Design of Gelatin Pouches for the Preservation of Flaxseed Oil during Storage
Abstract
1. Introduction
2. Material and Methods
2.1. Materials
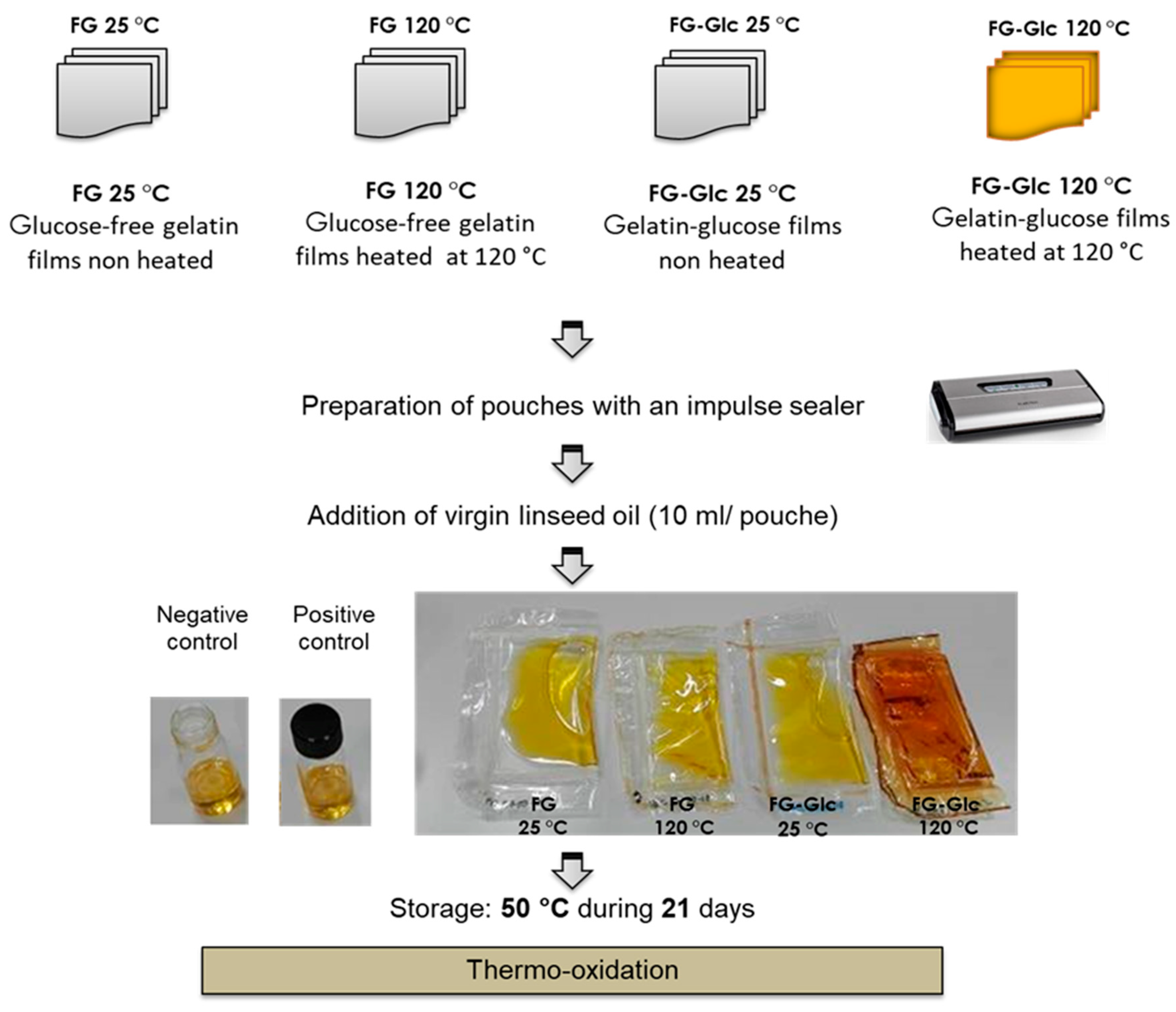
2.2. Film Preparation
2.3. Oxygen Transfer Through the Films
2.4. Packaging of Flaxseed Oil (FO) in Gelatin Pouches
2.5. Quality Changes of FO Packaged in Gelatin Pouches
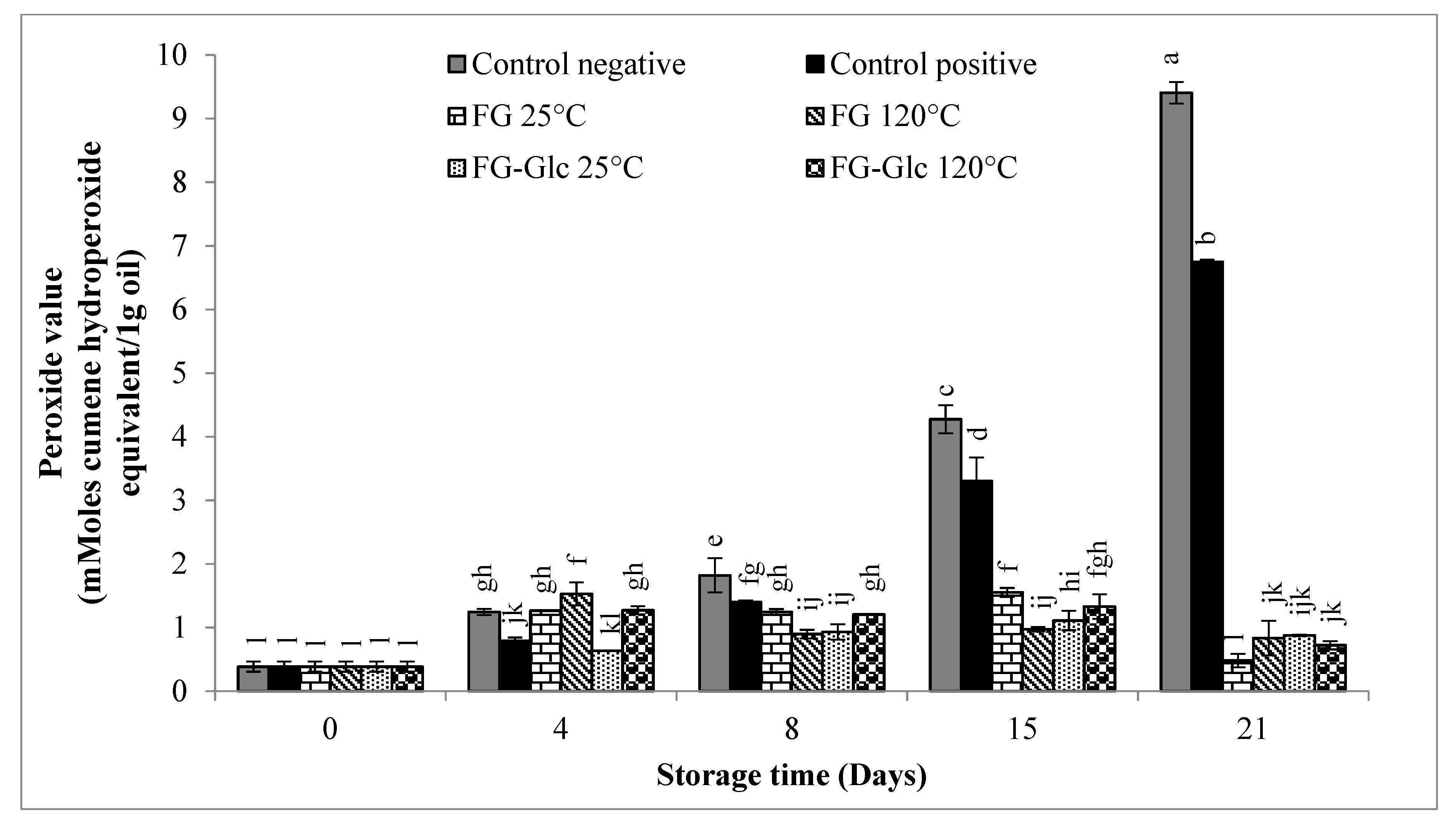
2.5.1. Peroxide Value (PV)
2.5.2. Concentration of Thiobarbituric Acid Reactive Substances (TBARs)
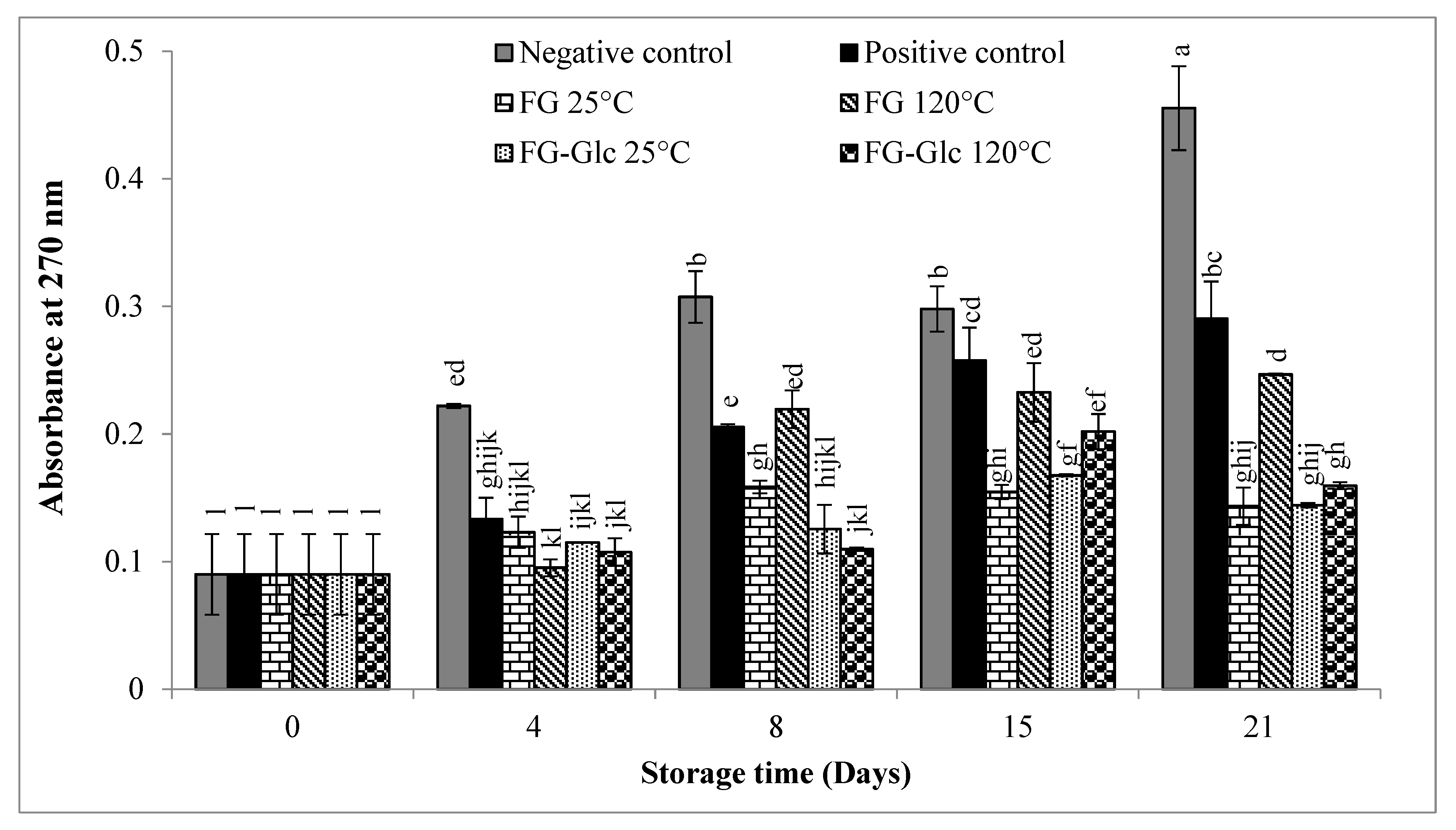
2.5.3. K270 Extinction Coefficient
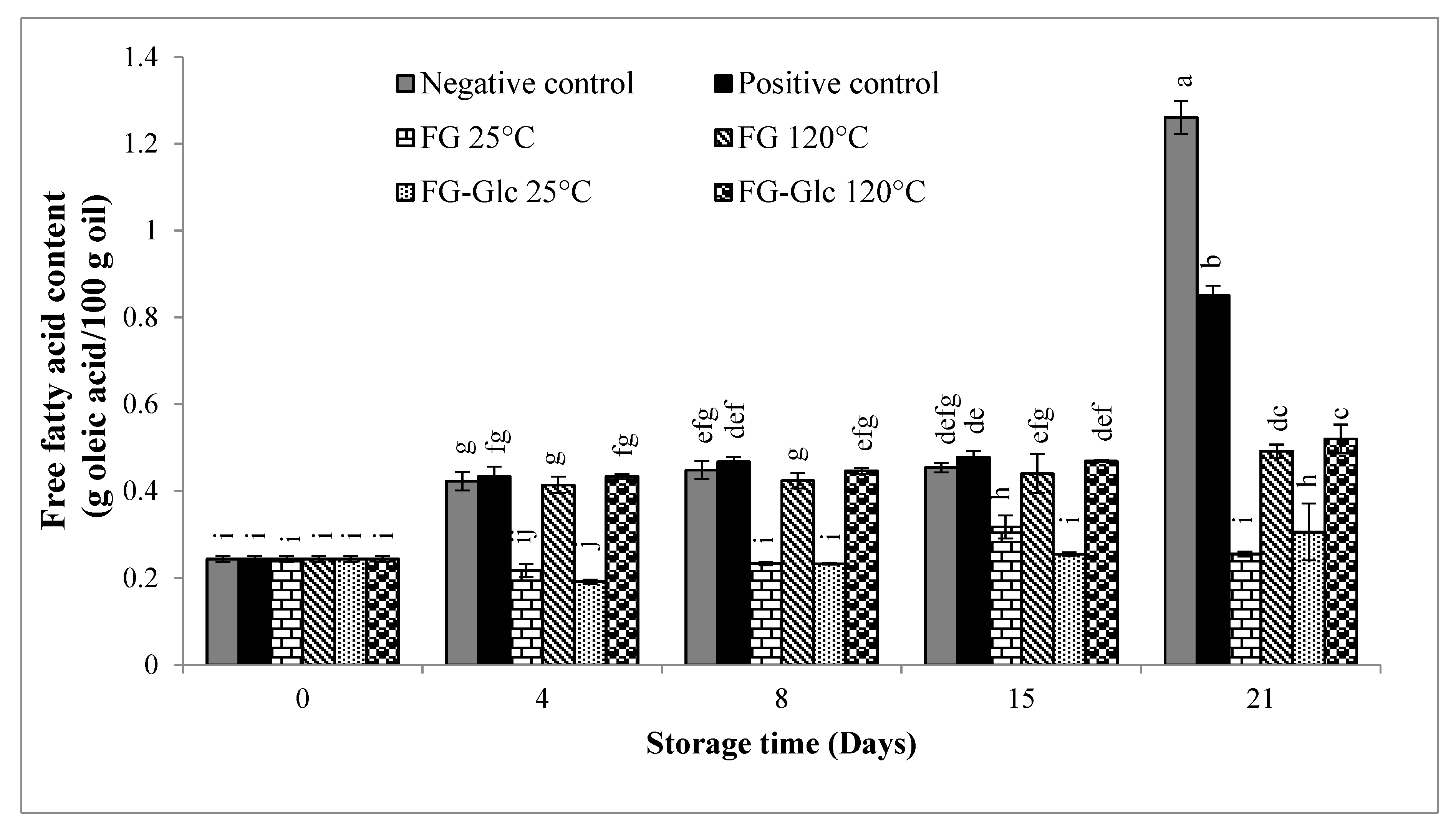
2.5.4. Free Fatty Acid Content (FFA)
2.5.5. Intrinsic Viscosity of FO
2.6. Changes of Gelatin Pouches before and after Application
2.6.1. Color
2.6.2. Light Transmission
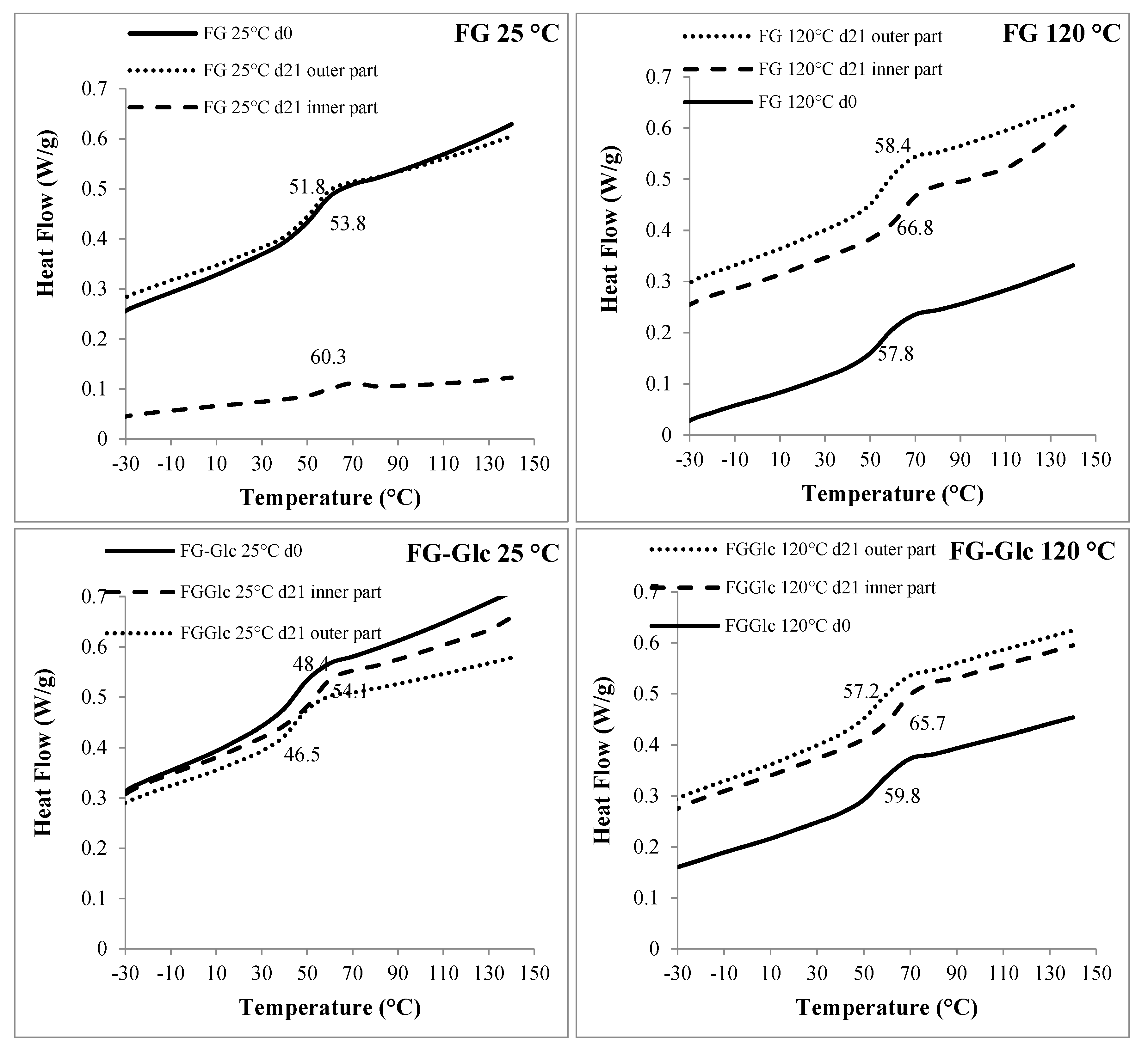
2.6.3. Differential Scanning Calorimetry (DSC)
2.7. Statistical Analysis
3. Results and Discussion
3.1. Quality Changes of Flaxseed Oil Packaged in Gelatin Pouches
3.1.1. Peroxide Value (PV)
3.1.2. Thiobarbituric Acid Reactive substances (TBARs)
3.1.3. K270 Extinction Coefficient
3.1.4. Free Fatty Acid (FFA) Content
3.1.5. Intrinsic Viscosity
3.1.6. Color
3.2. Physicochemical Properties of Gelatin Pouches Related to the Application with Flaxseed Oil
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Popa, V.M.; Gruia, A.; Raba, D.N.; Dumbrava, D.; Moldovan, C.; Bordean, D.; Mateescu, C. Fatty acids composition and oil characteristics of linseed (Linum usitatissimum L.) from Romania. J. Agroaliment. Process. Technol. 2012, 18, 136–140. [Google Scholar]
