Binary Additives Enhance Micro Arc Oxidation Coating on 6061Al Alloy with Improved Anti-Corrosion Property
Abstract
:1. Introduction
2. Experimental Details
2.1. Materials and Regents
2.2. Fabrication of MAO Coating
2.3. Characterization
2.4. Electrochemical Tests
3. Results and Discussion
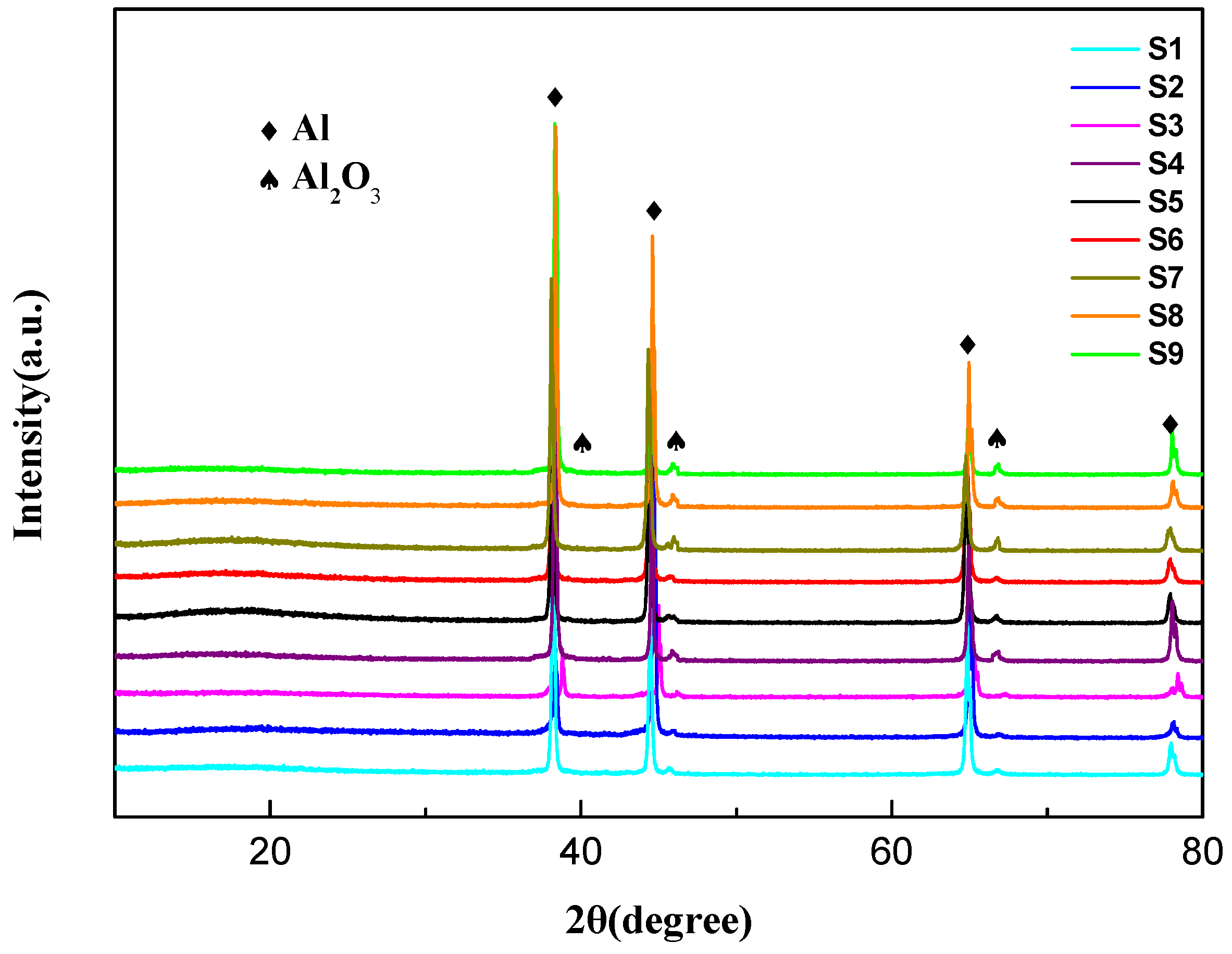
3.1. Thickness and Phase Structure
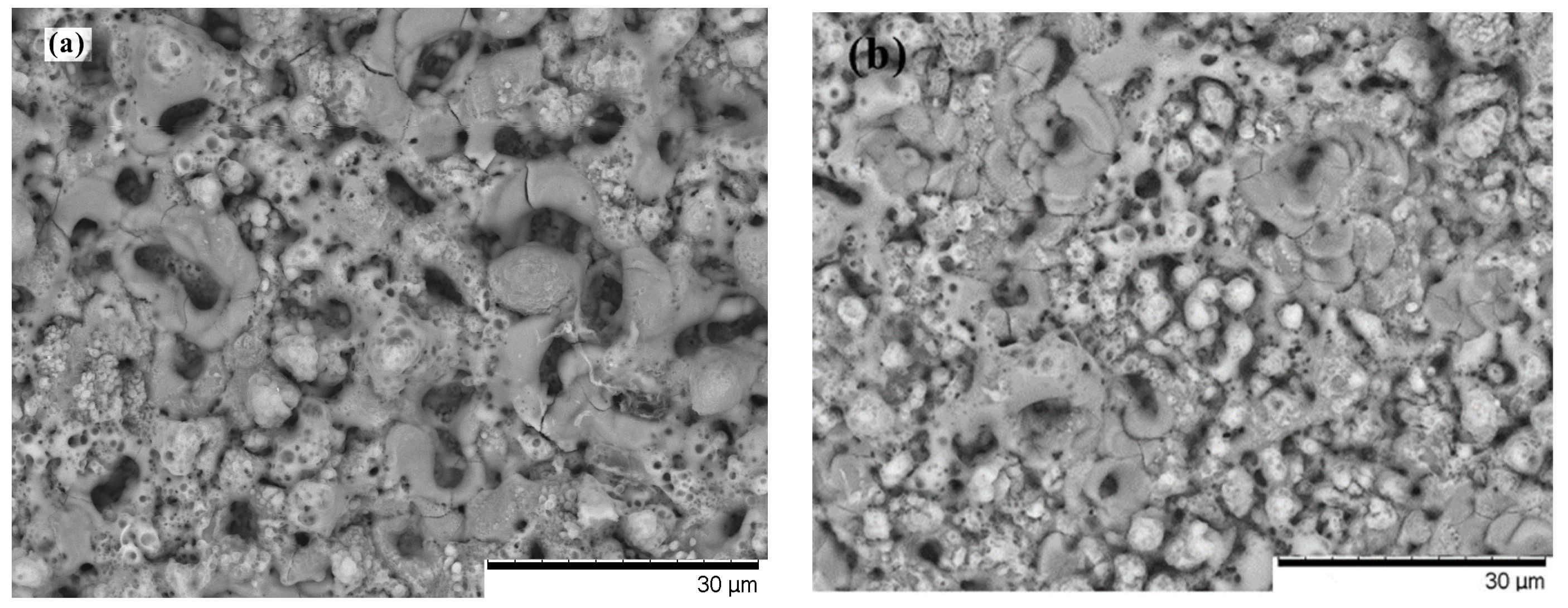
3.2. Microstructural Characteristics
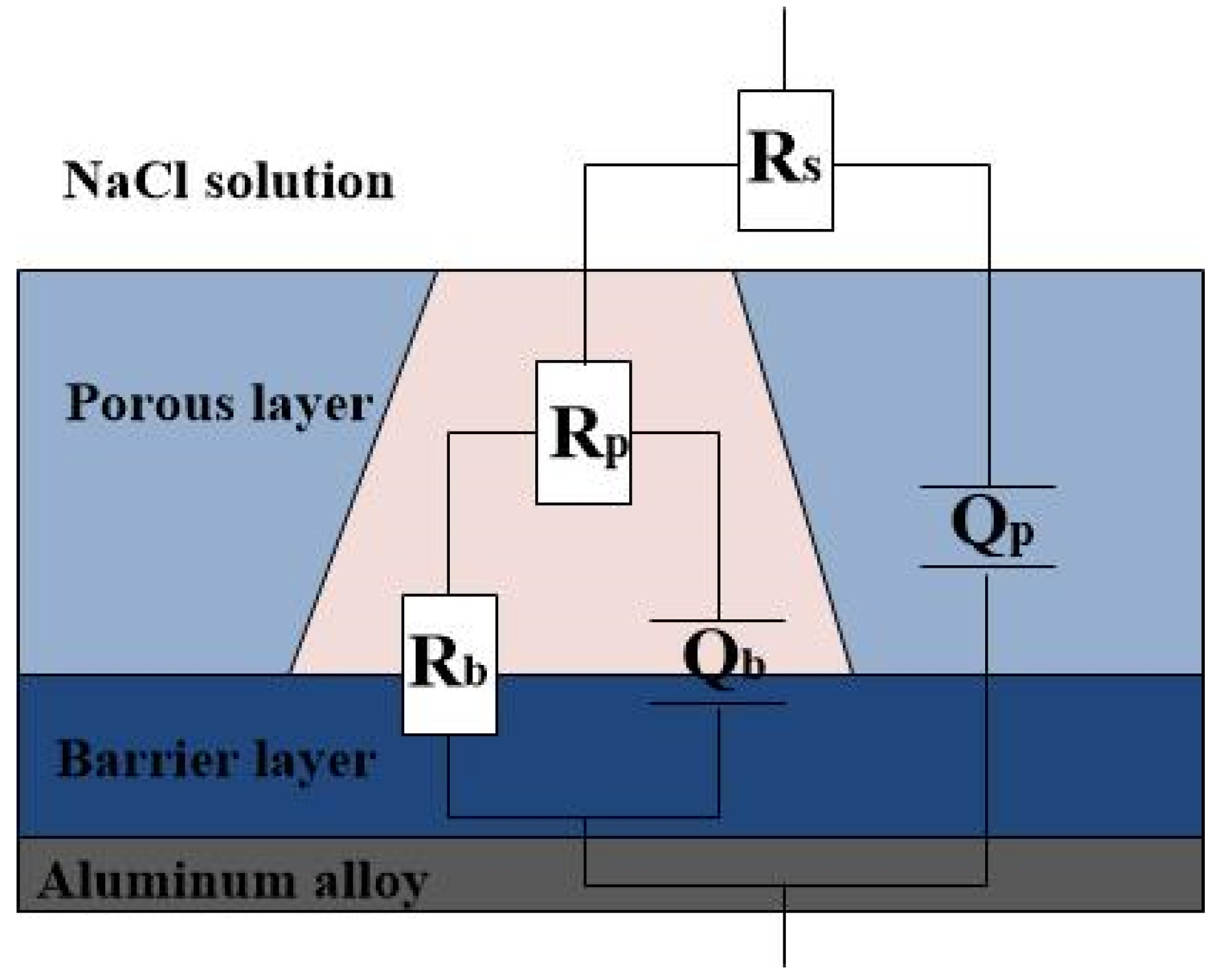
3.3. EIS Analysis
3.4. Potentiodynamic Polarization
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Md, S.U.R.; Jayahari, L. Study of mechanical properties and wear behaviour of aluminium 6061 matrix composites reinforced with steel machining chips. Mater. Today Proc. 2018, 5, 20117–20123. [Google Scholar] [CrossRef]
- Yang, L.H.; Wan, Y.X.; Qin, Z.L.; Xu, Q.J.; Min, Y.L. Fabrication and corrosion resistance of a graphene-tin oxide composite film on aluminium alloy 6061. Corros. Sci. 2018, 130, 85–94. [Google Scholar] [CrossRef]
- Zhang, B.B.; Xu, W.C.; Zhu, Q.J.; Sun, Y.Y.; Li, Y.T. Mechanically robust superhydrophobic porous anodized AA5083 for marine corrosion protection. Corros. Sci. 2019, 15, 108083. [Google Scholar] [CrossRef]
- Zhang, B.B.; Xu, W.C.; Zhu, Q.J.; Yuan, S.; Li, Y.T. Lotus-inspired multiscale superhydrophobic AA5083 resisting surface contamination and marine corrosion attack. Materials 2019, 12, 1592. [Google Scholar] [CrossRef] [Green Version]
- Ingrid, M.; Barbara, V. Conversion coatings based on rare earth nitrates and chlorides for corrosion protection of aluminum alloy 7075-T6. Corrosion 2017, 73, 822–843. [Google Scholar] [CrossRef]
