Characterization of Thermochemical and Thermomechanical Properties of Eyjafjallajökull Volcanic Ash Glass
Abstract
:1. Introduction
2. Experimental Procedure
3. Results
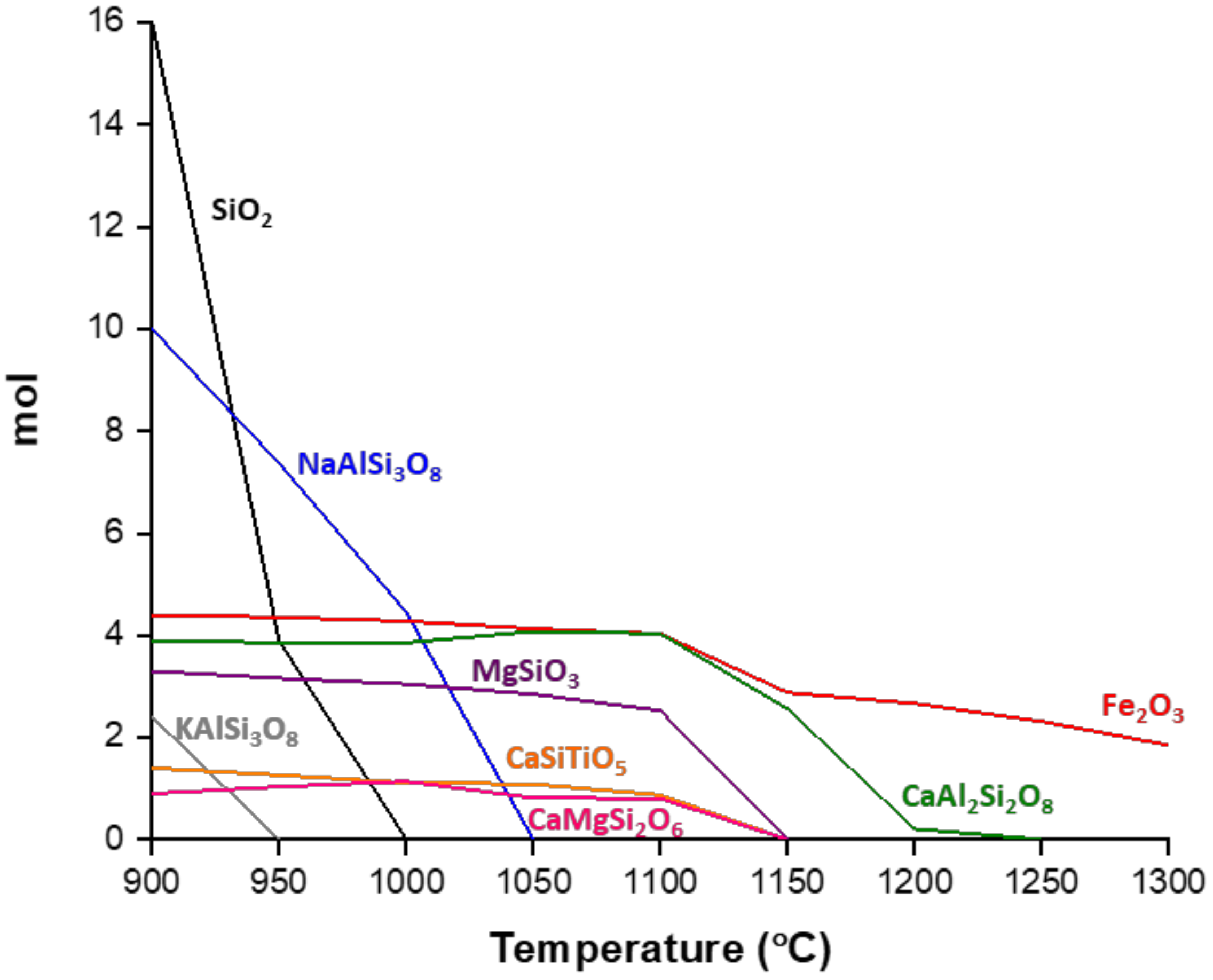
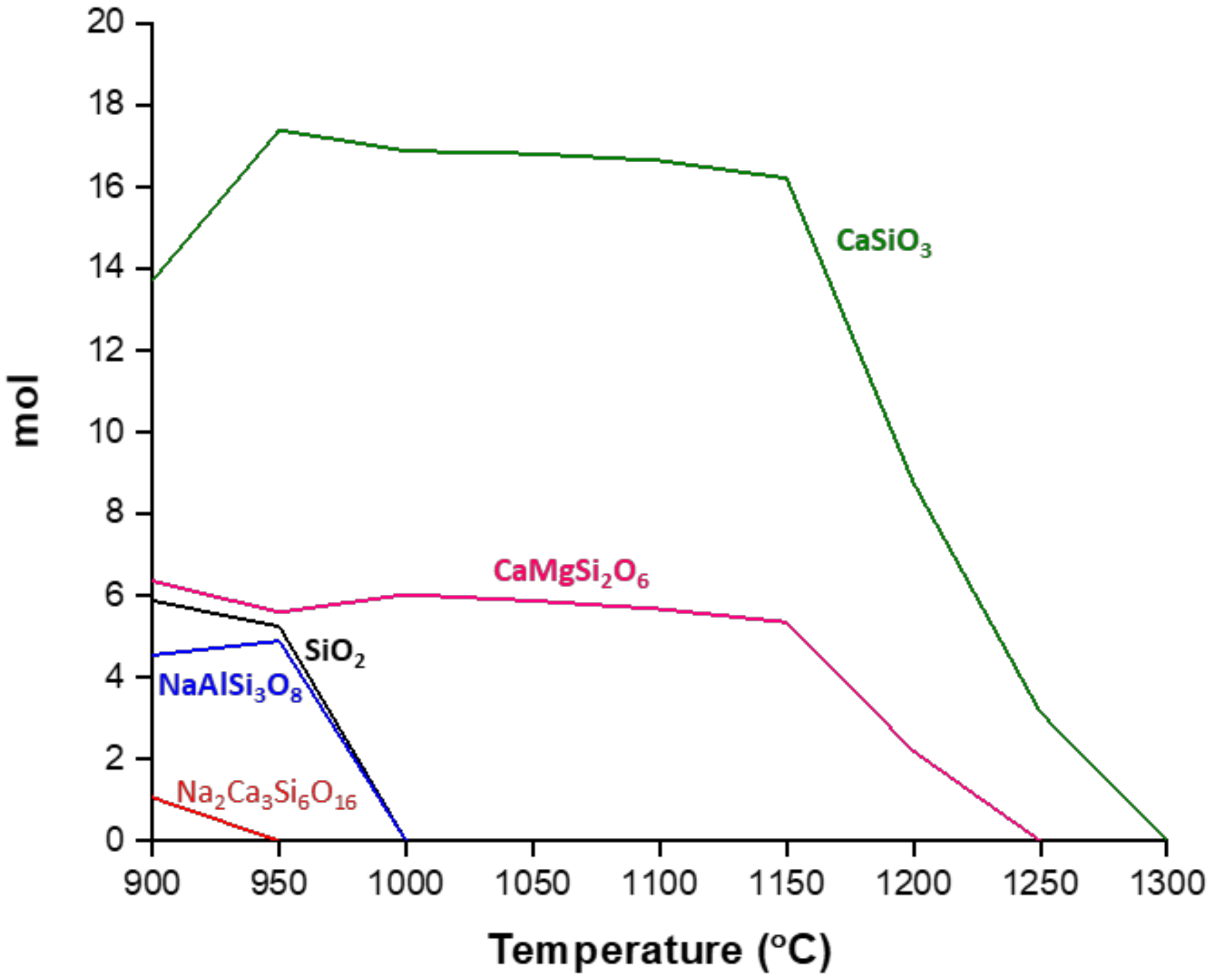
3.1. Crystallization Behavior—DTA
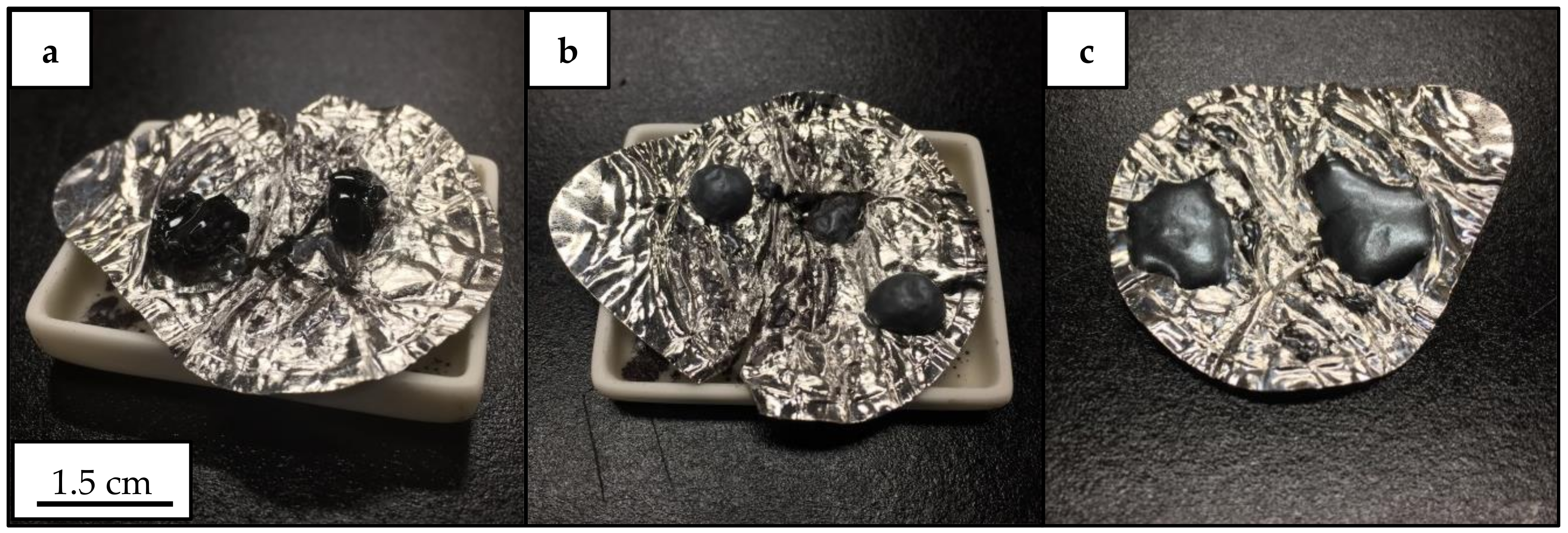
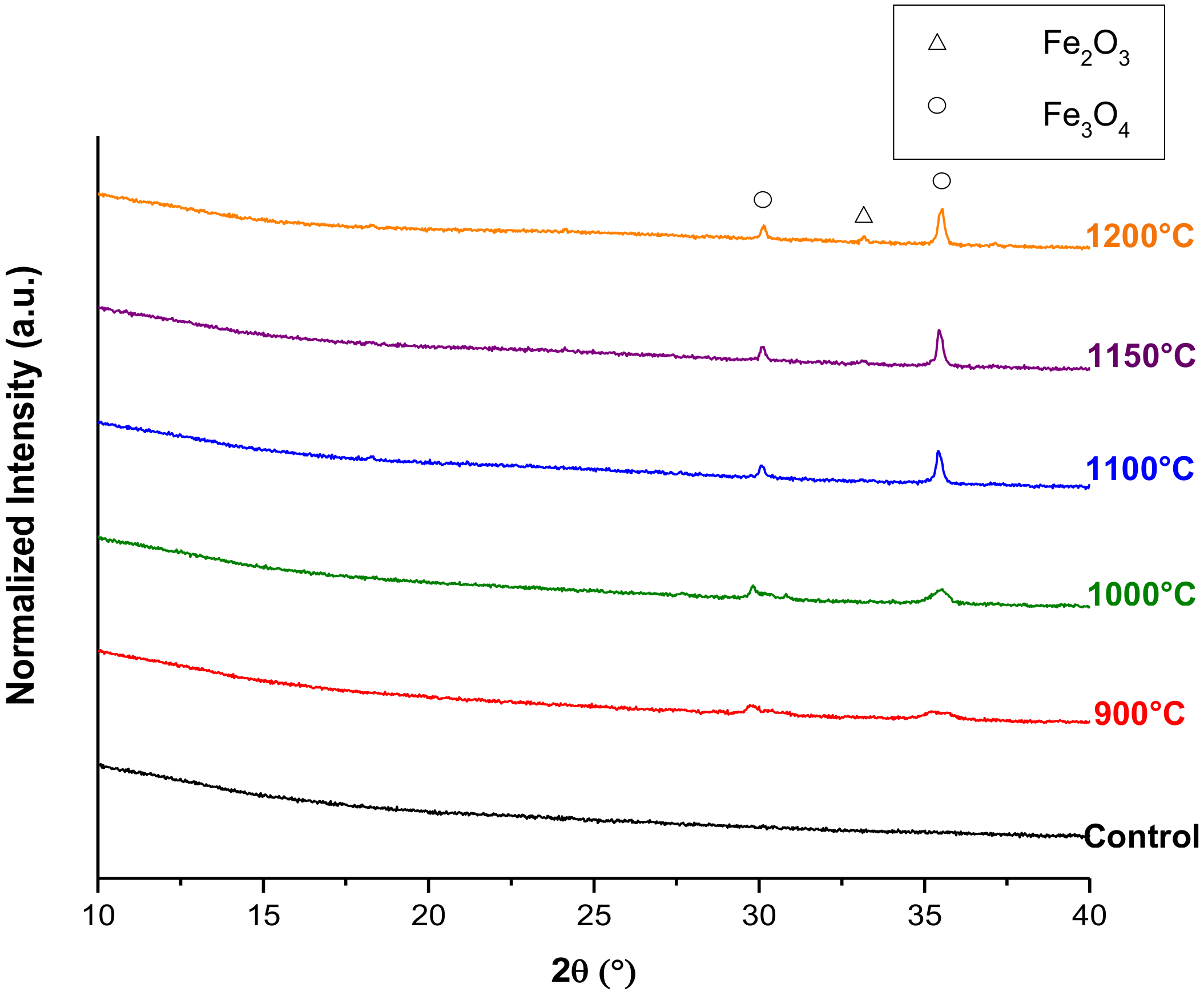
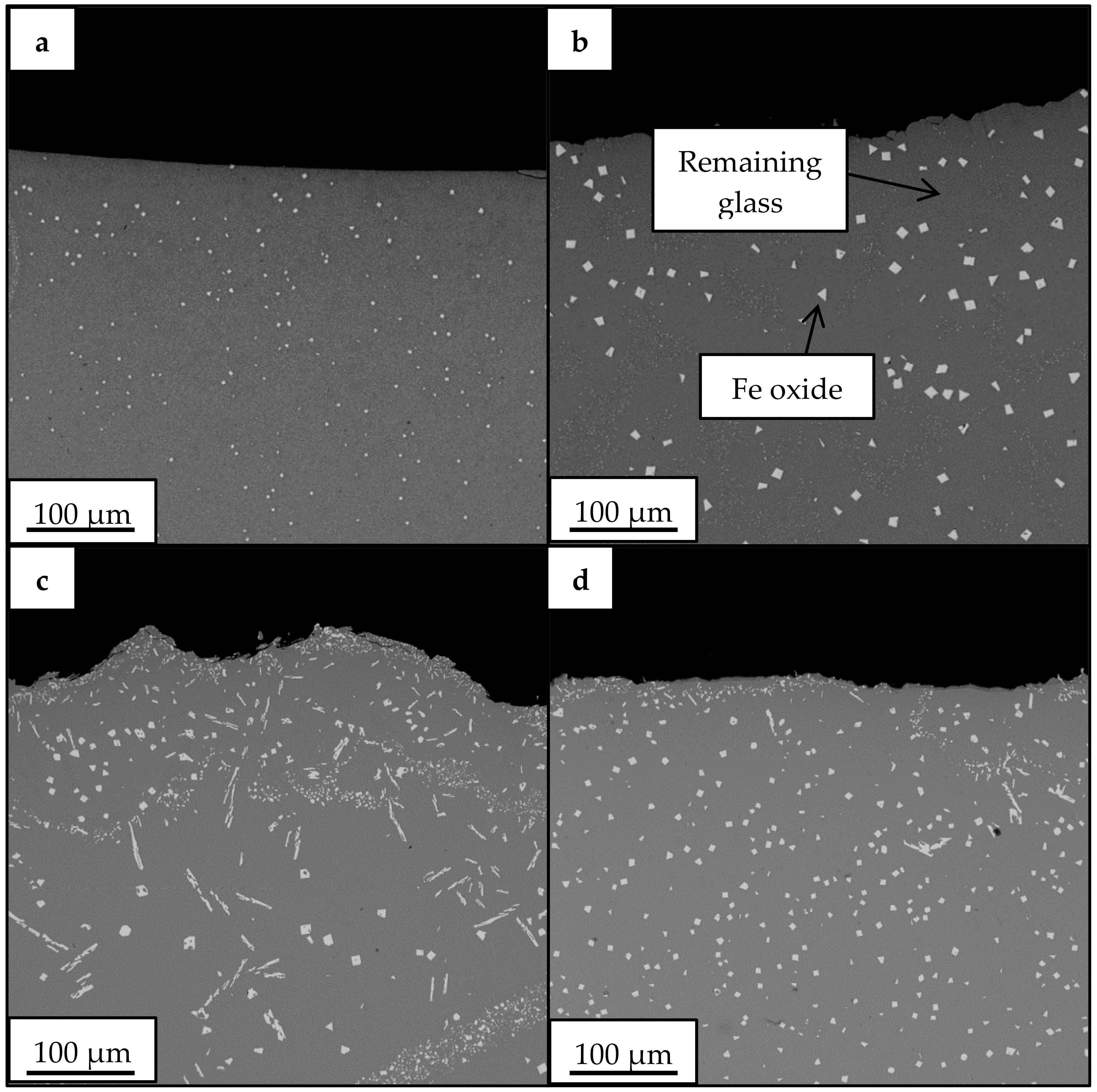
3.2. Crystallization Behavior—Box Furnace Experiments
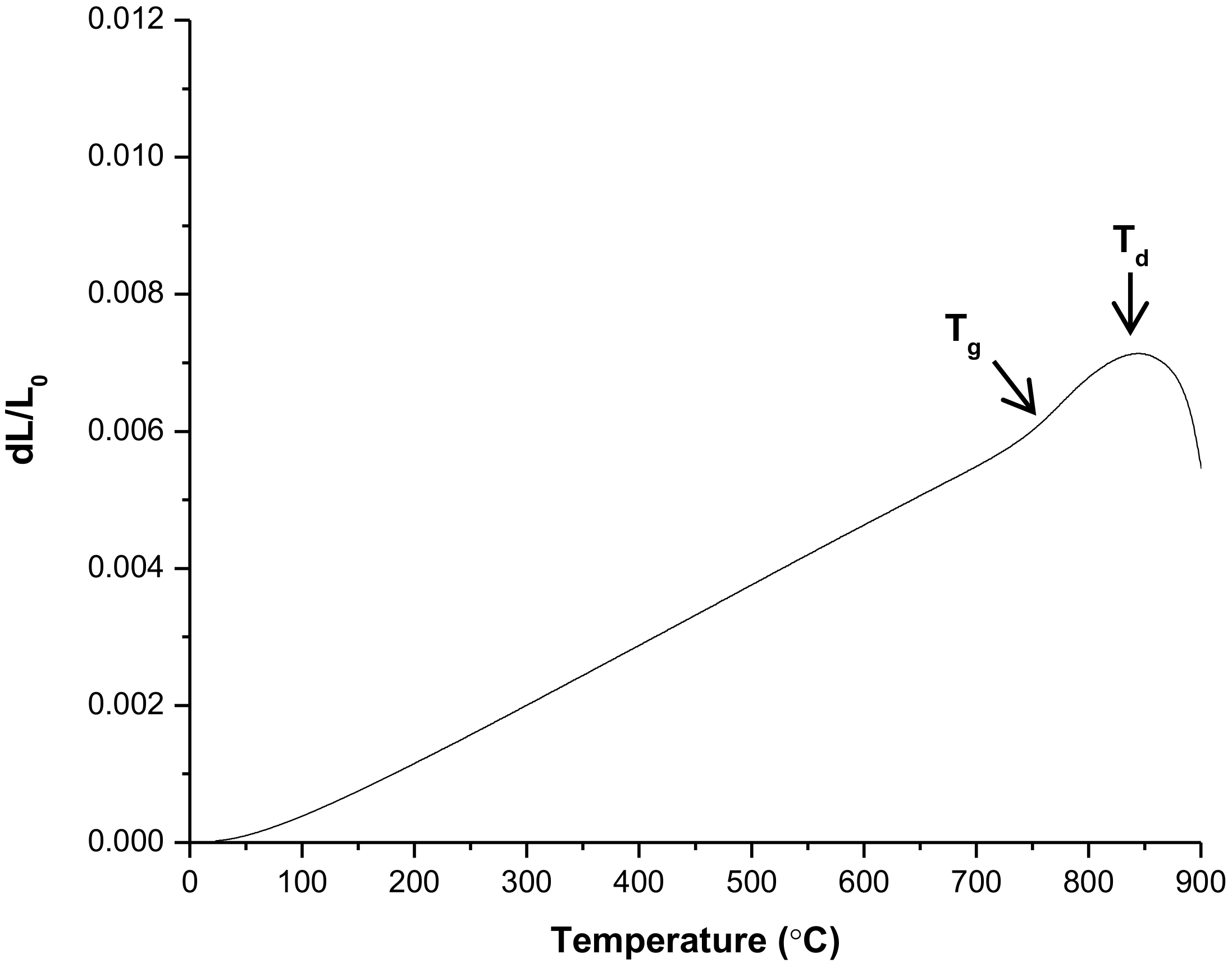
3.3. Thermal Behavior—Dilatometry
3.4. Density and Mechanical Properties
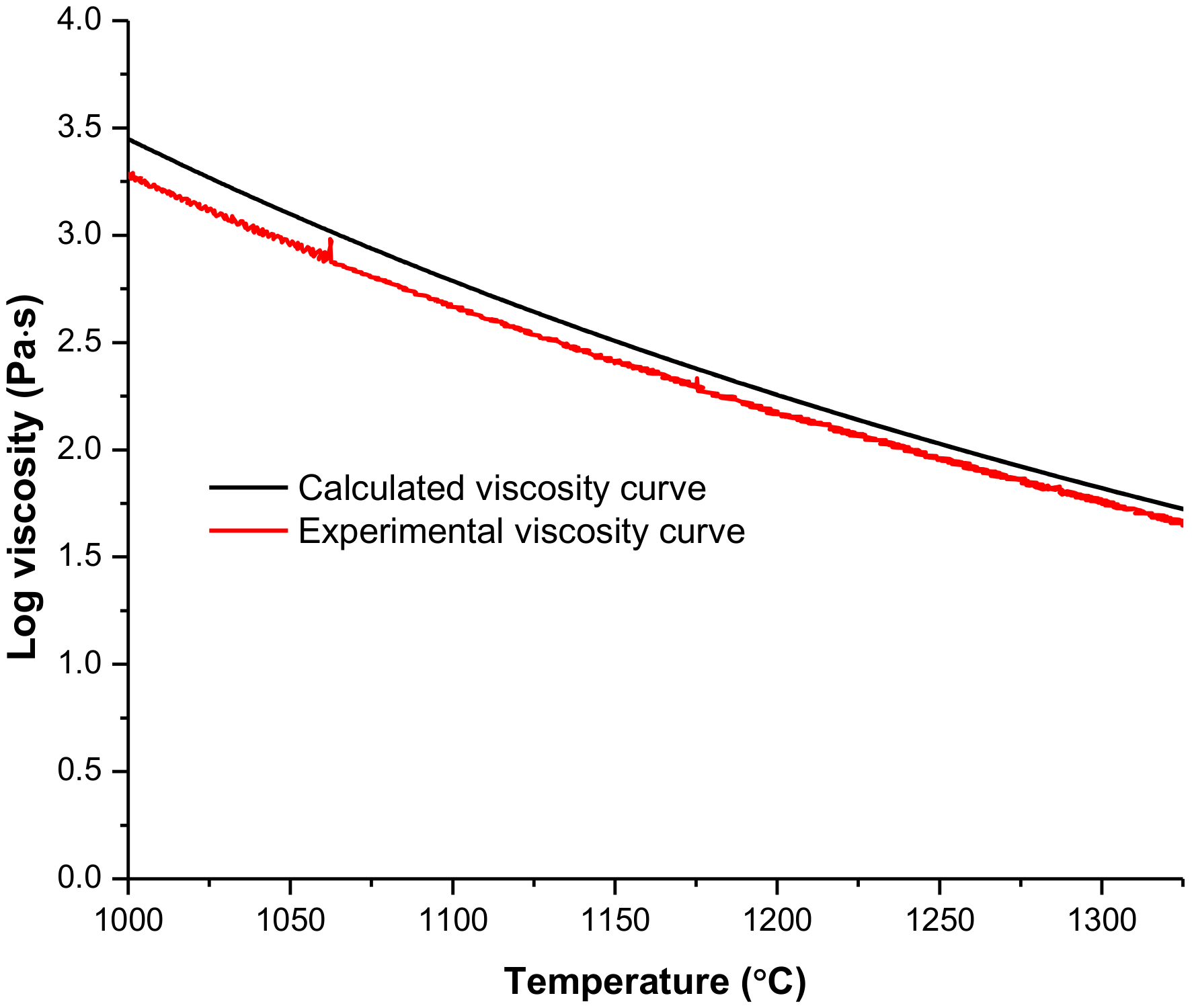
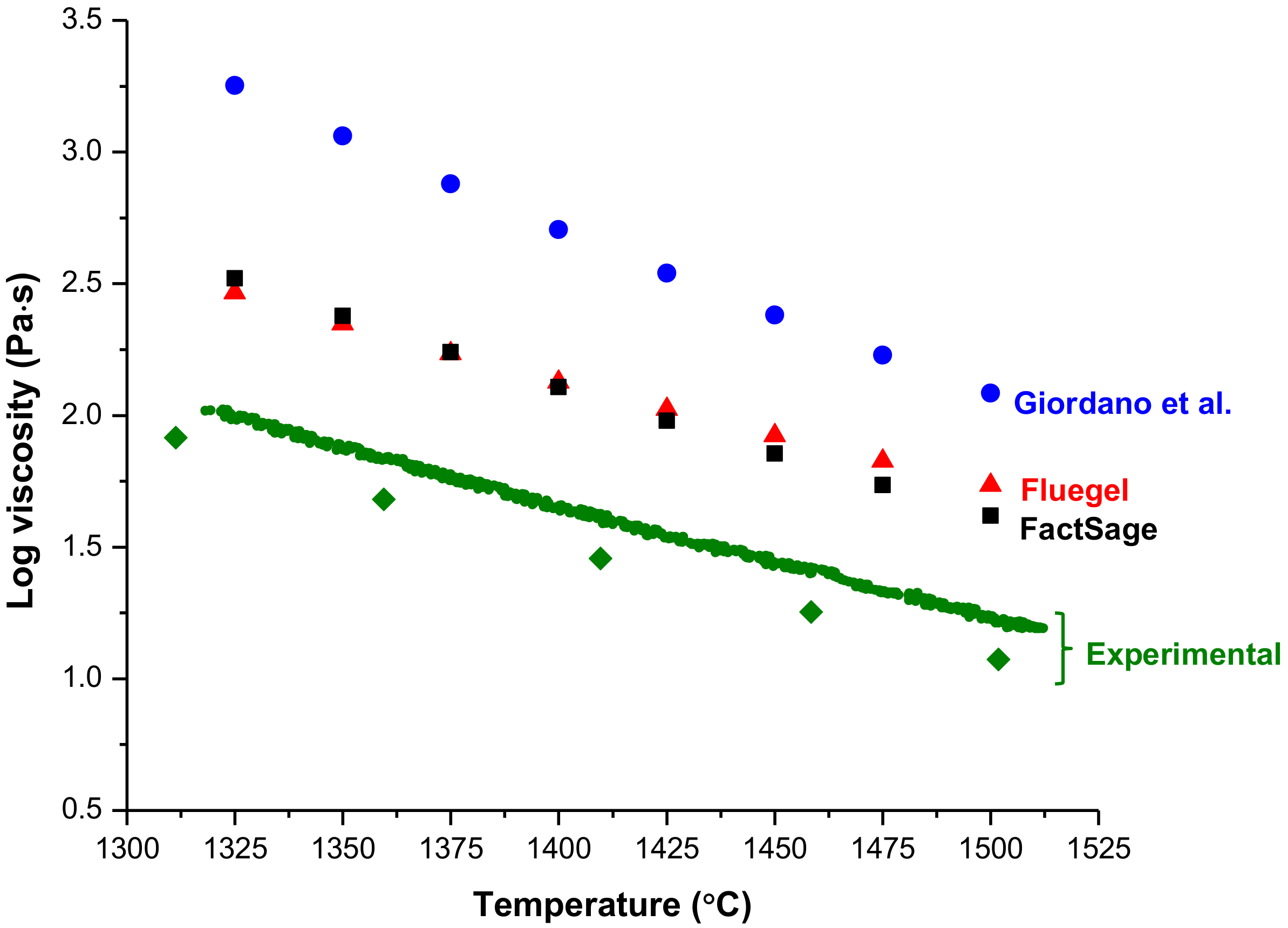
3.5. Viscosity
4. Discussion
5. Summary and Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- De Wet, D.; Taylor, R.; Stott, F. Corrosion mechanisms of ZrO2-Y2O3 thermal barrier coatings in the presence of molten middle-east sand. J. Phys. IV 1993, 3, 655–663. [Google Scholar] [CrossRef] [Green Version]
- Stott, F.H.; De Wet, D.; Taylor, R. Degradation of thermal barrier coatings at very high temperature. MRS Bull. 1994, 19, 46–49. [Google Scholar] [CrossRef]
- Clarke, D.R.; Oechsner, M.; Padture, N.P. Thermal-barrier coatings for more efficient gas turbine engines. MRS Bull. 2012, 37, 891–898. [Google Scholar] [CrossRef] [Green Version]
- Ohnabe, H.; Masaki, S.; Onozuka, M.; Miyahara, K.; Sasa, T. Potential application of ceramic matrix composites to aero-engine components. Compos. Part A 1999, 30, 489–496. [Google Scholar] [CrossRef]
- Lee, K.N. Environmental Barrier Coatings for SiCf/SiC. In Ceramic Matrix Composites: Materials, Modeling and Technology; Bansal, N.P., Lamon, J., Eds.; John Wiley and Sons: Hoboken, NJ, USA, 2014; pp. 430–451. [Google Scholar]
- Lee, K.N.; Fox, D.S.; Bansal, N.P. Rare earth silicate environmental barrier coatings for SiC/SiC composites and Si3N4 ceramics. J. Eur. Ceram. Soc. 2005, 25, 1705–1715. [Google Scholar] [CrossRef]
- Borom, M.P.; Johnson, C.A.; Peluso, L.A. Role of environment deposits and operating surface temperature in spallation of air plasma sprayed thermal barrier coatings. Surf. Coat. Technol. 1996, 86, 116–126. [Google Scholar] [CrossRef]
