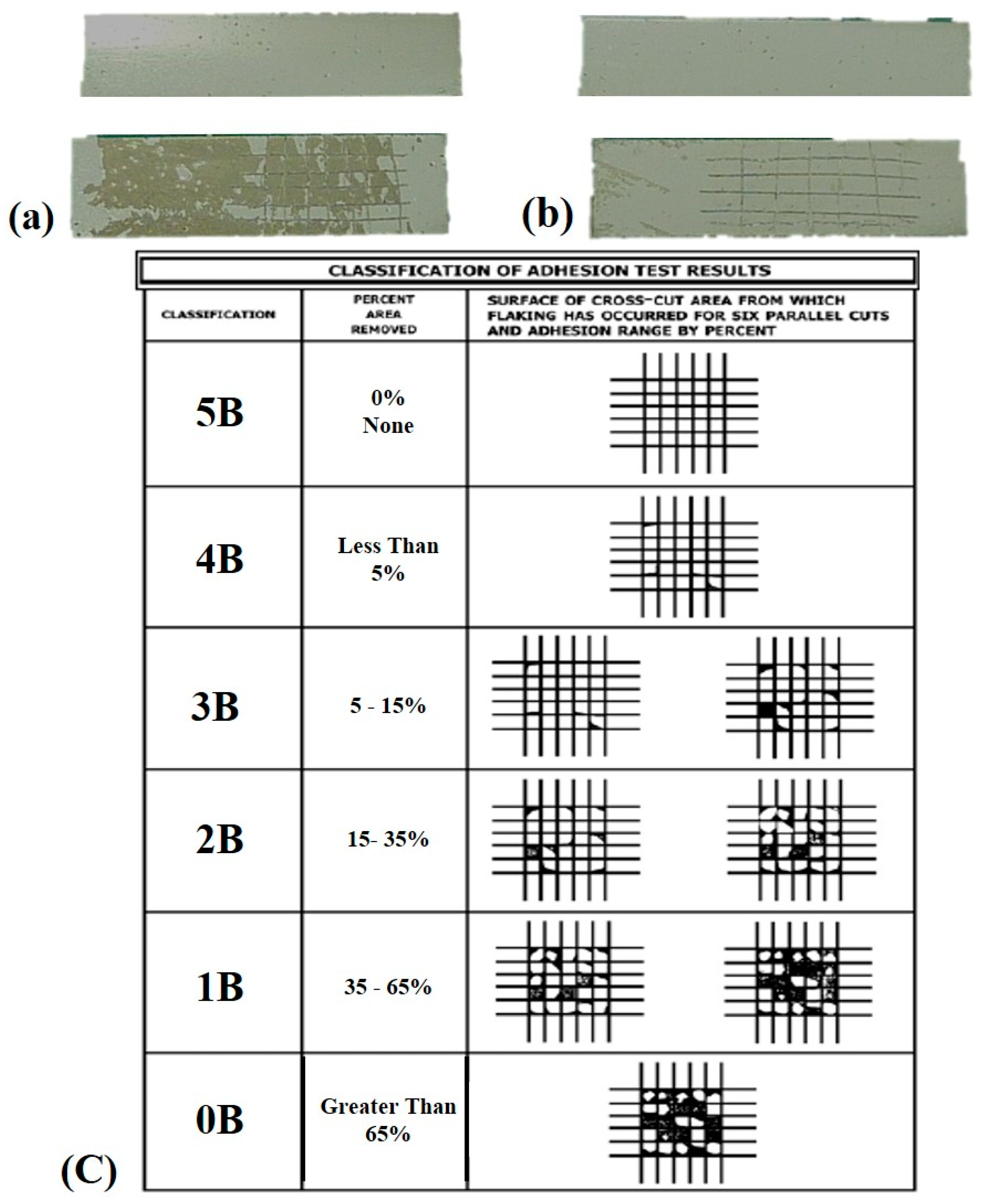
2.3. Characterization of Microstructure and Properties
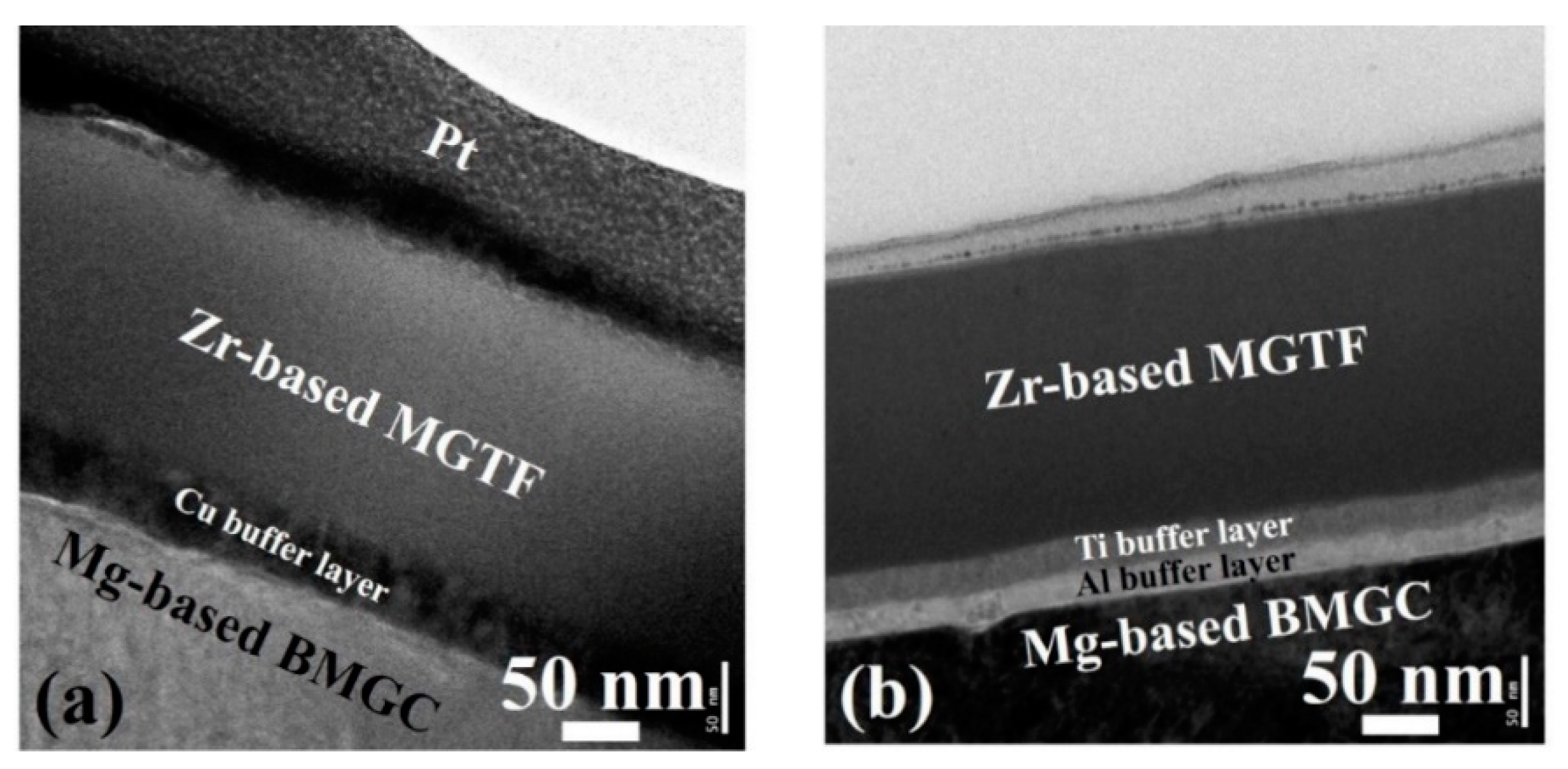
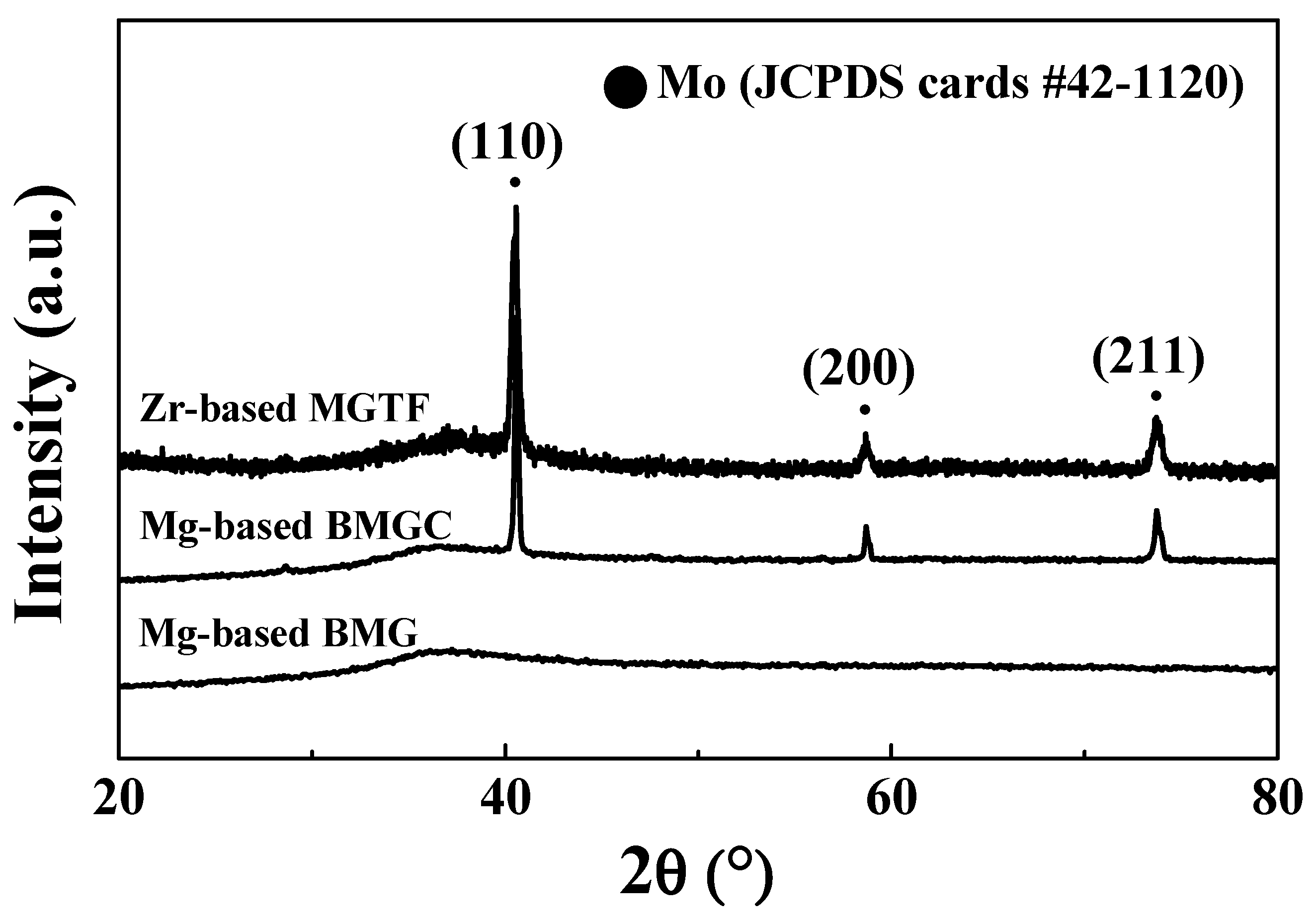
3. Results
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Johnson, W.L. Bulk Glass-forming metallic alloys: Science and technology. MRS Bull. 1999, 24, 42–56. [Google Scholar] [CrossRef]
- Inoue, A. Stabilization of metallic supercooled liquid and bulk amorphous alloys. Acta Mater. 2000, 48, 279–306. [Google Scholar] [CrossRef]
- Oak, J.J.; Louzguine, D.V.; Inoue, A. Investigation of glass-forming ability, deformation and corrosion behavior of Ni-free Ti-based BMG alloys designed for application as dental implants. Mater. Sci. Eng. C 2009, 29, 322–327. [Google Scholar] [CrossRef]
- Zhang, T.; Inoue, A. New bulk glassy Ni-based alloys with high strength of 3000 MPa. Mater. Trans. 2002, 43, 708–711. [Google Scholar] [CrossRef]
- Suryanarayana, C.; Inoue, A. Bulk Metallic Glasses; CRC Press/Taylor & Francis Group: Boca Raton, FL, USA, 2011. [Google Scholar]
- Zhu, S.L.; Wang, X.M.; Qin, F.X.; Inoue, A. A new Ti-based bulk glassy alloy with potential for biomedical application. Mater. Sci. Eng. A 2007, 459, 233–237. [Google Scholar] [CrossRef]
