Fluorescent Silica Hybrid Film-Forming Materials Based on Salicylaldazine
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis Methods
2.2.1. SAA Synthesis
2.2.2. Fluorescent Film-Forming Materials
2.3. Characterization Methods
3. Results and Discussion
3.1. Photophysical Properties of SAA in Solution
3.2. Photophysical Properties of Fluorescent Hybrid Materials
3.3. Structural Characterization by ATR FTIR Spectroscopy
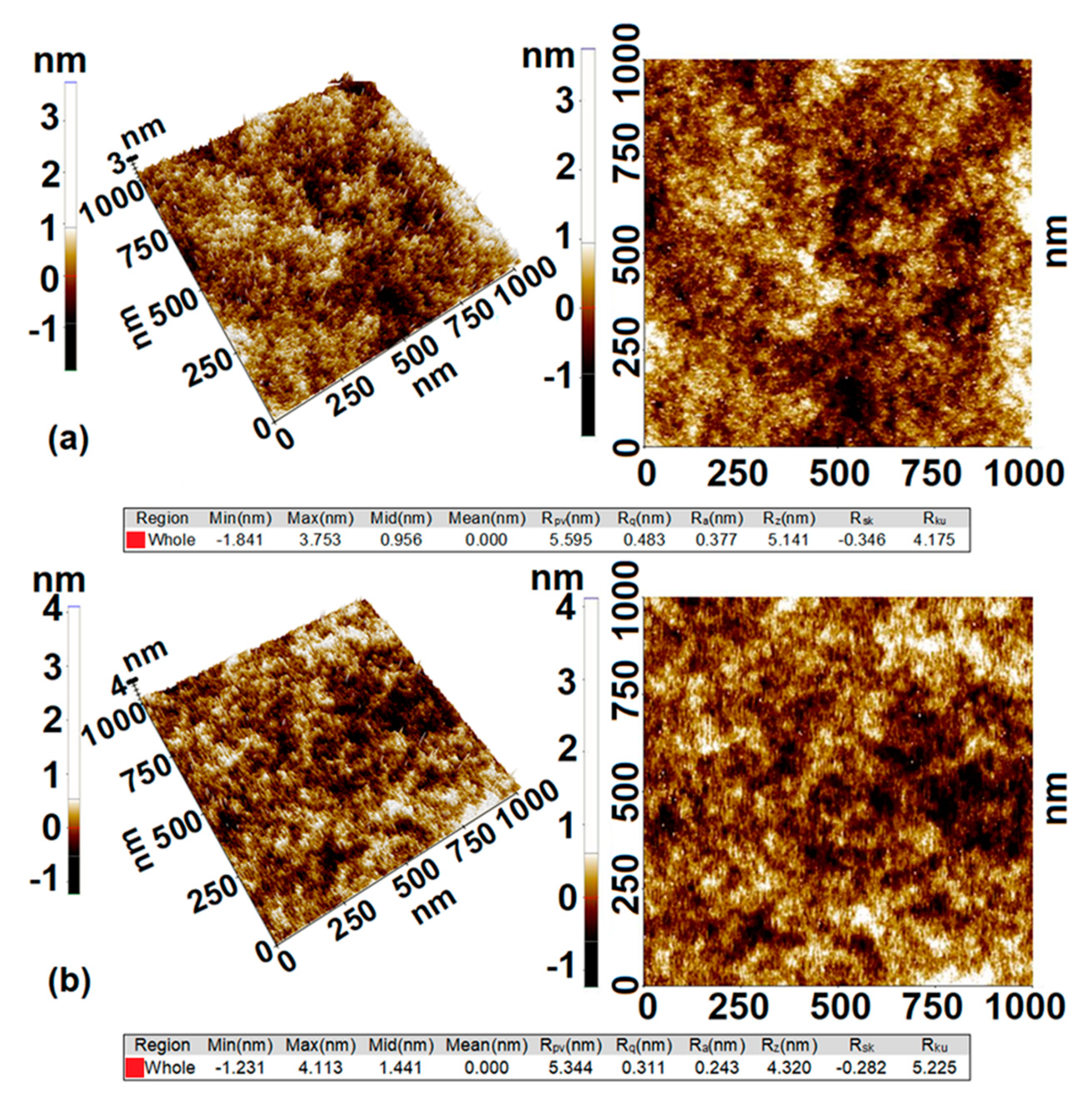
3.4. Properties of Fluorescent Dimethyl-Modified Silica Films
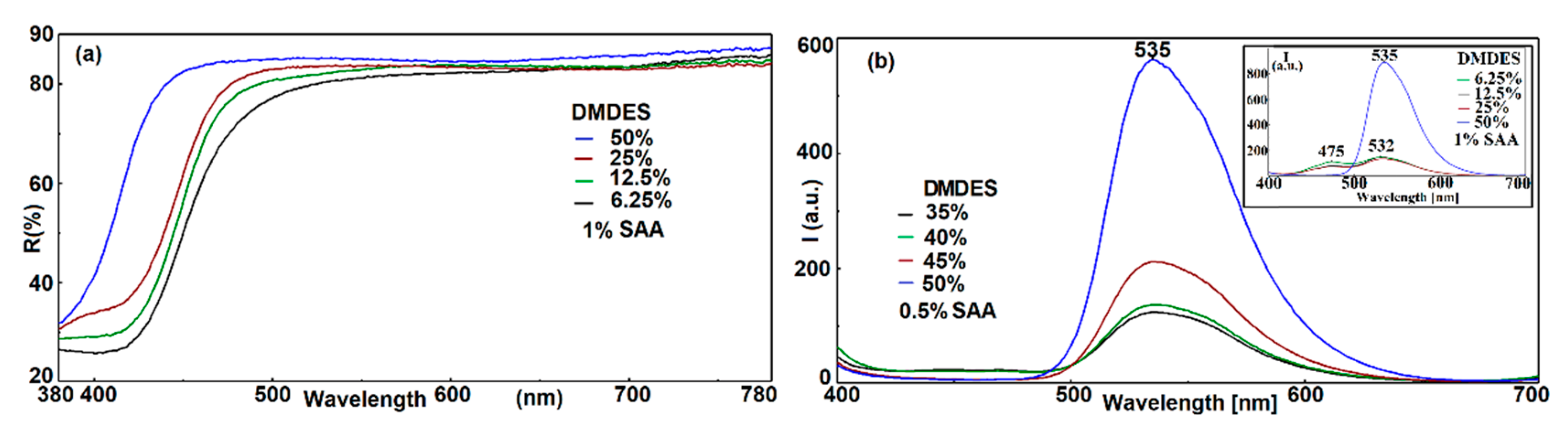
3.4.1. Influence of the Amount of DMDES
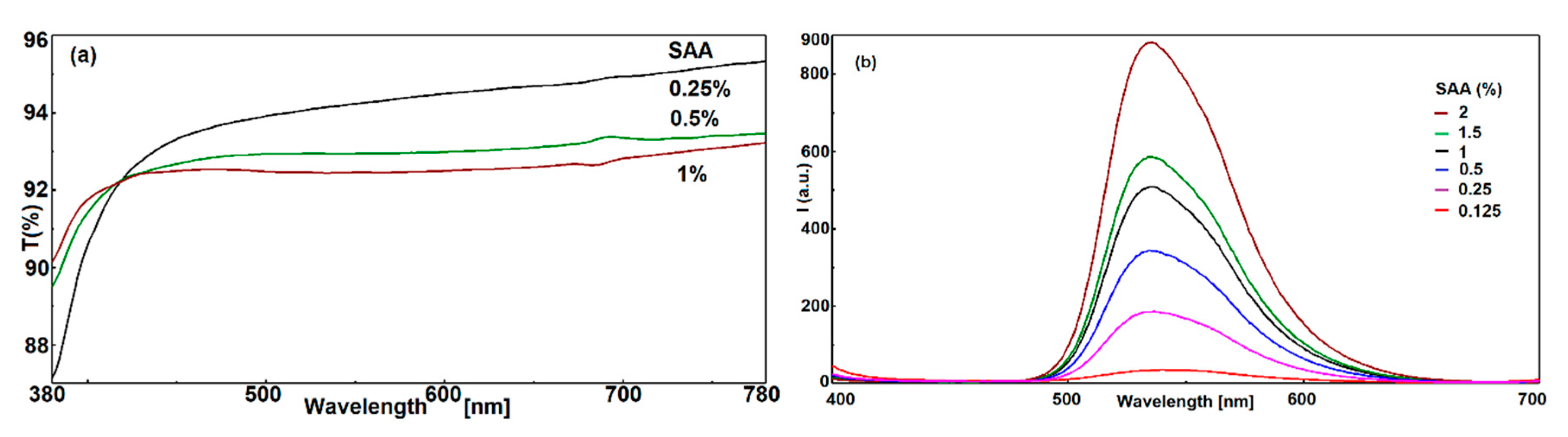
3.4.2. Influence of the Amount of SAA
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ammar, A.H.; El-Sayed, B.A.; El-Sayad, E.A. Structural and optical studies on ortho-hydroxy acetophenone azine thin films. J. Mater. Sci. 2002, 37, 3255–3260. [Google Scholar] [CrossRef]
- Satam, M.A.; Telore, R.D.; Sekar, N. Pigment Yellow 101 analogs from 3-(1,3-benzothiazol-2-yl)-2-hydroxynaphthalene-1-carbaldehyde—synthesis and characterization. Dyes Pigments 2015, 123, 274–284. [Google Scholar] [CrossRef]
- Satam, M.A.; Telore, R.D.; Sekar, N. Photophysical properties of Schiff’s bases from 3-(1,3-benzothiazol-2-yl)-2-hydroxy naphthalene-1-carbaldehyde. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2014, 132, 678–686. [Google Scholar] [CrossRef]
- Sedgwick, A.C.; Wu, L.; Han, H.-H.; Bull, S.D.; He, X.-P.; James, T.D.; Sessler, J.L.; Tang, B.Z.; Tian, H.; Yoon, J. Excited-state intramolecular proton-transfer (ESIPT) based fluorescence sensors and imaging agents. Chem. Soc. Rev. 2018, 47, 8842–8880. [Google Scholar] [CrossRef] [Green Version]
- Aiello, D.; Malfatti, L.; Kidchob, T.; Aiello, R.; Testa, F.; Aiello, I.; Ghedini, M.; La Deda, M.; Martino, T.; Casula, M.; et al. Blue-emitting mesoporous films prepared via incorporation of luminescent Schiff base zinc(II) complex. J. Sol-Gel Sci. Technol. 2008, 47, 283–289. [Google Scholar] [CrossRef]