- Sotomayor-Gerding, D.; Oomah, B.D.; Acevedo, F.; Morales, E.; Bustamante, M.; Shene, C.; Rubilar, M. High carotenoid bioaccessibility through linseed oil nanoemulsions with enhanced physical and oxidative stability. Food Chem. 2016, 199, 463–470. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Wang, Q.; Li, T.; Xia, N.; Xia, Q. Nanostructured lipid carrier (NLC) as a strategy for encapsulation of quercetin and linseed oil: Preparation and in vitro characterization studies. J. Food Eng. 2017, 215, 1–12. [Google Scholar] [CrossRef]
- Piornos, J.A.; Burgos-Díaz, C.; Morales, E.; Rubilar, M.; Acevedo, F. Highly efficient encapsulation of linseed oil into alginate/lupin protein beads: Optimization of the emulsion formulation. Food Hydrocolloid 2017, 63, 139–148. [Google Scholar] [CrossRef]
- Gray, J.I. Measurement of lipid oxidation: A review. J. Am. Oil Chem. Soc. 1978, 55, 539–546. [Google Scholar] [CrossRef]
- Sai-Ut, S.; Benjakul, S.; Rawdkuen, S. Retardation of lipid oxidation using gelatin film incorporated with longan seed extract compared with BHT. J. Food Sci. Technol. 2015, 52, 5842–5849. [Google Scholar] [CrossRef]
- Guillén, M.D.; Ruiz, A. Oxidation process of oils with high content of linoleic acyl groups and formation of toxic hydroperoxy- and hydroxyalkenals. A study by 1H nuclear magnetic resonance. J. Sci. Food Agric. 2005, 85, 2413–2420. [Google Scholar] [CrossRef]