- Inoue, A.; Shen, B.L.; Chang, C.T. Super-high strength of over 4000 MPa for Fe-based bulk glassy alloys in ((Fe1−xCox)0.75B0.2Si0.05)96Nb4 system. Acta Mater. 2004, 52, 4093–4099. [Google Scholar] [CrossRef]
- Lu, Z.P.; Liu, C.T.; Thompson, J.R.; Porter, W.D. Structural amorphous steels. Phys. Rev. Lett. 2004, 92, 245503. [Google Scholar] [CrossRef]
- Tsai, P.H.; Xiao, A.C.; Li, J.B.; Jang, J.S.C.; Chu, J.P.; Huang, J.C. Prominent Fe-based bulk amorphous steel alloy with large supercooled liquid region and superior corrosion resistance. J. Alloys Compd. 2014, 586, 94–98. [Google Scholar] [CrossRef]
- Amiya, K.; Inoue, A. Thermal stability and mechanical properties of Mg–Y–Cu–M (M = Ag, Pd) bulk amorphous Alloys. Mater. Trans. JIM 2000, 41, 1460–1462. [Google Scholar] [CrossRef][Green Version]
- Xu, Y.K.; Ma, H.; Xu, J.; Ma, E. Mg-based bulk metallic glass composites with plasticity and gigapascal strength. Acta Mater. 2005, 53, 1857–1866. [Google Scholar] [CrossRef]
- Ma, H.; Shi, L.L.; Xu, J.; Li, Y.; Ma, E. Discovering inch-diameter metallic glasses in three-dimensional composition space. Appl. Phys. Lett. 2005, 87, 181915. [Google Scholar] [CrossRef]