- Raditoiu, A.; Radițoiu, V.; Culita, D.C.; Baran, A.; Anghel, D.-F.; Spataru, C.I.; Amariutei, V.; Nicolae, C.A.; Wagner, L.E. Photophysical properties of some fluorescent materials containing 3-methoxy-7H-benzo[de]anthracen-7-one embedded in sol-gel silica hybrids. Opt. Mater. 2015, 45, 55–63. [Google Scholar] [CrossRef]
- Deshpande, A.V.; Jathar, L.V.; Rane, J.R. Effect of method of preparation and drying time on photophysical properties of coumarin 1 laser dye embedded in HCl catalysed sol-gel glasses. J. Non-Cryst. Solids 2010, 356, 1–7. [Google Scholar] [CrossRef]
- Sokolov, I.; Volkov, D.O. Ultrabright fluorescent mesoporous silica particles. J. Mater. Chem. 2010, 20, 4247–4250. [Google Scholar] [CrossRef]
- Rawat, M.S.M.; Mal, S.; Singh, P. Photochromism in anils—A review. Open Chem. J. 2015, 2, 7–19. [Google Scholar] [CrossRef] [Green Version]
- Lin, H.; Chang, X.; Yan, D.; Fang, W.-H.; Cui, G. Tuning excited-state-intramolecular-proton-transfer (ESIPT) process and emission by cocrystal formation: A combined experimental and theoretical study. Chem. Sci. 2017, 8, 2086–2090. [Google Scholar] [CrossRef] [Green Version]
- Chen, C.-C.; Dou, L.; Zhu, R.; Chung, C.-H.; Song, T.-B.; Zheng, Y.B.; Hawks, S.; Li, G.; Weiss, P.S.; Yang, Y. Visibly transparent polymer solar cells produced by solution processing. ACS Nano 2012, 6, 7185–7190. [Google Scholar] [CrossRef] [PubMed]
- Poojary, S.; Sunil, D.; Kekuda, D.; Sreenivasa, S. Fluorescent aromatic symmetrical azines: Synthesis and appraisal of their photophysical and electrochemical properties. Opt. Mater. 2018, 85, 1–7. [Google Scholar] [CrossRef]
- Tang, W.; Xiang, Y.; Tong, A. salicylaldehyde azines as fluorophores of aggregation-induced emission enhancement characteristics. J. Org. Chem. 2009, 74, 2163–2166. [Google Scholar] [CrossRef]