- Krämer, S.; Yang, J.; Levi, C.G. Infiltration-inhibiting reaction of gadolinium zirconate thermal barrier coatings with CMAS melts. J. Am. Ceram. Soc. 2008, 91, 576–583. [Google Scholar] [CrossRef]
- Poerschke, D.L.; Jackson, R.W.; Levi, C.G. Silicate deposit degradation of engineered coatings in gas turbines: Progress toward models and materials solutions. Annu. Rev. Mater. Res. 2017, 47, 297–330. [Google Scholar] [CrossRef]
- Stolzenburg, F.; Johnson, M.T.; Lee, K.N.; Jacobson, N.S.; Faber, K.T. The interaction of calcium magnesium-aluminosilicate with ytterbium silicate environmental barrier materials. Surf. Coat. Technol. 2015, 284, 44–50. [Google Scholar] [CrossRef] [Green Version]
- Zhao, H.; Richards, B.T.; Levi, C.G.; Wadley, H.N.G. Molten silicate reactions with plasma sprayed ytterbium silicate coatings. Surf. Coat. Technol. 2016, 288, 151–162. [Google Scholar] [CrossRef] [Green Version]
- Ahlborg, N.L.; Zhu, D. Calcium-magnesium aluminosilicate (CMAS) reactions and degradation mechanisms of advanced environmental barrier coatings. Surf. Coat. Technol. 2013, 237, 79–87. [Google Scholar] [CrossRef] [Green Version]
- Wiesner, V.L.; Harder, B.J.; Bansal, N.P. High-temperature interactions of desert sand CMAS glass with yttrium disilicate environmental barrier coating material. Ceram. Int. 2018, 44, 22738–22743. [Google Scholar] [CrossRef]
- Levi, C.G.; Hutchinson, J.W.; Vidal-Sétif, M.-H.; Johnson, C.A. Environmental degradation of thermal barrier coatings by molten deposits. MRS Bull. 2012, 37, 932–941. [Google Scholar] [CrossRef] [Green Version]
- Zaleski, E.M.; Ensslen, C.; Levi, C.G. Melting and crystallization of silicate systems relevant to thermal barrier coating damage. J. Am. Ceram. Soc. 2015, 98, 1642–1649. [Google Scholar] [CrossRef]
- Delmelle, P.; Lambert, M.; Dufrêne, Y.; Gerin, P.; Óskarsson, N. Gas/aerosol-ash interaction in volcanic plumes: New insights from surface analyses of fine ash particles. Earth Planet. Sci. Lett. 2007, 259, 159–170. [Google Scholar] [CrossRef]
- Wiesner, V.L.; Bansal, N.P. Crystallization kinetics of calcium-magnesium aluminosilicate (CMAS) glass. Surf. Coat. Technol. 2014, 259, 608–615. [Google Scholar] [CrossRef]
- Wiesner, V.L.; Bansal, N.P. Mechanical and thermal properties of calcium magnesium aluminosilicate (CMAS) glass. J. Eur. Ceram. Soc. 2015, 35, 2907–2914. [Google Scholar] [CrossRef]