- Reni, A.; Emilia, S.; Aleksandar, T.; Maria, D.; Dimitar, S. On the Role of pre-treatment of Aluminum Substrate on Deposition of Cerium Based Conversion Layers and Their Corrosion-Protective Ability. Int. J. Electrochem. Sci. 2018, 13, 5333–5351. [Google Scholar] [CrossRef]
- Kaseem, M.; Kamil, M.P.; Kwon, J.H.; Ko, Y.G. Effect of sodium benzoate on corrosion behavior of 6061 Al alloy processed by plasma electrolytic oxidation. Surf. Coat. Technol. 2015, 283, 268–273. [Google Scholar] [CrossRef]
- Barati, N.; Yerokhin, A.; Golestanifard, F.; Rastegari, S.; Meletis, E.I. Alumina- zirconia coatings produced by plasma electrolytic oxidation on Al alloy for corrosion resistance improvement. J. Alloy. Compd. 2017, 724, 435–442. [Google Scholar] [CrossRef]
- Ji, S.P.; Weng, Y.C.; Wu, Z.Z.; Ma, Z.Y.; Tian, X.B.; Ricky, K.Y.; Lin, H.; Wu, G.S.; Chu, P.K.; Pan, F. Excellent corrosion resistance of P and Fe modified micro-arc oxidation coating on Al alloy. J. Alloy. Compd. 2017, 710, 452–459. [Google Scholar] [CrossRef]
- Wang, W.B.; Dong, P.; Wang, H.Y.; Cheng, J.; Liu, S.L. Synergistic corrosion inhibition effect of molybdate and phosphate ions for anodic oxidation film formed on 2024 aluminum alloy. J. Wuhan Univ. Technol. Mat. Sci. Edit. 2019, 34, 426–432. [Google Scholar] [CrossRef]
- Wan, Y.; Wang, H.; Zhang, Y.D.; Wang, X.M.; Li, Y.B. Study on anodic oxidation and sealing of aluminum alloy. Int. J. Electrochem. Sci. 2018, 13, 2175–2185. [Google Scholar] [CrossRef]
- Wu, X.Q.; Yan, H.; Xin, Y. Study on microstructure and properties of laser cladding Al-Ti-C coating on aluminum alloy surface. J. Nanoelectron. Optoelectron. 2018, 13, 1258–1264. [Google Scholar] [CrossRef]