- Ksouda, G.; Hajji, M.; Sellimi, S.; Merlier, F.; Falcimaigne-Cordin, A.; Nasri, M.; Thomasset, B. A systematic comparison of 25 Tunisian plant species based on oil and phenolic contents, fatty acid composition and antioxidant activity. Ind. Crop. Prod. 2018, 123, 768–778. [Google Scholar] [CrossRef]
- Kahl, R.; Kappus, H. Toxicology of the synthetic antioxidants BHA and BHT in comparison with the natural antioxidant vitamin E. Z. Lebensm. Unters. Forsch. 1993, 196, 329–338. [Google Scholar] [CrossRef]
- Yu, R.; Mandlekar, S.; Tony Kong, A.N. Molecular mechanisms of butylated hydroxylanisole-induced toxicity: Induction of apoptosis through direct release of cytochrome c. Mol. Pharmacol. 2000, 58, 431–437. [Google Scholar] [CrossRef]
- Śpitalniak-Bajerska, K.; Szumny, A.; Kucharska, A.Z.; Kupczyński, R. Effect of natural antioxidants on the stability of linseed oil and fish stored under anaerobic conditions. J. Chem. 2018. [Google Scholar] [CrossRef]
- Varas Condori, M.A.; Pascual Chagman, G.J.; Barriga-Sanchez, M.; Villegas Vilchez, L.F.; Ursetta, S.; Guevara Perez, A.; Hidalgo, A. Effect of tomato (Solanum lycopersicum L.) lycopene-rich extract on the kinetics of rancidity and shelf-life of linseed (Linum usitatissimum L.) oil. Food Chem. 2020, 302, 125327. [Google Scholar] [CrossRef]
- Taktak, W.; Nasri, R.; Lopez-Rubio, A.; Hamdi, M.; Gomez-Mascaraque, L.G.; Ben Amor, N.; Kabadou, A.; Li, S.; Nasri, M.; Chaâbouni-Karra, M. Improved antioxidant activity and oxidative stability of spray dried European eel (Anguilla anguilla) oil microcapsules: Effect of emulsification process and eel protein isolate concentration. Mater. Sci. Eng. C 2019, 104, 109867. [Google Scholar] [CrossRef]