- Bansal, N.P.; Choi, S.R. Properties of CMAS glass from desert sand. Ceram. Int. 2015, 41, 3901–3909. [Google Scholar] [CrossRef]
- ASTM C1259-14 Standard Test Method for Dynamic Young’s Modulus, Shear Modulus, and Poisson’s Ratio for Advanced Ceramics by Impulse Excitation of Vibration; ASTM International: Conshohocken, PA, USA, 2014.
- ASTM C1327-08 Standard Test Method for Vickers Indentation Hardness of Advanced Ceramics; ASTM International: Conshohocken, PA, USA, 2008.
- Lamkin, M.A.; Riley, F.L.; Fordham, R.J. Oxygen mobility in silicon dioxide and silicate glasses: A review. J. Eur. Ceram. Soc. 1992, 10, 347–367. [Google Scholar] [CrossRef]
- Monazam, E.R.; Breault, R.W.; Siriwardane, R. Kinetics of magnetite (Fe3O4) oxidation to hematite (Fe2O3) in air for chemical looping combustion. Ind. Eng. Chem. Res. 2014, 53, 13320–13328. [Google Scholar] [CrossRef]
- Forsmo, S.P.E.; Forsmo, S.-E.; Samskog, P.-O.; Björkman, B.M.T. Mechanisms in oxidation and sintering of magnetite iron ore green pellets. Powder Technol. 2008, 183, 247–259. [Google Scholar] [CrossRef]
- Bale, C.W.; Bélisle, E.; Chartrand, P.; Decterov, S.A.; Eriksson, G.; Hack, K.; Jung, I.-H.; Kang, Y.B.; Melançon, J.; Pelton, A.D.; et al. FactSage thermochemical software and databases-recent developments. Calphad 2009, 33, 295–311. [Google Scholar] [CrossRef]
- Miyoshi, T.; Sagawa, N.; Sassa, T. Study on fracture toughness evaluation for structural ceramics. Trans. Jpn. Soc. Mech. Eng. 1985, 51, 2487–2489. [Google Scholar] [CrossRef] [Green Version]
- Marshall, D.; Evans, A. Reply to “Comment on ‘Elastic/plastic indentation damage in ceramics: The median/radial crack system’”. J. Am. Ceram. Soc. 1981, 64, C182–C183. [Google Scholar]
- Anstis, G.; Chantikul, P.; Lawn, B.R.; Marshall, R. A critical evaluation of indentation techniques for measuring fracture toughness: I. Direct crack measurements. J. Am. Ceram. Soc. 1981, 64, 533–538. [Google Scholar] [CrossRef]
- Salem, J.; Jenkins, M. Applying ASTM International C1421 to glasses and optical ceramics. In Proceedings of the 42nd International Conference on Advanced Ceramics and Composites, Ceramic Engineering and Science Proceedings; Salem, J., Koch, D., Mechnich, P., Kusnezoff, M., Bansal, N.P., LaSalvia, J.C., Balaya, P., Fu, Z., Ohii, T., Wiesner, V., et al., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2019; Volume 39, pp. 27–40. [Google Scholar]