- Ni, C.; Shi, Y.; Liu, J.; Huang, G.Z. Characterization of Al0.5FeCu0.7NiCoCr high-entropy alloy coating on aluminum alloy by laser cladding. Opt. Laser Technol. 2018, 105, 257–263. [Google Scholar] [CrossRef]
- Chang, S.Y.; Lin, H.K.; Tsao, L.C.; Huang, W.T.; Huang, Y.T. Effect of voltage on microstructure and corrosion resistance of microarc oxidation coatings on CP-Ti. Corros. Eng. Sci. Technol. 2014, 49, 17–22. [Google Scholar] [CrossRef]
- Shokouhfar, M.; Allahkaram, S.R. Formation mechanism and surface characterization of ceramic composite coatings on pure titanium prepared by micro-arc oxidation in electrolytes containing nanoparticles. Surf. Coat. Technol. 2016, 291, 396–406. [Google Scholar] [CrossRef]
- Krishna, L.R.; Poshal, G.; Jyothirmayi, A.; Sundararajan, G. Relative hardness and corrosion behavior of micro arc oxidation coatings deposited on binary and ternary magnesium alloys. Mater. Des. 2015, 77, 6–14. [Google Scholar] [CrossRef]
- Oh, Y.J.; Mun, J.I.; Kim, J.H. Effects of alloying elements on microstructure and protective properties of Al2O3 coatings formed on aluminum alloy substrates by plasma electrolysis. Surf. Coat. Technol. 2009, 204, 141–148. [Google Scholar] [CrossRef]
- Gao, H.T.; Zhang, M.; Yang, X.; Huang, P.; Xu, K.W. Effect of Na2SiO3 solution concentration of micro-arc oxidation process on lap-shear strength of adhesive-bonded magnesium alloys. Appl. Surf. Sci. 2014, 314, 447–452. [Google Scholar] [CrossRef]
- Karbowniczek, J.; Muhaffel, F.; Cempura, G.; Cimenoglu, H.; Czyrska-Filemonowicz, A. Influence of electrolyte composition on microstructure, adhesion and bioactivity of micro-arc oxidation coatings produced on biomedical Ti6Al7Nb alloy. Surf. Coat. Technol. 2017, 321, 97–107. [Google Scholar] [CrossRef]