- Nilsuwan, K.; Benjakul, S.; Prodpran, T.; de la Caba, K. Fish gelatin monolayer and bilayer films incorporated with epigallocatechin gallate: Properties and their use as pouches for storage of chicken skin oil. Food Hydrocoll. 2019, 89, 783–791. [Google Scholar] [CrossRef]
- Kchaou, H.; Benbettaïeb, N.; Jridi, M.; Nasri, M.; Debeaufort, F. Influence of Maillard reaction and temperature on functional, structure and bioactive properties of fish gelatin films. Food Hydrocoll. 2019, 97, 105196. [Google Scholar] [CrossRef]
- Kchaou, H.; Benbettaïeb, N.; Jridi, M.; Abdelhedi, O.; Karbowiak, T.; Brachais, C.-H.; Léonard, M.-L.; Debeaufort, F.; Nasri, M. Enhancement of structural, functional and antioxidant properties of fish gelatin films using Maillard reactions. Food Hydrocoll. 2018, 83, 326–339. [Google Scholar] [CrossRef]
- Uluata, S.; McClements, D.J.; Decker, E.A. How the multiple antioxidant properties of ascorbic acid affect lipid oxidation in oil-in-water emulsions. J. Agric. Food Chem. 2015, 63, 1819–1824. [Google Scholar] [CrossRef]
- Qiu, C.; Zhao, M.; Decker, E.A.; McClements, D.J. Influence of protein type on oxidation and digestibility of fish oil-in-water emulsions: Gliadin, caseinate, and whey protein. Food Chem. 2015, 175, 249–257. [Google Scholar] [CrossRef]
- Boscou, D. Olive oil: Chemistry and technology. Champaign Illinois. Am. Oil Chem. Soc. 1996, 69, 552–556. [Google Scholar]