- Giordano, D.; Russell, J.K.; Dingwell, D.B. Viscosity of magmatic liquids: A model. Earth Planet. Sci. Lett. 2008, 271, 123–134. [Google Scholar] [CrossRef]
- Vogel, D.H. Temperaturabhängigkeitsgesetz der Viskosität von Flüssigkeiten. Phys. Z. 1921, 22, 645–646. [Google Scholar]
- Fulcher, G.S. Analysis of recent measurements of the viscosity of glasses. J. Am. Ceram. Soc. 1925, 8, 339–355. [Google Scholar] [CrossRef]
- Tammann, G.; Hesse, W. Die abhängigkeit der viskosität von der temperatur bei unterkühlten flüssigkeiten. Z. Anorgan. Allg. Chem. 1926, 156, 245–257. [Google Scholar] [CrossRef]
- Fluegel, A. Glass viscosity calculation based on a global statistical modeling approach. Glass Technol. Eur. J. Glass Sci. Technol. Part A 2007, 48, 13–30. [Google Scholar]
- Wiesner, V.L.; Vempati, U.K.; Bansal, N.P. High temperature viscosity of calcium magnesium aluminosilicate glass from synthetic sand. Scr. Mater. 2016, 124, 189–192. [Google Scholar] [CrossRef]
- Wiesner, V.L.; Vempati, U.K.; Bansal, N.P. Corrigendum to “High temperature viscosity of calcium-magnesium-aluminosilicate glass from synthetic sand” [Scripta Mater. 2016, 124, 189–192]. Scr. Mater. 2017, 130, 298. [Google Scholar] [CrossRef]
- Krämer, S.; Yang, J.; Levi, C.G.; Johnson, C.A. Thermochemical interaction of thermal barrier coatings with molten CaO-MgO-Al2O3-SiO2 (CMAS) deposits. J. Am. Ceram. Soc. 2006, 89, 3167–3175. [Google Scholar] [CrossRef]
- Kucuk, A.; Clare, A.; Jones, L. An estimation of the surface tension for silicate glass melts at 1400 °C using statistical analysis. Glass Technol. 1999, 40, 149–153. [Google Scholar]
- Mechnich, P.; Braue, W.; Schulz, U. High-temperature corrosion of EB-PVD yttria partially stabilized zirconia thermal barrier coatings with an artificial volcanic ash overlay. J. Am. Ceram. Soc. 2011, 94, 925–931. [Google Scholar] [CrossRef]
- Jang, B.-K.; Feng, F.-J.; Suzuta, K.; Tanaka, H.; Matsushita, Y.; Lee, K.-S.; Ueno, S. Corrosion behavior of volcanic ash and calcium magnesium aluminosilicate on Yb2SiO5 environmental barrier coatings. J. Ceram. Soc. Jpn. 2017, 125, 326–332. [Google Scholar] [CrossRef] [Green Version]