- Martin, J.; Leone, P.; Nominé, A.; Veys-Renaux, D.; Henrion, G.; Belmonte, T. Influence of electrolyte ageing on the Plasma Electrolytic Oxidation of aluminium. Surf. Coat. Technol. 2015, 269, 36–46. [Google Scholar] [CrossRef]
- Bosta, M.M.S.A.; Mab, K.J.; Chien, H.H. The effect of MAO processing time on surface properties and low temperature infrared emissivity of ceramic coating on aluminium 6061 alloy. Infrared Phys. Technol. 2013, 60, 323–334. [Google Scholar] [CrossRef]
- Martin, J.; Melhem, A.; Shchedrina, I.; Duchanoy, T.; Nominé, A.; Henrion, G.; Czerwiec, T.; Belmonte, T. Effects of electrical parameters on plasma electrolytic oxidation of aluminium. Surf. Coat. Technol. 2013, 221, 70–76. [Google Scholar] [CrossRef]
- Bosta, M.M.S.A.; Ma, K.J. Influence of electrolyte temperature on properties and infrared emissivity of MAO ceramic coating on 6061 aluminum alloy. Infrared Phys. Technol. 2014, 67, 63–72. [Google Scholar] [CrossRef]
- Kaseem, M.; Min, J.H.; Ko, Y.G. Corrosion behavior of Al-1wt% Mg-0.85wt%Si alloy coated by microarc-oxidation using TiO2 and Na2MoO4 additives: Role of current density. J. Alloy. Compd. 2017, 723, 448–455. [Google Scholar] [CrossRef]
- Ezhilselvi, V.; Nithin, J.; Balaraju, J.N.; Subramanian, S. The influence of current density on the morphology and corrosion properties of MAO coatings on AZ31B magnesium alloy. Surf. Coat. Technol. 2016, 288, 221–229. [Google Scholar] [CrossRef]
- Xiang, N.; Song, R.G.; Zhuang, J.J.; Song, R.X.; Lu, X.Y.; Su, X.P. Effects of current density on microstructure and properties of plasma electrolytic oxidation ceramic coatings formed on 6063 aluminum alloy. Trans. Nonferrous Met. Soc. China 2016, 26, 806–813. [Google Scholar] [CrossRef]