- Park, E.S.; Kyeong, J.S.; Kim, D.H. Enhanced glass forming ability and plasticity in Mg-based bulk metallic glasses. Mater. Sci. Eng. A 2007, 449, 225–229. [Google Scholar] [CrossRef]
- Pan, D.G.; Zhang, H.F.; Wang, A.M.; Hu, Z.Q. Enhanced plasticity in Mg-based bulk metallic glass composite reinforced with ductile Nb particles. Appl. Phys. Lett. 2006, 89, 261904. [Google Scholar] [CrossRef]
- Hui, X.; Dong, W.; Chen, G.L.; Yao, K.F. Formation, microstructure and properties of long-period order structure reinforced Mg-based bulk metallic glass composites. Acta Mater. 2007, 55, 907–920. [Google Scholar] [CrossRef]
- Jang, J.S.C.; Ciou, J.Y.; Hung, T.H.; Huang, J.C.; Du, X.H. Enhanced mechanical performance of Mg metallic glass with porous Mo particles. Appl. Phys. Lett. 2008, 92, 011930. [Google Scholar] [CrossRef]
- Jang, J.S.C.; Li, T.H.; Jian, S.R.; Huang, J.C.; Nieh, T.G. Effects of characteristics of Mo dispersions on the plasticity of Mg-based bulk metallic glass composites. Intermetallics 2011, 19, 738–743. [Google Scholar] [CrossRef]
- Jang, J.S.C.; Li, J.B.; Lee, S.L.; Chang, Y.S.; Jian, S.R.; Huang, J.C.; Nieh, T.G. Prominent plasticity of Mg-based bulk metallic glass composites by ex-situ spherical Ti particles. Intermetallics 2012, 30, 25–29. [Google Scholar] [CrossRef]