- Mechnich, P.; Braue, W. Volcanic ash-induced decomposition of EB-PVD Gd2Zr2O7 thermal barrier coatings to Gd-oxyapatite, zircon, and Gd,Fe-zirconolite. J. Am. Ceram. Soc. 2013, 96, 1958–1965. [Google Scholar] [CrossRef] [Green Version]
- Evans, A.G.; Mumm, D.R.; Hutchinson, J.W.; Meier, G.H.; Pettit, F.S. Mechanisms controlling the durability of thermal barrier coatings. Prog. Mater. Sci. 2001, 46, 505–553. [Google Scholar] [CrossRef]
- Touloukian, Y.S.; Kirby, R.K.; Taylor, R.E.; Lee, T.Y.R. Thermal Expansion-Non Metallic Solids; IFI/Plenum: New York, NY, USA, 1977; Volume 13. [Google Scholar]
- Fukuda, K.; Matsubara, H. Anisotropic thermal expansion in yttrium silicate. J. Mater. Res. 2003, 18, 1715–1722. [Google Scholar] [CrossRef]
- NETL. The Gas Turbine Handbook; United States Department of Energy (DOE): Morgantown, WV, USA, 2006. [Google Scholar]
- Sun, Z.; Zhou, Y.; Wang, J.; Li, M. Thermal properties and thermal shock resistance of γ- Y2Si2O7. J. Am. Ceram. Soc. 2008, 91, 2623–2629. [Google Scholar] [CrossRef]
- Fernández-Carrión, A.J.; Allix, M.; Becerro, A.I. Thermal expansion of rare-earth pyrosilicates. J. Am. Ceram. Soc. 2013, 96, 2298–2305. [Google Scholar] [CrossRef]
- Takeda, M.; Onishi, T.; Nakakubo, S.; Fujimoto, S. Physical properties of iron-oxide scales on Si containing steels at high temperature. Mater. Trans. 2009, 50, 2242–2246. [Google Scholar] [CrossRef] [Green Version]
- Tribaudino, M.; Angel, R.J.; Cámara, F.; Nestola, F.; Pasqual, D.; Margiolaki, I. Thermal expansion of plagioclase feldspars. Mineral. Petrol. 2010, 160, 899–908. [Google Scholar] [CrossRef]
- Vassen, R.; Cao, X.; Tietz, F.; Basu, D.; Stöver, D. Zirconates as new materials for thermal barrier coatings. J. Am. Ceram. Soc. 2000, 83, 2023–2028. [Google Scholar] [CrossRef]
- Van Dijk, M.P.; de Vries, K.J.; Burggraaf, A.J. Oxygen ion and mixed conductivity in compounds with the fluorite and pyrochlore structure. Solid State Ion. 1983, 9–10, 913–919. [Google Scholar] [CrossRef] [Green Version]
- Schmitt, M.P.; Stokes, J.L.; Gorin, B.L.; Rai, A.K.; Zhu, D.; Eden, T.J.; Wolfe, D.E. Effect of Gd content on mechanical properties and erosion durability of sub-stoichiometric Gd2Zr2O7. Surf. Coat. Technol. 2017, 313, 177–183. [Google Scholar] [CrossRef] [Green Version]
