Evolution of the Microstructure and Properties of Pre-Boronized Coatings During Pack-Cementation Chromizing
Abstract
1. Introduction
2. Experimental Details
2.1. Sample Preparation
2.2. Characterization and Properties Test
3. Results and Discussion
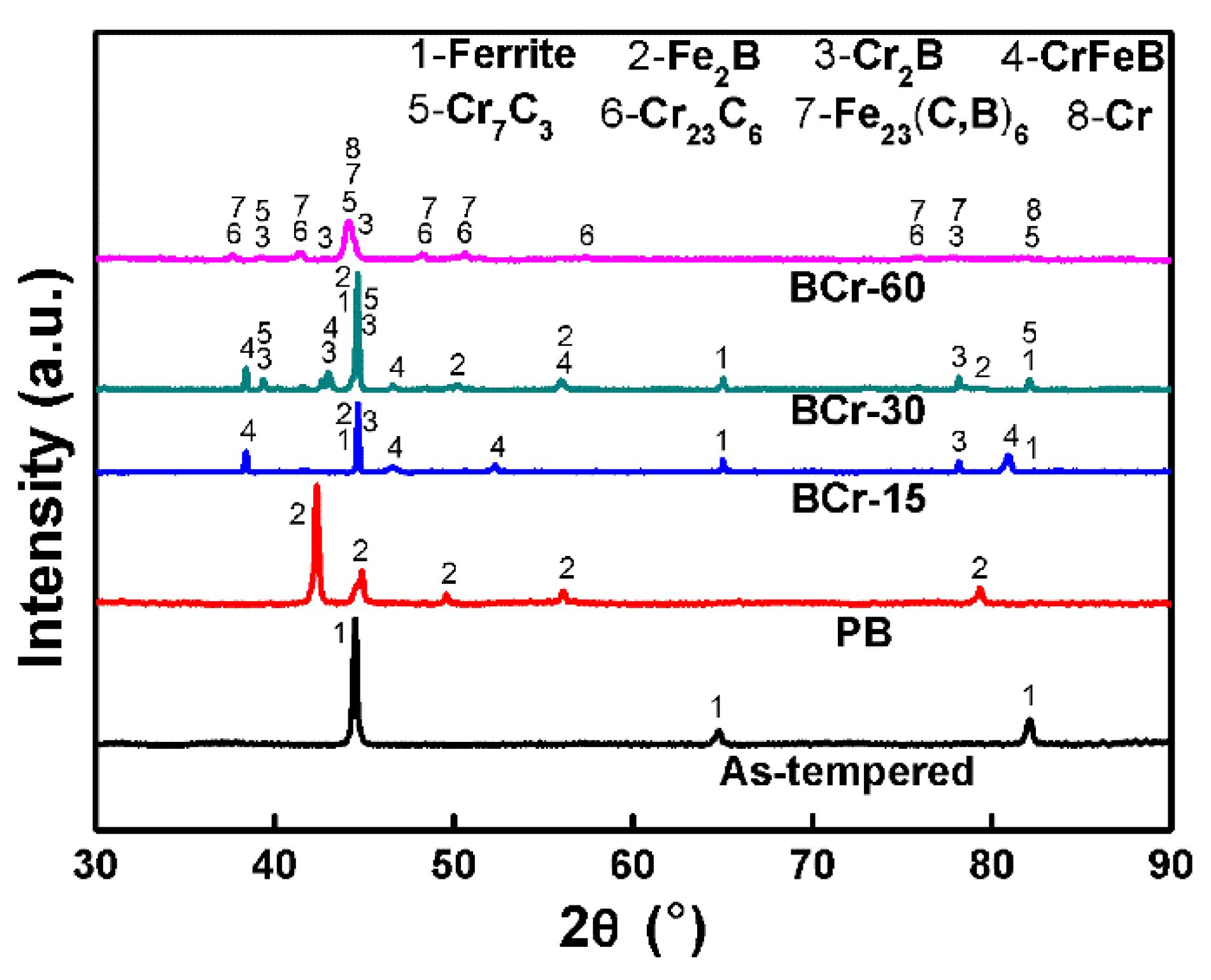
3.1. Phase Evolution
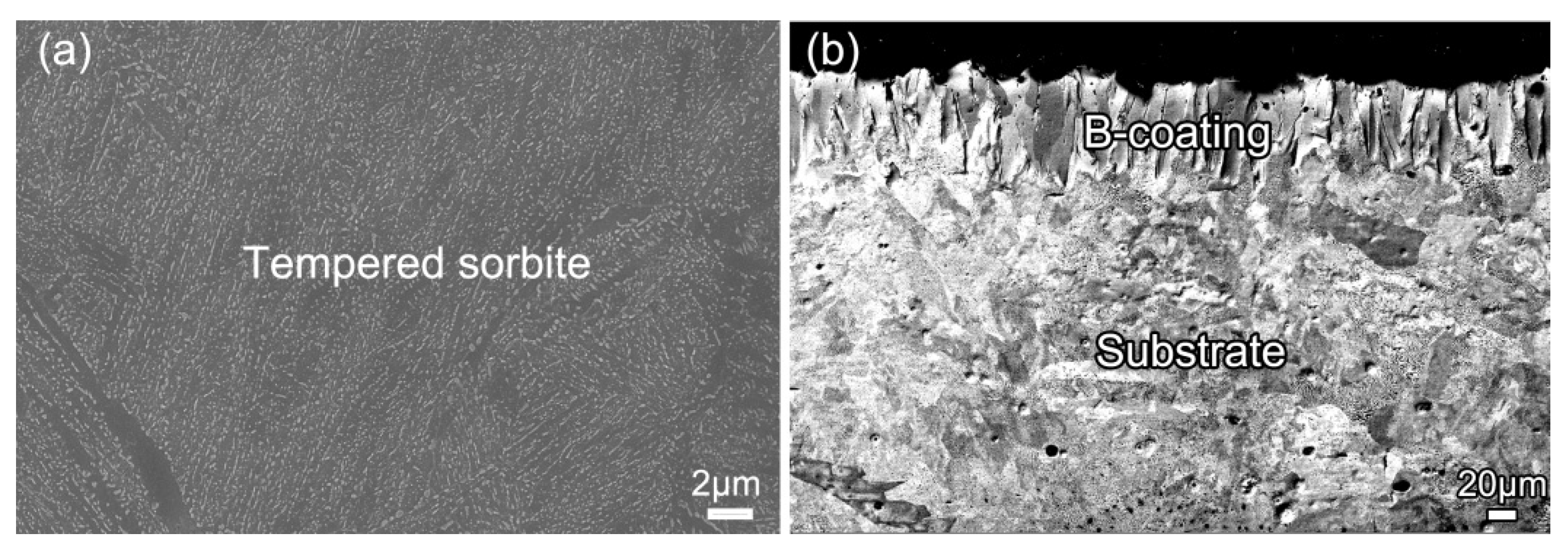