- Kinaka, M.; Kato, H.; Hasegawa, M.; Inoue, A. High specific strength Mg-based bulk metallic glass matrix composite highly ductilized by Ti dispersoid. Mater. Sci. Eng. A 2008, 494, 299–303. [Google Scholar] [CrossRef]
- Mizutania, Y.; Kim, S.J.; Ichino, R.; Okido, M. Anodizing of Mg alloys in alkaline solutions. Surf. Coat. Tech. 2003, 169–170, 143–146. [Google Scholar] [CrossRef]
- Abulsain, M.; Berkani, A.; Bonilla, F.A.; Liu, Y.; Arenas, M.A.; Skeldon, P.; Thompson, G.E.; Bailey, P.; Noakes, T.C.Q.; Shimizu, K.; et al. Anodic oxidation of Mg–Cu and Mg–Zn alloys. Electrochim. Acta 2004, 49, 899–904. [Google Scholar] [CrossRef]
- Zhang, Y.; Yan, C. Development of anodic film on Mg alloy AZ91D. Surf. Coat. Tech. 2006, 201, 2381–2386. [Google Scholar] [CrossRef]
- Guo, H.F.; An, M.Z.; Huo, H.B.; Xu, S.; Wu, L.J. Microstructure characteristic of ceramic coatings fabricated on magnesium alloys by micro-arc oxidation in alkaline silicate solutions. Appl. Surf. Sci. 2006, 252, 7911–7916. [Google Scholar] [CrossRef]