- Sun, Z.; Zhou, Y.; Wang, J.; Li, M. γ-Y2Si2O7, a machinable silicate ceramic: Mechanical properties and machinability. J. Am. Ceram. Soc. 2007, 90, 2535–2541. [Google Scholar] [CrossRef]
- Zhou, Y.-C.; Zhao, C.; Wang, F.; Sun, Y.-J.; Zheng, L.-Y.; Wang, X.-H. Theoretical prediction and experimental investigation on the thermal and mechanical properties of bulk β- Yb2Si2O7. J. Am. Ceram. Soc. 2013, 96, 3891–3900. [Google Scholar] [CrossRef]
- Sun, Z.; Wang, J.; Li, M.; Zhou, Y. Mechanical properties and damage tolerance of Y2SiO5. J. Eur. Ceram. Soc. 2008, 28, 2895–2901. [Google Scholar] [CrossRef]
- Lu, M.-H.; Xiang, H.-M.; Feng, Z.-H.; Wang, X.-Y.; Zhou, Y.-C. Mechanical and thermal properties of Yb2SiO5: A promising material for T/EBCs applications. J. Am. Ceram. Soc. 2016, 99, 1401–1411. [Google Scholar] [CrossRef]
- Stolper, E. Water in silicate glasses: An infrared spectroscopic study. Contrib. Mineral. Petrol. 1982, 81, 1–17. [Google Scholar] [CrossRef]
- Cook, G.B.; Cooper, R.F. Chemical diffusion and crystalline nucleation during oxidation of ferrous iron-bearing magnesium aluminosilicate glass. J. Non Cryst. Solids 1990, 120, 207–222. [Google Scholar] [CrossRef]

| CaO | MgO | Al2O3 | SiO2 | Fe2O3 | TiO2 | Na2O | K2O | Trace Oxides | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Volcanic ash glass | mol % | 6.2 | 4.2 | 10.1 | 67.5 | 4.4 | 1.4 | 5.0 | 1.2 | – |
| wt % | 4.9 | 2.4 | 14.6 | 57.3 | 10.0 | 1.6 | 4.4 | 1.6 | bal. | |
| Synthetic sand glass | mol % | 23.3 | 6.4 | 3.1 | 62.5 | 0.04 | – | 4.1 | 0.5 | – |
| wt % | 21.9 | 4.3 | 5.4 | 63 | 0.1 | – | 4.3 | 0.8 | bal. | |
| Desert sand glass | mol % | 27.8 | 4 | 5 | 61.6 | 0.6 | – | – | 1 | – |
| wt % | 25.2 | 2.6 | 8.2 | 59.8 | 1.6 | – | – | 1.5 | bal. | |
| Bulk | Approximate Temperature (°C) | Powder | Approximate Temperature (°C) | |
|---|---|---|---|---|
| On heating | ||||
| Onset Melting (endo) | 3B | 1050 °C (peak melting at 1350 °C) | 4P | 950–1000 °C |
| Crystallization (exo) | 1B, 2B | 900, 1100 °C | 1P, 2P, 3P | 900, 1225, 1275 °C |
| On cooling | ||||
| Crystallization (exo) | 4B | 900 °C | 5P | 900 °C |
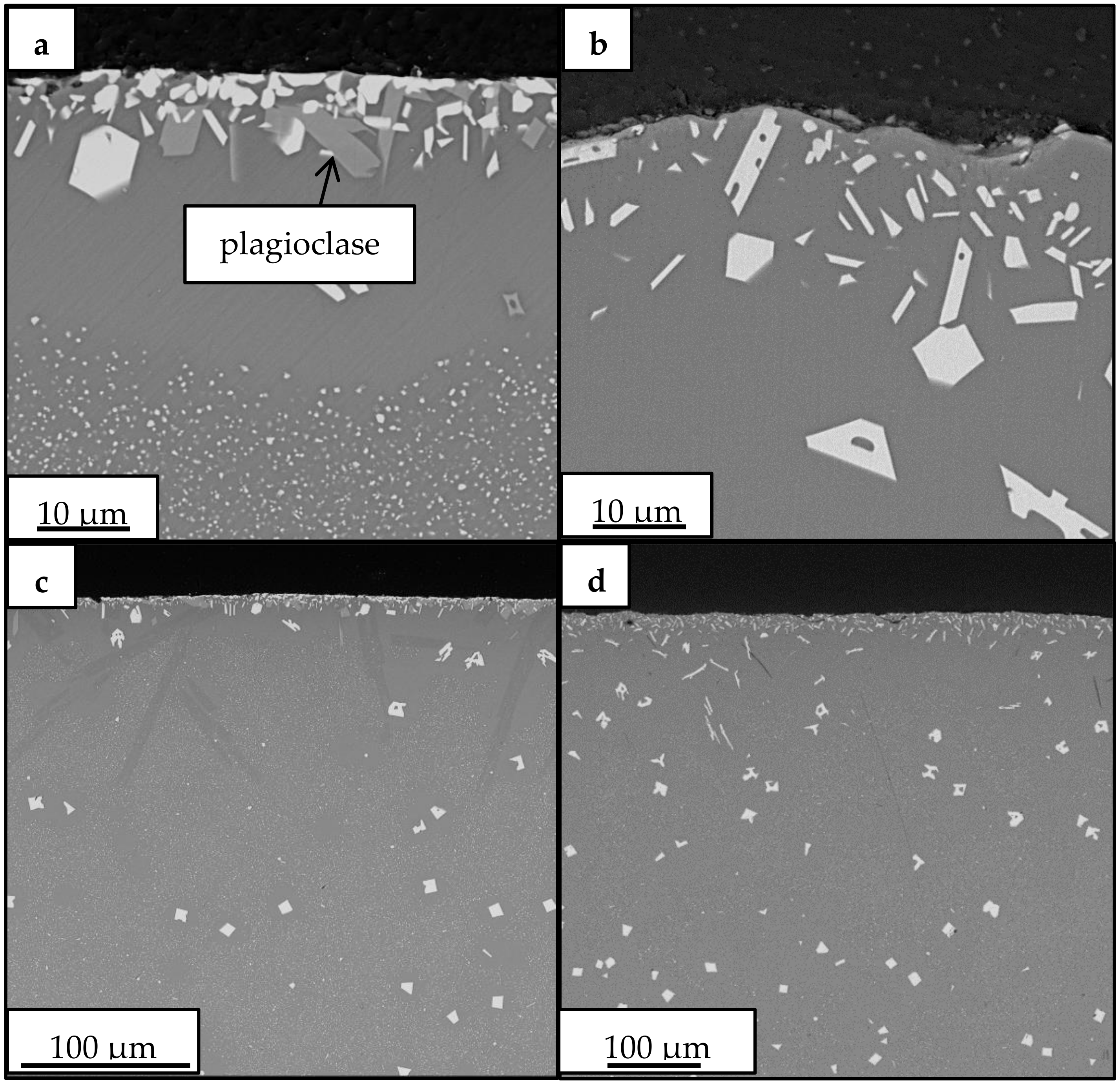