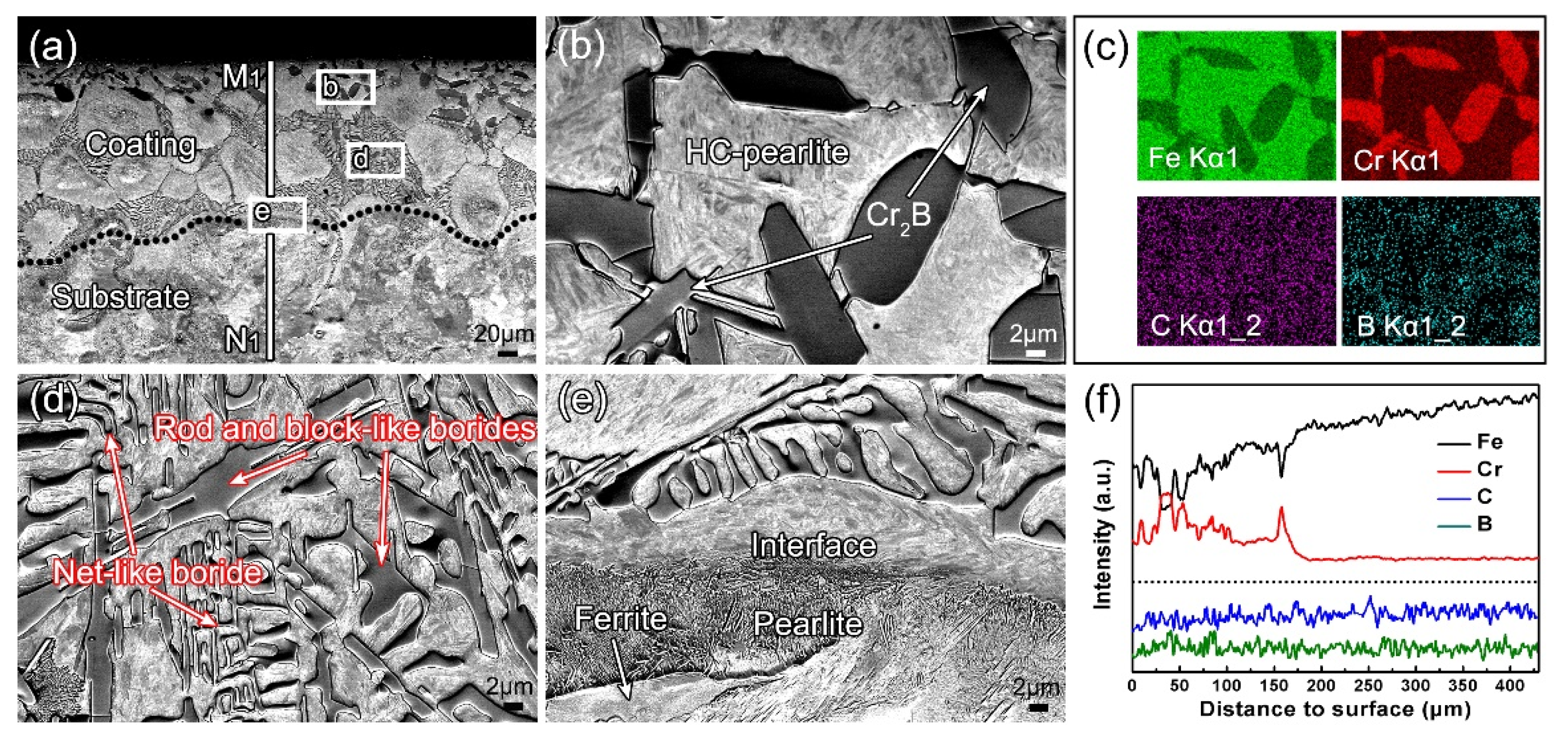
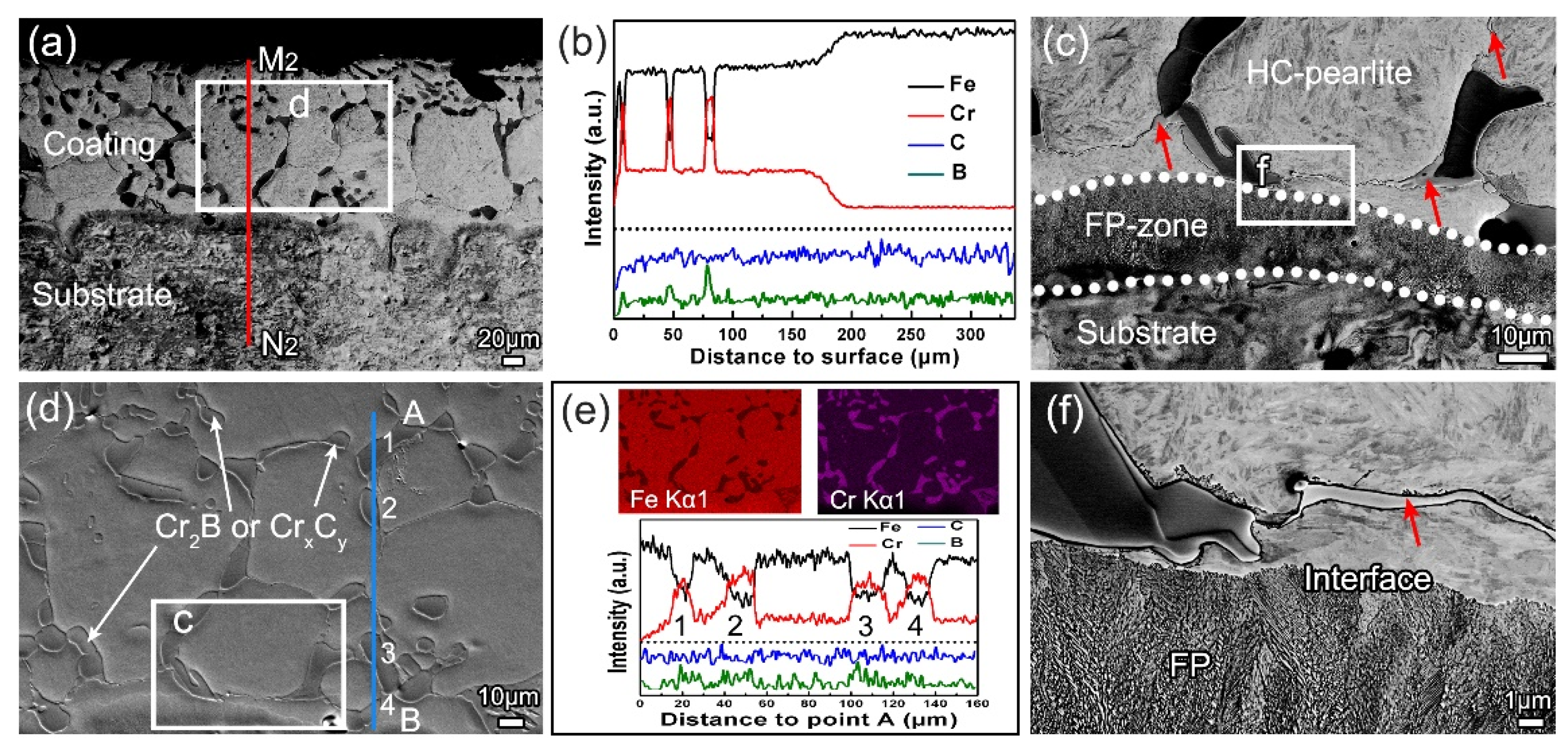