- Chen, F.; Zhou, H.; Yao, B.; Qin, Z.; Zhang, Q. Corrosion resistance property of the ceramic coating obtained through microarc oxidation on the AZ31 magnesium alloy surfaces. Surf. Coat. Tech. 2007, 201, 4905–4908. [Google Scholar] [CrossRef]
- Chu, J.P.; Jang, J.S.C.; Huang, J.C.; Chou, H.S.; Yang, Y.; Ye, J.C.; Wang, Y.C.; Lee, J.W.; Liu, F.X.; Liaw, P.K.; et al. Thin film metallic glasses: Unique properties and potential applications. Thin Solid Film. 2012, 520, 5097–5122. [Google Scholar] [CrossRef]
- Chieh, T.C.; Chu, J.; Liu, C.T.; Wu, J.K. Corrosion of Zr52.5Cu17.9Ni14.6Al10Ti5 bulk metallic glasses in aqueous solutions. Mater. Lett. 2003, 57, 3022–3025. [Google Scholar] [CrossRef]
- Lin, C.H.; Huang, C.H.; Chuang, J.F.; Lee, H.C.; Liu, M.C.; Du, X.H.; Huang, J.C.; Jang, J.S.C.; Chen, C.H. Simulated body-fluid tests and electrochemical investigations on biocompatibility of metallic glasses. Mater. Sci. Eng. C 2012, 32, 2578–2582. [Google Scholar] [CrossRef]
- Deng, Y.L.; Lee, J.W.; Lou, B.S.; Duh, J.G.; Chu, J.P.; Jang, J.S.C. The fabrication and property evaluation of Zr–Ti–B–Si thin film metallic glass materials. Surf. Coat. Tech. 2014, 259, 115–122. [Google Scholar] [CrossRef]