- Wang, J.H.; Du, M.H.; Han, F.Z.; Yang, J. Effects of the ratio of anodic and cathodic currents on the characteristics of micro-arc oxidation ceramic coatings on Al alloys. Appl. Surf. Sci. 2014, 292, 658–664. [Google Scholar] [CrossRef]
- Xin, S.G.; Song, L.X.; Zhao, R.G.; Hu, X.F. Influence of cathodic current on composition, structure and properties of Al2O3 coatings on aluminum alloy prepared by micro-arc oxidation process. Thin Solid Films 2006, 515, 326–332. [Google Scholar] [CrossRef]
- Wang, S.L.; Zhang, P.; Du, Y.H.; Wang, Y.J.; Hao, Z.Q. Quick surface treatment of AZ31B by ac micro-arc oxidation. J. Wuhan Univ. Technol.-Mat. Sci. Edit. 2014, 29, 773–779. [Google Scholar] [CrossRef]
- Shen, D.J.; Cai, J.R.; Li, G.L.; He, D.L.; Wu, L.L.; Ma, H.J.; Xia, Y.H.; Chen, H.; Yang, Y.Q. Effect of ultrasonic on microstructure and growth characteristics of micro-arc oxidation ceramic coatings on 6061 aluminum alloy. Vacuum 2014, 99, 143–148. [Google Scholar] [CrossRef]
- Lu, P.; Fan, H.; Liu, Y.; Cao, L.; Wu, X.; Xu, X. Controllable biodegradability, drug release behavior and hemocompatibility of PTX-eluting magnesium stents. Colloid Surf. B Biointerfaces 2011, 83, 3–28. [Google Scholar] [CrossRef] [PubMed]
- Prasad Rao, K.; Janaki Ram, G.D.; Stucker, B.E. Improvement in corrosion resistance of friction stir welded aluminum alloys with micro arc oxidation coatings. Scripta Mater. 2008, 58, 998–1001. [Google Scholar] [CrossRef]
- Ma, H.J.; Li, D.L.; Liu, C.; Huang, Z.Q.; He, D.L.; Yan, Q.; Liu, P.; Nash, P.; Shen, D.J. An investigation of (NaPO3)6 effects and mechanisms during micro-arc oxidation of AZ31 magnesium alloy. Surf. Coat. Technol. 2015, 266, 151–159. [Google Scholar] [CrossRef]