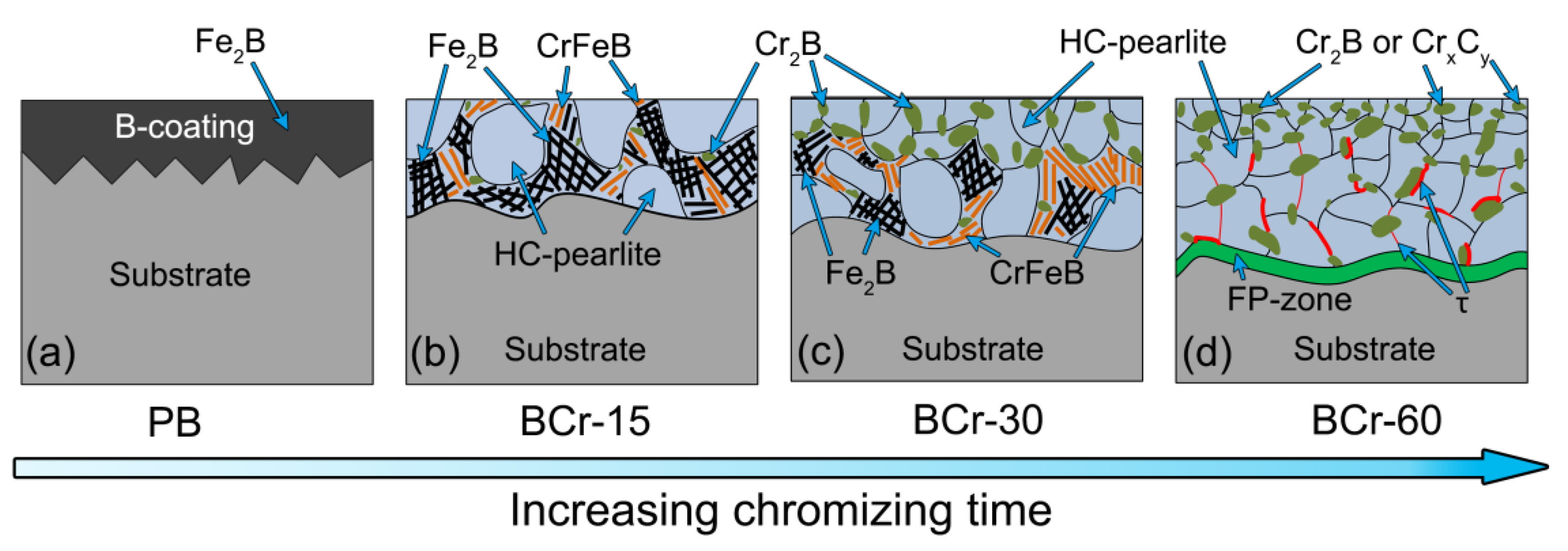
3.2. Microstructure Evolution
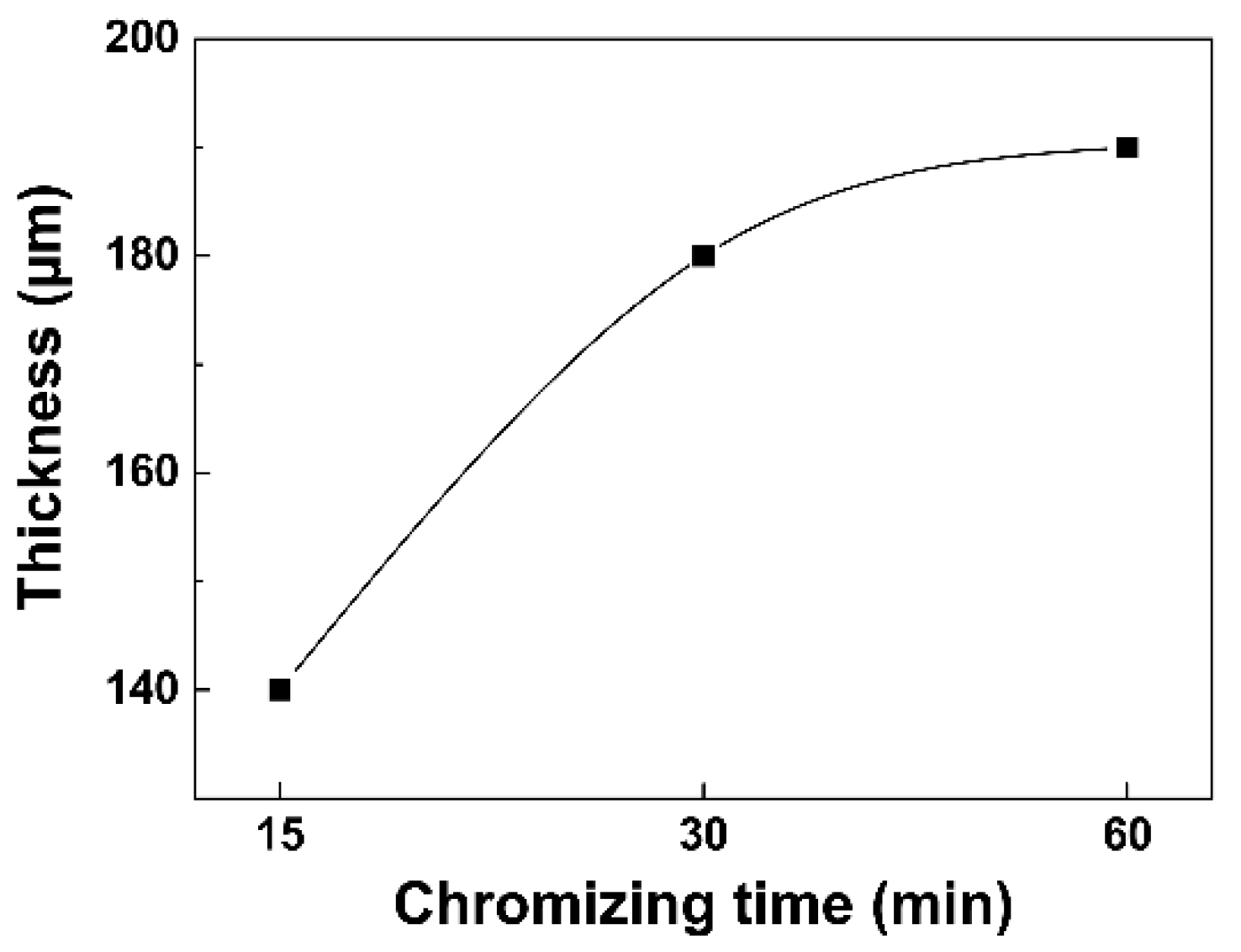