- ASTM D3359-09 Standard Test Methods for Measuring Adhesion by Tape Test; ASTM: PA, USA. Available online: https://www.astm.org/DATABASE.CART/HISTORICAL/D3359-09.htm (accessed on 12 November 2020).
- ASTM E855-08 Standard Test Methods For Bend Testing Of Metallic Flat Materials For Spring Applications Involving Static Loading; ASTM: PA, USA. Available online: https://webstore.ansi.org/standards/astm/astme85508 (accessed on 12 November 2020).
- Chang, Y.Z.; Tsai, P.H.; Li, J.B.; Lin, H.C.; Jang, J.S.C.; Li, C.; Chen, G.J.; Chen, Y.C.; Chu, J.P.; Liaw, P.K. Zr-based metallic glass thin film coating for fatigue-properties improvement of 7075-T6 aluminum alloy. Thin Solid Films 2013, 544, 331–334. [Google Scholar] [CrossRef]
- Chu, J.P.; Greene, J.E.; Jang, J.S.C.; Huang, J.C.; Shen, Y.L.; Liaw, P.K.; Yokoyama, Y.; Inoue, A.; Nieh, T.G. Bendable bulk metallic glass: Effects of a thin, adhesive, strong, and ductile coating. Acta Mater. 2012, 60, 3226–3238. [Google Scholar] [CrossRef]
- Chu, J.P.; Huang, J.C.; Jang, J.S.C.; Wang, Y.C.; Liaw, P.K. Thin film metallic glasses: Preparations, properties, and applications. JOM 2010, 62, 19–24. [Google Scholar] [CrossRef]
- Schroers, J.; Johnson, W.L. Ductile bulk metallic glass. Phys. Rev. Lett. 2004, 93, 255506. [Google Scholar] [CrossRef] [PubMed]