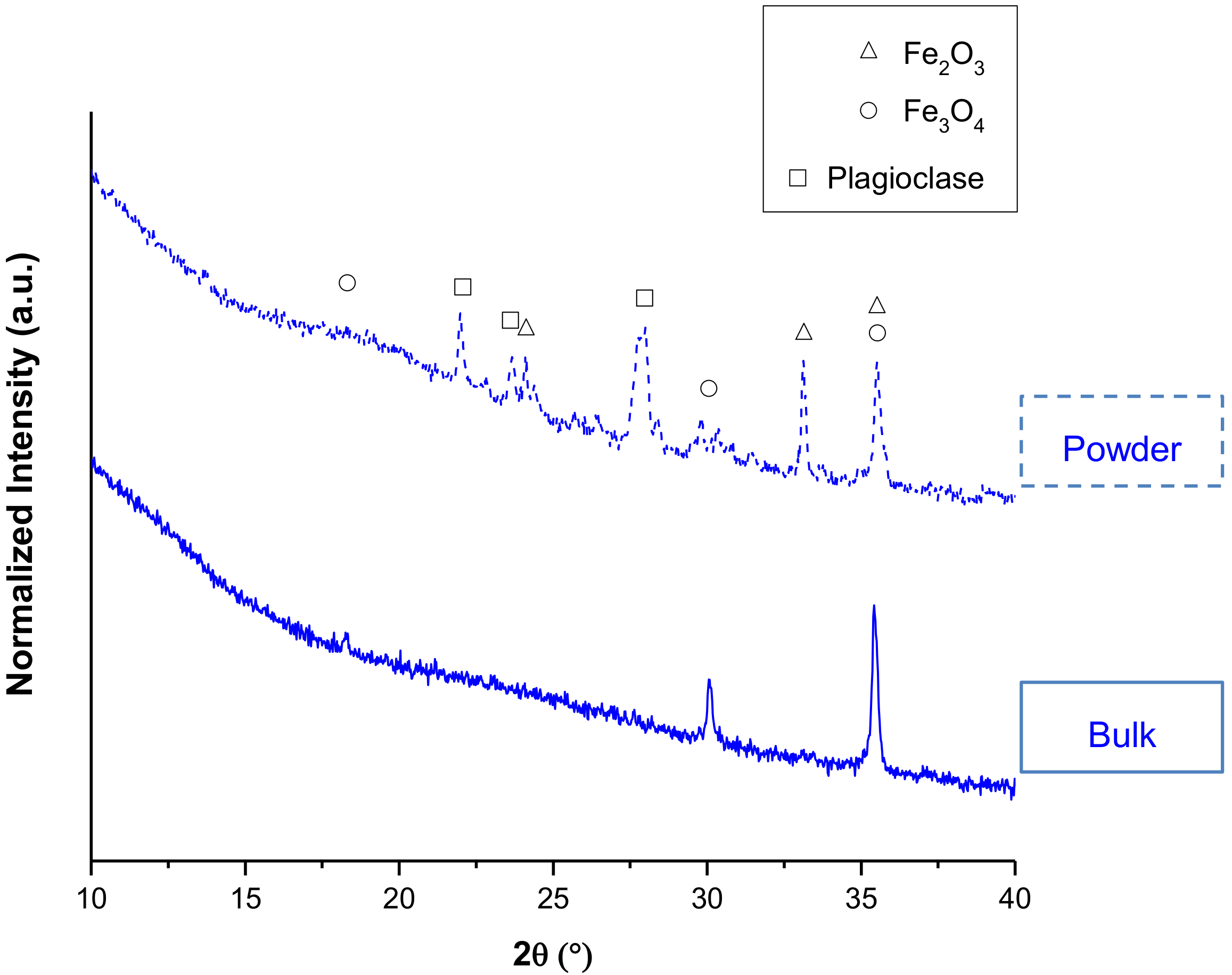
| wt % Amorphous | wt % Fe3O4 | wt % Fe2O3 | wt % Plagioclase | |
|---|---|---|---|---|
| 1100 °C | ||||
| 1 h | 88.7 ± 0.1 | 9.9 ± 0.1 | 1.4 ± 0.1 | 0.0 |
| 10 h | 87.8 ± 0.2 | 8.9 ± 0.1 | 1.0 ± 0.1 | 2.3 ± 0.2 |
| 20 h | 89.4 ± 0.1 | 5.9 ± 0.1 | 2.3 ± 0.1 | 2.4 ± 0.1 |
| 40 h | 81.7 ± 0.3 | 4.6 ± 0.1 | 3.0 ± 0.1 | 10.7 ± 0.3 |
| 1150 °C | ||||
| 1 h | 90.7 ± 0.1 | 8.5 ± 0.1 | 0.8 ± 0.0 | 0.0 |
| 10 h | 94.2 ± 0.1 | 2.8 ± 0.0 | 3.0 ± 0.0 | 0.0 |
| 20 h | 94.5 ± 0.1 | 1.6 ± 0.1 | 3.9 ± 0.0 | 0.0 |
| 40 h | 95.7 ± 0.0 | 0.0 | 4.3 ± 0.0 | 0.0 |
| 1200 °C | ||||
| 1 h | 90.6 ± 0.1 | 7.7 ± 0.1 | 1.8 ± 0.1 | 0.0 |
| 10 h | 95.5 ± 0.1 | 0.9 ± 0.1 | 3.6 ± 0.0 | 0.0 |
| 20 h | 95.4 ± 0.1 | 1.4 ± 0.0 | 3.2 ± 0.0 | 0.0 |
| wt % Amorphous | wt % Fe3O4 | wt % Fe2O3 | wt % Plagioclase | |
|---|---|---|---|---|
| 1100 °C | ||||
| 1 h | 50.6 ± 0.5 | 3.9 ± 0.1 | 7.2 ± 0.1 | 38.3 ± 0.5 |
| 1150 °C | ||||
| 1 h | 65.5 ± 0.3 | 0.5 ± 0.1 | 7.1 ± 0.1 | 26.8 ± 0.3 |
| Property | Volcanic Ash Glass | Synthetic Sand Glass * | Desert Sand Glass ± |
|---|---|---|---|
| Melting (Tm, °C) | ~1300–1350 °C | 1176 | – |
| Density (g/cm3) | 2.52 | 2.63 | 2.69 |
| Glass transition temperature (Tg, °C) | 741 | 694 | 706 |
| Dilatometric softening point (Td, °C) | 844 | 751 | 764 |
| Coefficient of thermal expansion (CTE α, K−1) | 7.00 × 10−6 (~25–720 °C) | 9.32 × 10−6 (~25–690 °C) | 9.8 × 10−6 (~25–700 °C) |
| Young’s Modulus (E, GPa) | 75 | 84.3 | 92.3 |
| Poisson’s ratio (ν) | 0.24 | 0.26 | 0.28 |
| Indent Load, P (N) | Vickers Hardness, HV (GPa) | Indentation Fracture Toughness, KC (MPa √m) Calculated Using Equations (3)–(5) | ||
|---|---|---|---|---|
| Miyoshi et al. [26] | Marshall and Evans [27] | Anstis et al. [28] | ||
| Volcanic ash glass (current study) | ||||
| 1.96 | 7.02 ± 0.48 | n/a | n/a | n/a |
| 2.94 | 6.22 ± 0.28 | 1.60 ± 0.24 | 1.83 ± 0.28 | 1.43 ± 0.22 |
| 4.9 | 6.99 ± 0.35 | 1.31 ± 0.08 | 1.53 ± 0.09 | 1.17 ± 0.07 |
| 9.8 | 7.03 ± 0.37 | 1.26 ± 0.13 | 1.47 ± 0.15 | 1.12 ± 0.12 |
| Grand mean | 6.75 ± 0.40 | 1.39 ± 0.18 | 1.61 ± 0.19 | 1.24 ± 0.17 |
| Synthetic sand glass [17,18] | ||||
| 1.96 | 6.12 ± 0.19 | 0.72 ± 0.04 | 0.82 ± 0.04 | 0.62 ± 0.04 |
| 2.94 | 6.28 ± 0.19 | 0.74 ± 0.05 | 0.86 ± 0.05 | 0.68 ± 0.04 |