- Purcar, V.; Rădiţoiu, V.; Dumitru, A.; Nicolae, C.-A.; Frone, A.N.; Anastasescu, M.; Rădiţoiu, A.; Raduly, M.F.; Gabor, R.A.; Căprărescu, S. Antireflective coating based on TiO2 nanoparticles modified with coupling agents via acid-catalyzed sol–gel method. Appl. Surf. Sci. 2019, 487, 819–824. [Google Scholar] [CrossRef]
- Ziółek, M.; Filipczak, K.; Maciejewski, A. Spectroscopic and photophysical properties of salicylaldehyde azine (SAA) as a photochromic Schiff base suitable for heterogeneous studies. Chem. Phys. Lett. 2008, 464, 181–186. [Google Scholar] [CrossRef]
- Rodríguez-Córdoba, W.; Zugazagoitia, J.S.; Collado-Fregoso, E.; Peón, J. Excited state intramolecular proton transfer in schiff bases. decay of the locally excited enol state observed by femtosecond resolved fluorescence. J. Phys. Chem. A 2007, 111, 6241–6247. [Google Scholar] [CrossRef]
- Plotner, J.; Dreuw, A. Solid state fluorescence of pigment yellow 101 and derivatives: A conserved property of the individual molecules. Phys. Chem. Chem. Phys. 2006, 8, 1197–1204. [Google Scholar] [CrossRef]
- Massue, J.; Felouat, A.; Vérité, P.M.; Jacquemin, D.; Cyprych, K.; Durko, M.; Sznitko, L.; Mysliwiec, J.; Ulrich, G. An extended excited-state intramolecular proton transfer (ESIPT) emitter for random lasing applications. Phys. Chem. Chem. Phys. 2018, 20, 19958–19963. [Google Scholar] [CrossRef]
- Zhang, Z.; Gorman, B.P.; Dong, H.; Orozco-Teran, R.A.; Mueller, D.W.; Reidy, R.F. Investigation of polymerization and cyclization of dimethyldiethoxysilane. J. Sol-Gel Sci. Technol. 2003, 28, 159–165. [Google Scholar] [CrossRef]
- Nocun, M.; Cholewa-Kowalska, K.; Łączka, M. Structure of hybrids based on TEOS-cyclic forms of siloxane system. J. Mol. Struct. 2009, 938, 24–28. [Google Scholar] [CrossRef]
- Mah, S.K.; Chung, I.J. Effects of dimethyldiethoxysilane addition on tetraethylorthosilicate sol-gel process. J. Non-Cryst. Solids 1995, 183, 252–259. [Google Scholar] [CrossRef]