- Conner, R.D.; Li, Y.; Nix, W.D.; Johnson, W.L. Shear band spacing under bending of Zr-based metallic glass plates. Acta Mater. 2004, 52, 2429–2434. [Google Scholar] [CrossRef]

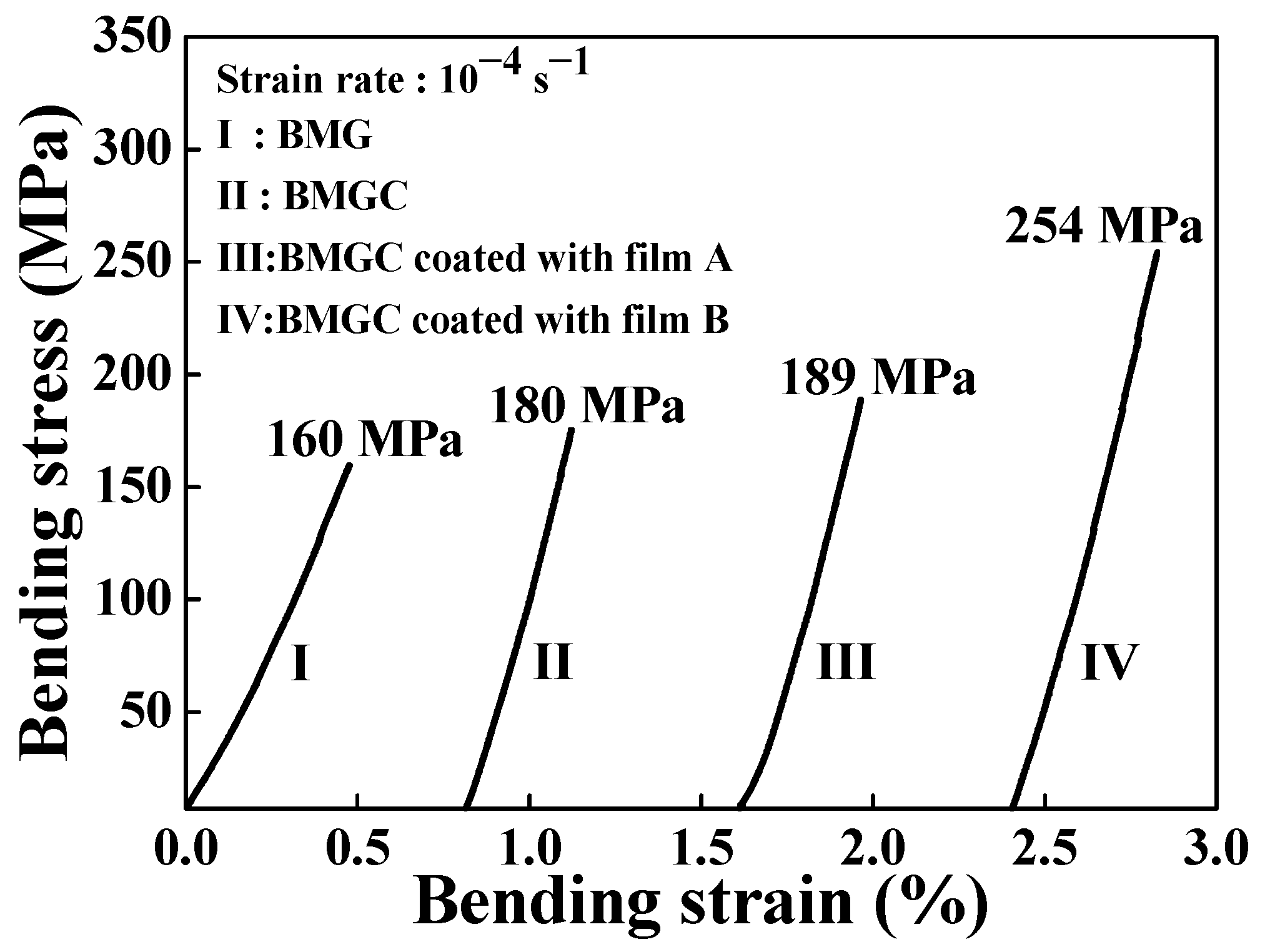
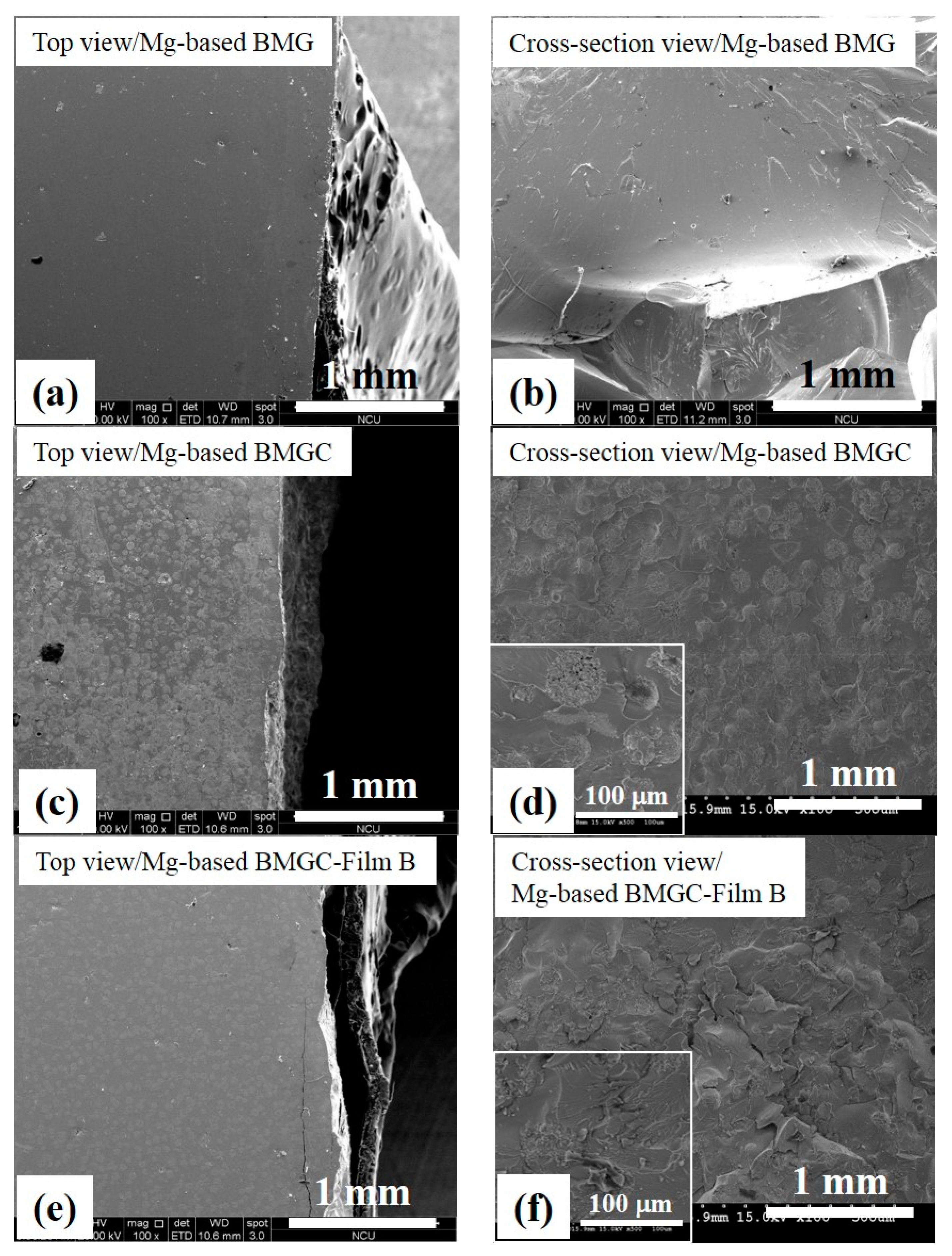
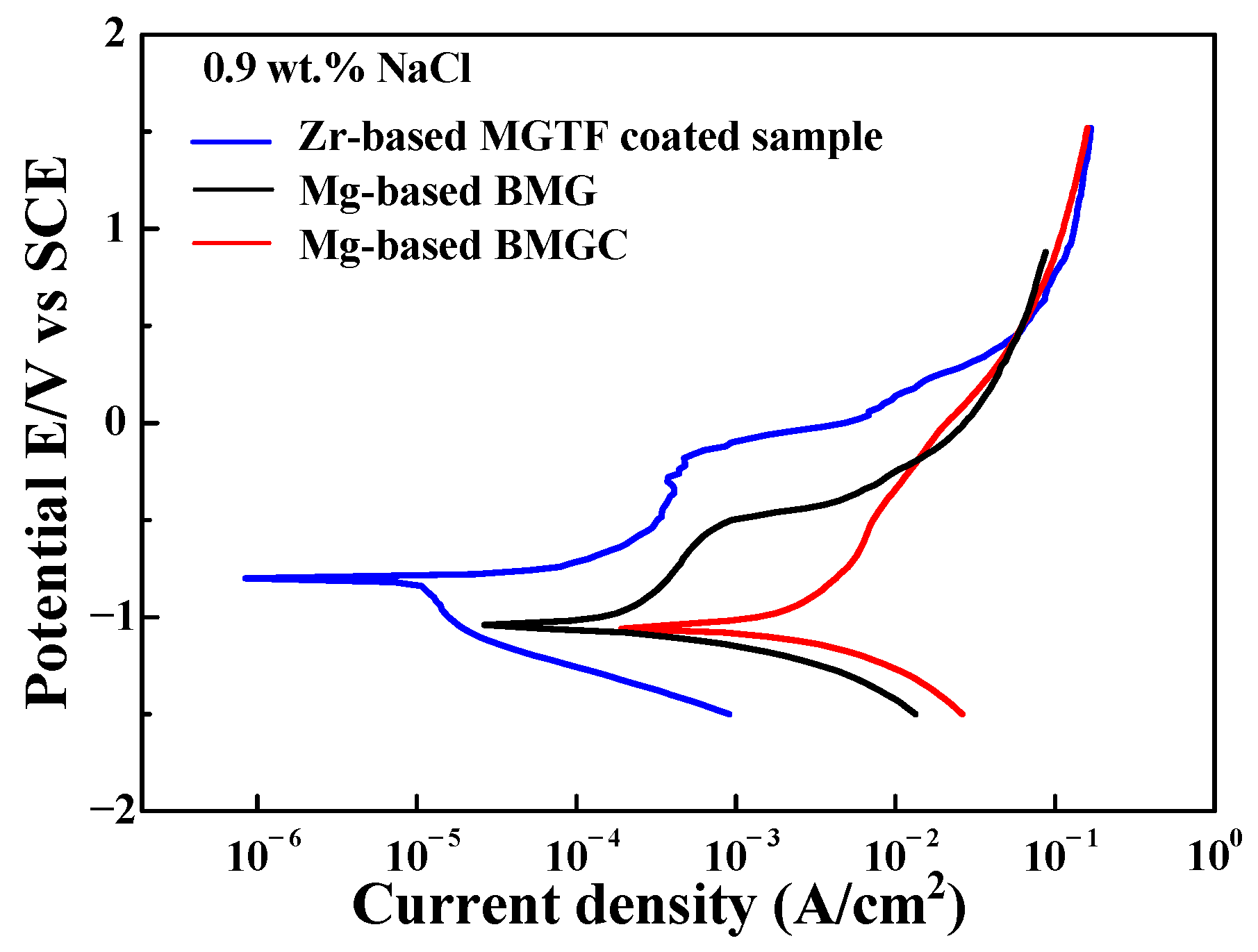
| Material | (at.%) | Mg | Cu | Gd |
|---|---|---|---|---|
| Mg-based BMG | Design composition | 58 | 31 | 11 |
| Average | 51.94 | 34.78 | 13.28 | |
| Deviation | 0.3 | 0.38 | 0.02 | |
| Mg-based BMGC | Design composition | 58 | 31 | 11 |
| Average | 53.7 | 34.09 | 12.22 | |
| Deviation | 0.82 | 0.58 | 0.02 |
| Material | (at.%) | Zr | Cu | Ni | Al | Si |
|---|---|---|---|---|---|---|
| Zr-based MGTF | Design composition | 52.73 | 29.85 | 8.96 | 7.96 | 0.5 |
| Average | 52.44 | 31.69 | 12.05 | 3.69 | 0.13 | |
| Deviation | 0.59 | 0.19 | 0.02 | 0.25 | 0.01 |
| Material | Ecorr (V) | Icorr (A/cm2) |
|---|---|---|
| Mg-based BMG | −1.04 | 9.07 × 10−5 |
| Mg-based BMGC | −1.06 | 5.38 × 10−4 |