| 4.9 | 6.04 ± 0.18 | 0.66 ± 0.05 | 0.74 ± 0.05 | 0.6 |
| 9.8 | 6.12 ± 0.13 | 0.62 ± 0.04 | 0.72 ± 0.04 | 0.58 ± 0.04 |
| Grand mean | 6.14 ± 0.10 | 0.69 ± 0.06 | 0.79 ± 0.07 | 0.62 ± 0.04 |
| Desert sand glass [19] | ||||
| 1.96 | 5.90 ± 0.10 | 0.70 ± 0.03 | 0.70 ± 0.03 | 0.60 ± 0.03 |
| 2.94 | 6.60 ± 0.10 | 0.80 ± 0.05 | 0.90 ± 0.10 | 0.70 ± 0.05 |
| 4.9 | 6.40 ± 0.10 | 0.75 ± 0.05 | 0.80 ± 0.10 | 0.65 ± 0.05 |
| 9.8 | 6.20 ± 0.10 | 0.65 ± 0.05 | 0.70 ± 0.10 | 0.60 ± 0.10 |
| Grand mean | 6.28 ± 0.30 | 0.73 ± 0.06 | 0.78 ± 0.10 | 0.64 ± 0.05 |
| Synthetic Sand | Volcanic Ash | |||||||
|---|---|---|---|---|---|---|---|---|
| Giordano | Fluegel | FactSage | Experimental | Giordano | Fluegel | FactSage | Experimental | |
| η 1300 °C | 274.9 | 33.6 | 78.1 | 19.5 (1318 °C) | 2848 | 389 | 465 | 82.3 (1311 °C) |
| η 1400 °C | 70.3 | 11.5 | 19.6 | 7.1 (1411 °C) | 507.3 | 134.0 | 127.9 | 28.6 (1410 °C) |
| η 1500 °C | 22.3 | 4.8 | 6.2 | 3.3 (1519 °C) | 121.4 | 54.4 | 41.7 | 11.8 (1502 °C) |
| Infiltration Time (1400 °C) | 3.8 min | n/a | 1.1 min | 0.41 min (1411 °C) | 29.2 min | 7.7 min | 7.3 min | 1.6 min (1410 °C) |
| Temperature (°C) | Logη (Pa·s) Using Fe2O3 | Logη (Pa·s) Using FeO |
|---|---|---|
| 1500 | 1.62 | 1.44 |
| 1475 | 1.74 | 1.55 |
| 1450 | 1.85 | 1.66 |
| 1425 | 1.98 | 1.78 |
| 1400 | 2.11 | 1.90 |
| 1375 | 2.24 | 2.03 |
| 1350 | 2.38 | 2.16 |
| 1325 | 2.52 | 2.30 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Webster, R.I.; Bansal, N.P.; Salem, J.A.; Opila, E.J.; Wiesner, V.L. Characterization of Thermochemical and Thermomechanical Properties of Eyjafjallajökull Volcanic Ash Glass. Coatings 2020, 10, 100. https://doi.org/10.3390/coatings10020100
Webster RI, Bansal NP, Salem JA, Opila EJ, Wiesner VL. Characterization of Thermochemical and Thermomechanical Properties of Eyjafjallajökull Volcanic Ash Glass. Coatings. 2020; 10(2):100. https://doi.org/10.3390/coatings10020100
Chicago/Turabian StyleWebster, Rebekah I., Narottam P. Bansal, Jonathan A. Salem, Elizabeth J. Opila, and Valerie L. Wiesner. 2020. "Characterization of Thermochemical and Thermomechanical Properties of Eyjafjallajökull Volcanic Ash Glass" Coatings 10, no. 2: 100. https://doi.org/10.3390/coatings10020100