- Wang, F.; Liu, J.; Luo, Z.; Zhang, Q.; Wang, P.; Liang, X.; Li, C.; Chen, J. Effects of dimethyldiethoxysilane addition on the sol–gel process of tetraethylorthosilicate. J. Non-Cryst. Solids 2007, 353, 321–326. [Google Scholar] [CrossRef]
- Iacono, S.T.; Budy, S.M.; Mabry, J.M.; Smith, D.W. Synthesis, characterization, and surface morphology of pendant polyhedral oligomeric silsesquioxane perfluorocyclobutyl aryl ether copolymers. Macomolecules 2007, 40, 9517–9522. [Google Scholar] [CrossRef]
- Guo, L.; Hyeon-Lee, J.; Beaucage, G. Structural analysis of poly(dimethylsiloxane) modified silica xerogels. J. Non-Cryst. Solids 1999, 243, 61–69. [Google Scholar] [CrossRef]
- Tamayo, A.; Rubio, J. Structure modification by solvent addition into TEOS/PDMS hybrid materials. J. Non-Cryst. Solids 2010, 356, 1742–1748. [Google Scholar] [CrossRef]
- Zheng, C.; Lin, A.; Zhen, X.; Feng, M.; Huang, J.; Zhan, H. Structural and textural evolution of dimethyl-modified silica xerogels. Mater. Lett. 2007, 61, 2927–2930. [Google Scholar] [CrossRef]
- Xu, J.; Liu, Y.; Du, W.; Lei, W.; Si, X.; Zhou, T.; Lin, J.; Peng, L. Superhydrophobic silica antireflective coatings with high transmittance via one-step sol–gel process. Thin Solid Films 2017, 631, 193–199. [Google Scholar] [CrossRef]
- Dreuw, A.; Plötner, J.; Lorenz, L.; Wachtveitl, J.; Djanhan, J.E.; Brüning, J.; Metz, T.; Bolte, M.; Schmidt, M.U. Molecular mechanism of the solid-state fluorescence behavior of the organic pigment yellow 101 and its derivatives. Angew. Chem. Int. Ed. 2005, 44, 7783–7786. [Google Scholar] [CrossRef]


| Solvent (Polarity Index) | DMF (6.4) | EtOH (5.2) | THF (4.0) | Hx (0.0) |
|---|---|---|---|---|
| Absorption wavelength (λabs, nm)/Optical density (a.u.) | 357/2.2 | 357/2.3 | 359/2.02 | 360/2.2 |
| Fluorescence wavelengths (λ1f/λ2fl, nm)/Intensity (a.u.) | 547/418 201/88 | 547/418 14/3 | 547/418 252/98 | 547/418 22/7 |
| Stokes shift (SS, nm) | 190 | 190 | 188 | 187 |