| Zr-based MGTF | −0.8 | 9.06 × 10−6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsai, P.-H.; Lee, C.-I.; Song, S.-M.; Liao, Y.-C.; Li, T.-H.; Jang, J.S.-C.; Chu, J.P. Improved Mechanical Properties and Corrosion Resistance of Mg-Based Bulk Metallic Glass Composite by Coating with Zr-Based Metallic Glass Thin Film. Coatings 2020, 10, 1212. https://doi.org/10.3390/coatings10121212
Tsai P-H, Lee C-I, Song S-M, Liao Y-C, Li T-H, Jang JS-C, Chu JP. Improved Mechanical Properties and Corrosion Resistance of Mg-Based Bulk Metallic Glass Composite by Coating with Zr-Based Metallic Glass Thin Film. Coatings. 2020; 10(12):1212. https://doi.org/10.3390/coatings10121212
Chicago/Turabian StyleTsai, Pei-Hua, Chung-I Lee, Sin-Mao Song, Yu-Chin Liao, Tsung-Hsiung Li, Jason Shian-Ching Jang, and Jinn P. Chu. 2020. "Improved Mechanical Properties and Corrosion Resistance of Mg-Based Bulk Metallic Glass Composite by Coating with Zr-Based Metallic Glass Thin Film" Coatings 10, no. 12: 1212. https://doi.org/10.3390/coatings10121212
APA StyleTsai, P.-H., Lee, C.-I., Song, S.-M., Liao, Y.-C., Li, T.-H., Jang, J. S.-C., & Chu, J. P. (2020). Improved Mechanical Properties and Corrosion Resistance of Mg-Based Bulk Metallic Glass Composite by Coating with Zr-Based Metallic Glass Thin Film. Coatings, 10(12), 1212. https://doi.org/10.3390/coatings10121212