- Babaei, M.; Dehghanian, C.; Vanaki, M. Effect of additive on electrochemical corrosion properties of plasma electrolytic oxidation coatings formed on CP Ti under different processing frequency. Appl. Surf. Sci. 2015, 357, 712–720. [Google Scholar] [CrossRef]
- Tran, Q.P.; Sun, J.K.; Kuo, Y.C.; Tseng, C.Y.; He, J.L.; Chin, T.S. Anomalous layer-thickening during micro-arc oxidation of 6061 Al alloy. J. Alloy. Compd. 2017, 697, 326–332. [Google Scholar] [CrossRef]
- Tang, M.Q.; Feng, Z.Q.; Li, G.; Zhang, Z.Z.; Zhang, R.Z. High-corrosion resistance of the microarc oxidation coatings on magnesium alloy obtained in potassium fluotitanate electrolytes. Surf. Coat. Technol. 2015, 264, 105–113. [Google Scholar] [CrossRef]
- Zhu, Q.J.; Wang, B.B.; Zhao, X.; Zhang, B.B. Robust micro arc oxidation coatings on 6061 aluminum alloys via surface thickening and microvoid reducing approach. Solid state Phenom. 2018, 279, 148–152. [Google Scholar] [CrossRef]




| Element | Mg | Si | Fe | Cu | Mn | Cr | Zn | Ti | Al |
|---|---|---|---|---|---|---|---|---|---|
| wt.% | 0.80 | 0.40 | 0.70 | 0.20 | 0.15 | 0.05 | 0.25 | 0.15 | Balance |
| Sample | (NaPO3)6 | H3BO3 |
|---|---|---|
| S0 | 0 | 0 |
| S1 | 0.5 | 1.0 |
| S2 | 0.5 | 1.5 |
| S3 | 0.5 | 2.0 |
| S4 | 0.75 | 1.0 |
| S5 | 0.75 | 1.5 |
| S6 | 0.75 | 2.0 |
| S7 | 1.0 | 1.0 |
| S8 | 1.0 | 1.5 |
| S9 | 1.0 | 2.0 |
| Sample | Rs (Ω∙cm2) | Qp (F∙cm2) | Rp (Ω∙cm2) | Qb (F∙cm2) | Rb (Ω∙cm2) |
|---|---|---|---|---|---|
| S1 | 24.05 | 6.669 × 10−8 | 7.779 × 106 | 7.11 × 10−7 | 2.466 × 107 |
| S2 | 9.434 | 4.218 × 10−8 | 1.419 × 107 | 1.032 × 10−7 | 1.307 × 108 |