| Sample | G0 | G1 | G2 | G3 | G4 | G5 | G6 |
|---|---|---|---|---|---|---|---|
| Absorption wavelength (λabs, nm) | 347 | 362 | 358 | 359 | 364 | 363 | 347 |
| Fluorescence wavelength (λfl1, nm)/(Intensity, a.u.) | 440 (20) | 438 (7) | 439 (13) | 438 (8) | 434 (17) | 437 (7) | 434 (56) |
| Fluorescence wavelength (λfl2, nm)/(Intensity, a.u.) | 534 (217) | 543 (185) | 536 (219) | 546 (93) | 545 (155) | 536 (243) | 533 (89) |
| Stokes shift (SS, nm) | 187 | 181 | 198 | 187 | 181 | 173 | 186 |
| Sample absorbance (%) | 82.7 | 88.9 | 90.2 | 81.1 | 85.4 | 83.5 | 89.7 |
| External quantum efficiency (%) | 0.01 | 3.06 | 2.13 | 3.38 | 1.73 | 2.69 | 1.64 |
| Internal quantum efficiency (%) | 0.02 | 3.46 | 2.37 | 4.18 | 2.03 | 3.44 | 1.83 |
| DMDES (%) | SBJH (m2·g−1) | VBJH × 103 (cm3·g−1) | Dpore (nm) | μpore S (m2·g−1) | μpore V × 103 (cm3·g−1) |
|---|---|---|---|---|---|
| 0 | 1.72 | 2.5 | 3.3 | 3.05 | 1.1 |
| 12.5 | 2.05 | 2.8 | 3.3 | 2.66 | 0.9 |
| 25 | 1.79 | 2.2 | 3.1 | 6.54 | 2.1 |
| 50 | 3.55 | 4.8 | 3.3 | 8 | 2.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Raditoiu, A.; Raditoiu, V.; Raduly, F.M.; Ispas, G.C.; Purcar, V.; Frone, A.N.; Manea, R.; Wagner, L.E.; Anastasescu, M. Fluorescent Silica Hybrid Film-Forming Materials Based on Salicylaldazine. Coatings 2020, 10, 1255. https://doi.org/10.3390/coatings10121255
Raditoiu A, Raditoiu V, Raduly FM, Ispas GC, Purcar V, Frone AN, Manea R, Wagner LE, Anastasescu M. Fluorescent Silica Hybrid Film-Forming Materials Based on Salicylaldazine. Coatings. 2020; 10(12):1255. https://doi.org/10.3390/coatings10121255
Chicago/Turabian StyleRaditoiu, Alina, Valentin Raditoiu, Florentina Monica Raduly, Georgiana Cornelia Ispas, Violeta Purcar, Adriana Nicoleta Frone, Raluca Manea, Luminita Eugenia Wagner, and Mihai Anastasescu. 2020. "Fluorescent Silica Hybrid Film-Forming Materials Based on Salicylaldazine" Coatings 10, no. 12: 1255. https://doi.org/10.3390/coatings10121255