- Takeungwongtrakul, S.; Benjakul, S.; H-kittikun, A. Lipids from cephalothorax and hepatopancreas of Pacific white shrimp (Litopenaeus vannamei): Compositions and deterioration as affected by iced storage. Food Chem. 2012, 134, 2066–2074. [Google Scholar] [CrossRef]
- Shahidi, F.; Zhong, Y. Lipid oxidation and improving the oxidative stability. Chem. Soc. Rev. 2010, 39, 4067–4079. [Google Scholar] [CrossRef] [PubMed]
- Stoll, L.; Da Silva, A.M.; Iahnke AO, E.S.; Haas Costa, T.M.; Flores, S.H.; Rios, A.D.O. Active biodegradable film with encapsulated anthocyanins: Effect on the quality attributes of extra-virgin olive oil during storage. J. Food Process. Preserv. 2017, 41, 13218–13225. [Google Scholar] [CrossRef]
- Cho, S.Y.; Lee, S.Y.; Rhee, C. Edible oxygen barrier bilayer film pouches from corn zein and soy protein isolate for olive oil packaging. Lwt Food Sci. Technol. 2010, 43, 1234–1239. [Google Scholar] [CrossRef]
- Nawar, W.W. Lipids. In Food Chemistry; Fennema, O.R., Ed.; Marcel Dekker, Inc.: New York, NY, USA, 1996; pp. 225–319. [Google Scholar]
- Wolff, J.-P. Manuel d’analyse des corps gras, Edition; Azoulay: Paris, France, 1968. [Google Scholar]
- Pacheco-Aguilar, R.; Lugo-Sánchez, M.; Robles-Burgueño, M. Postmortem biochemical and functional characteristic of Monterey sardine muscle stored at 0 °C. J. Food Sci. 2000, 65, 40–47. [Google Scholar] [CrossRef]
- Roos, Y.H. Relaxations, Glass Transition and Engineering Properties of Food Solids. In Advances in Food Process Engineering Research and Applications; Springer: Berlin, Germany, 2013. [Google Scholar]