| S3 | 27.09 | 6.676 × 10−8 | 5.698 × 106 | 2.73 × 10−7 | 3.281 × 107 |
| S4 | 9.319 | 5.069 × 10−8 | 1.193 × 107 | 1.871 × 10−7 | 2.083 × 108 |
| S5 | 39.88 | 4.368 × 10−8 | 2.111 × 107 | 1.063 × 10−7 | 3.814 × 108 |
| S6 | 23.36 | 4.639 × 10−8 | 7.776 × 106 | 3.385 × 10−7 | 1.877 × 108 |
| S7 | 38.14 | 4.12 × 10−8 | 4.424 × 106 | 5.375 × 10−7 | 1.59 × 107 |
| S8 | 9.368 | 4.804 × 10−8 | 1.282 × 107 | 1.615 × 10−7 | 2.011 × 108 |
| S9 | 29.23 | 6.184 × 10−8 | 4.524 × 106 | 1.701 × 10−7 | 5.294 × 106 |
| Sample | Ecorr (V) | Icorr (A/cm2) | βa (V) | βc (V) | RP (Ω∙cm2) |
|---|---|---|---|---|---|
| 6061 | −672.7 | 1.209 × 10−6 | 0.0504 | 0.3478 | 1.581 × 104 |
| S5 | −511.5 | 2.981 × 10−9 | 0.1603 | 0.1491 | 1.142 × 107 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, Q.; Zhang, B.; Zhao, X.; Wang, B. Binary Additives Enhance Micro Arc Oxidation Coating on 6061Al Alloy with Improved Anti-Corrosion Property. Coatings 2020, 10, 128. https://doi.org/10.3390/coatings10020128
Zhu Q, Zhang B, Zhao X, Wang B. Binary Additives Enhance Micro Arc Oxidation Coating on 6061Al Alloy with Improved Anti-Corrosion Property. Coatings. 2020; 10(2):128. https://doi.org/10.3390/coatings10020128
Chicago/Turabian StyleZhu, Qingjun, Binbin Zhang, Xia Zhao, and Binbin Wang. 2020. "Binary Additives Enhance Micro Arc Oxidation Coating on 6061Al Alloy with Improved Anti-Corrosion Property" Coatings 10, no. 2: 128. https://doi.org/10.3390/coatings10020128
APA StyleZhu, Q., Zhang, B., Zhao, X., & Wang, B. (2020). Binary Additives Enhance Micro Arc Oxidation Coating on 6061Al Alloy with Improved Anti-Corrosion Property. Coatings, 10(2), 128. https://doi.org/10.3390/coatings10020128