- Choo, W.S.; Birch, E.J.; Dufour, J.P. Physicochemical and stability characteristics of flaxseed oils during pan-heating. J. Am. Oil Chem. Soc. 2007, 84, 735–740. [Google Scholar] [CrossRef]
- Miller, K.S.; Krochta, J.M. Oxygen and aroma barrier properties of edible films: A review. Trends Food Sci. Technol. 1997, 8, 228–237. [Google Scholar] [CrossRef]
- Ayranci, E.; Tunc, S. A method for the measurement of the oxygen permeability and the development of edible films to reduce the rate of oxidative reactions in fresh foods. Food Chem. 2003, 80, 423–431. [Google Scholar] [CrossRef]
- Cerqueira MA, P.R.; Pereira RN, C.; da Silva Ramos, O.L.; Teixeira JA, C.; Vicente, A.A. Edible Food Packaging: Materials and Processing Technologies; CRC Press: Boca Raton, FL, USA, 2016. [Google Scholar]
- Lee, Y.-Y.; Tang, T.-K.; Phuah, E.-T.; Alitheen NB, M.; Tan, C.-P.; Lai, O.-M. New functionalities of Maillard reaction products as emulsifiers and encapsulating agents, and the processing parameters: A brief review. J. Sci. Food Agric. 2017, 97, 1379–1385. [Google Scholar] [CrossRef]
- Etxabide, A.; Uranga, J.; Guerrero, P.; de la Caba, K. Improvement of barrier properties of fish gelatin films promoted by gelatin glycation with lactose at high temperatures. Lwt Food Sci. Technol. 2015, 63, 315–321. [Google Scholar] [CrossRef]
- Nooshkam, M.; Madadlou, A. Maillard conjugation of lactulose with potentially bioactive peptides. Food Chem. 2016, 192, 831–836. [Google Scholar] [CrossRef]
- Taktak, W.; Kchaou, H.; Hamdi, M.; Li, S.; Nasri, M.; Chaâbouni-Karra, M.; Nasri, R. Design of bioinspired emulsified composite European Eel gelatin and protein isolate-based food packaging film: Thermal, microstructural, mechanical, and biological features. Coatings 2020, 10, 26. [Google Scholar] [CrossRef]
- Rogers, C.E. Permeation of gases and vapors in polymers. In Polymer Permeability; Comyn, J., Ed.; Elsevier Applied Science: London, UK, 1985; pp. 11–73. [Google Scholar]

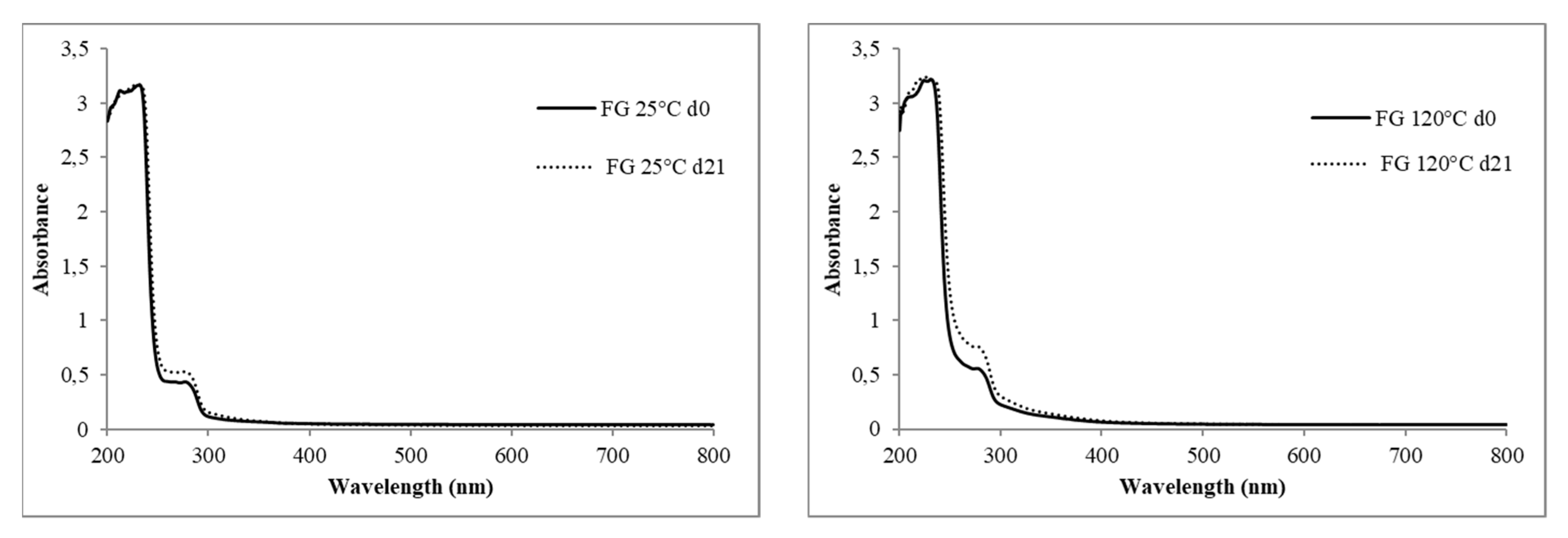

| Oil Samples | T2 (ms) | |
|---|---|---|
| Flaxseed Oil d0 | 159.2 ± 1.0 c | |
| Flaxseed oil d21 packaged in | FG 25 °C | 167.5 ± 0.5 a,b |
| FG 120 °C | 168.5 ± 0.5 a | |
| FG-Glc 25 °C | 165.5 ± 0.5 a,b | |
| FG-Glc 120 °C | 167.5 ± 0.5 a,b | |
| Positive control | 163.5 ± 1.5 b,c | |
| Negative control | 160.0 ± 3.0 c | |
| Samples | L | a | b | |
|---|---|---|---|---|
| FO | d0 | 48.44 ± 0.05 b | −3.71 ± 0.07 e | 14.34 ± 0.28 b |
| FO-FG 25 °C | d21 | 48.44 ± 0.08 b | −3.42 ± 0.08 c | 15.18 ± 0.14 a |
| FO-FG 120 °C | d21 | 48.29 ± 0.06 b | −3.42 ± 0.05 c | 15.42 ± 0.08 a |
| FO-FG-Glc 25 °C | d21 | 48.48 ± 0.02 b | −3.58 ± 0.02 d | 15.58 ± 0.23 a |
| FO-FG-Glc 120 °C | d21 | 48.47 ± 0.14 b | −3.48 ± 0.04 c,d | 14.52 ± 0.08 b |
| Positive control | d21 | 49.05 ± 0.14 a | −0.27 ± 0.05 a | 3.47 ± 0.42 c |
| Negative control | d21 | 49.05 ± 0.34 a | −0.48 ± 0.08 b | 3.68 ± 0.39 c |
| FG 25 °C | d0 | 89.4 ± 0.03 a | 1.67 ± 0.04 a | −3.3 ± 0.10 a |
| d21 | 89.59 ± 0.17 a | 1.64 ± 0.03 a | −3.16 ± 0.04 a | |
| FG 120 °C | d0 | 89.19 ± 0.02 a | 1.14 ± 0.02 a | −1.87 ± 0.10 a |
| d21 | 89.05 ± 0.11 a | 1.04 ± 0.03 a | −1.51 ± 0.04 a | |
| FG-Glc 25 °C | d0 | 89.46 ± 0.10 a | 1.65 ± 0.05 a | −3.39 ± 0.03 b |
| d21 | 88.3 ± 0.08 b | 0.59 ± 0.01 b | 2.72 ± 0.08 a | |
| FG-Glc 120 °C | d0 | 78.31 ± 0.2 a | 1.14 ± 0.09 a | 28.47 ± 0.28 a |
| d21 | 78.27 ± 0.07 a | 1.2 ± 0.12 a | 28.23 ± 0.16 a | |
| Pouches | Tg (°C) | |
|---|---|---|
| FG 25 °C | d0 | 53.8 |
| d21 inner part | 60.3 | |
| d21 outer part | 51.8 | |
| FG 120 °C | d0 | 57.8 |
| d21 inner part | 66.6 | |
| d21 outer part | 58.4 | |
| FG-Glc 25 °C | d0 | 48.4 |
| d21 inner part | 54.1 | |
| d21 outer part | 46.5 | |
| FG-Glc 120 °C | d0 | 59.8 |
| d21 inner part | 65.7 | |
| d21 outer part | 57.2 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kchaou, H.; Jridi, M.; Nasri, M.; Debeaufort, F. Design of Gelatin Pouches for the Preservation of Flaxseed Oil during Storage. Coatings 2020, 10, 150. https://doi.org/10.3390/coatings10020150
Kchaou H, Jridi M, Nasri M, Debeaufort F. Design of Gelatin Pouches for the Preservation of Flaxseed Oil during Storage. Coatings. 2020; 10(2):150. https://doi.org/10.3390/coatings10020150
Chicago/Turabian StyleKchaou, Hela, Mourad Jridi, Moncef Nasri, and Frédéric Debeaufort. 2020. "Design of Gelatin Pouches for the Preservation of Flaxseed Oil during Storage" Coatings 10, no. 2: 150. https://doi.org/10.3390/coatings10020150
APA StyleKchaou, H., Jridi, M., Nasri, M., & Debeaufort, F. (2020). Design of Gelatin Pouches for the Preservation of Flaxseed Oil during Storage. Coatings, 10(2), 150. https://doi.org/10.3390/coatings10020150