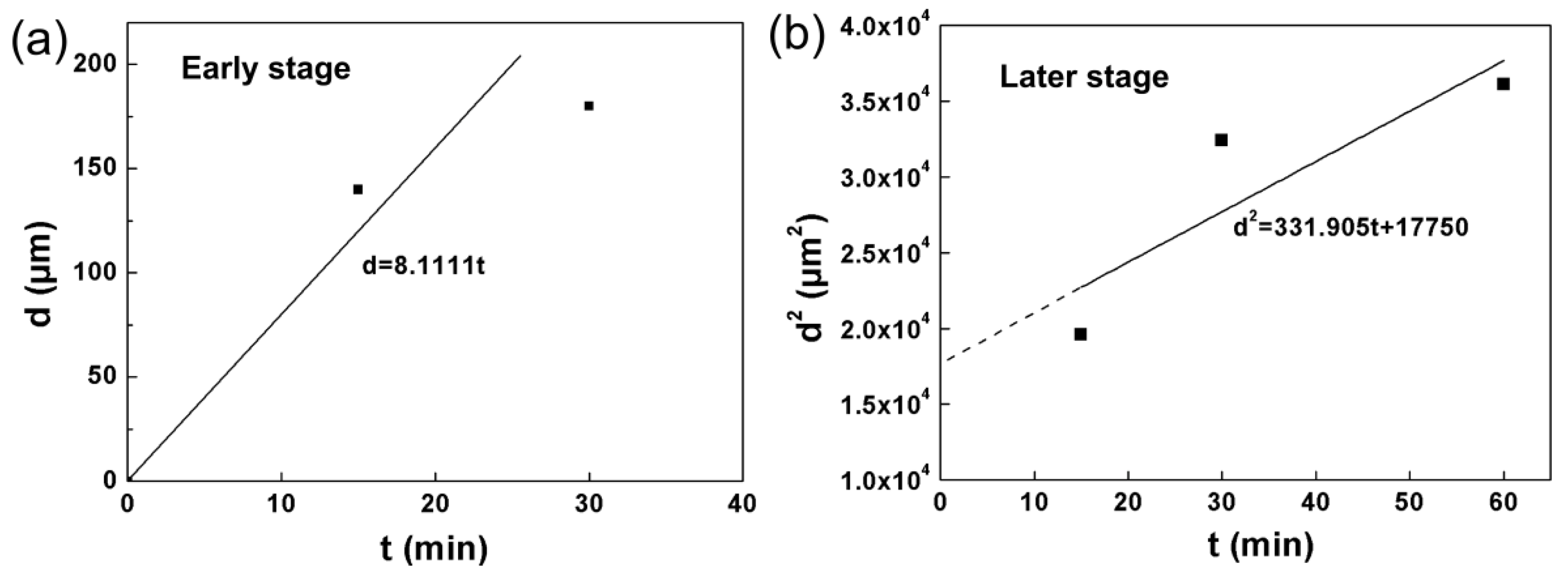
3.3. Growth Kinetics and Evolution Mechanism Analysis
3.4. Performance Comparison
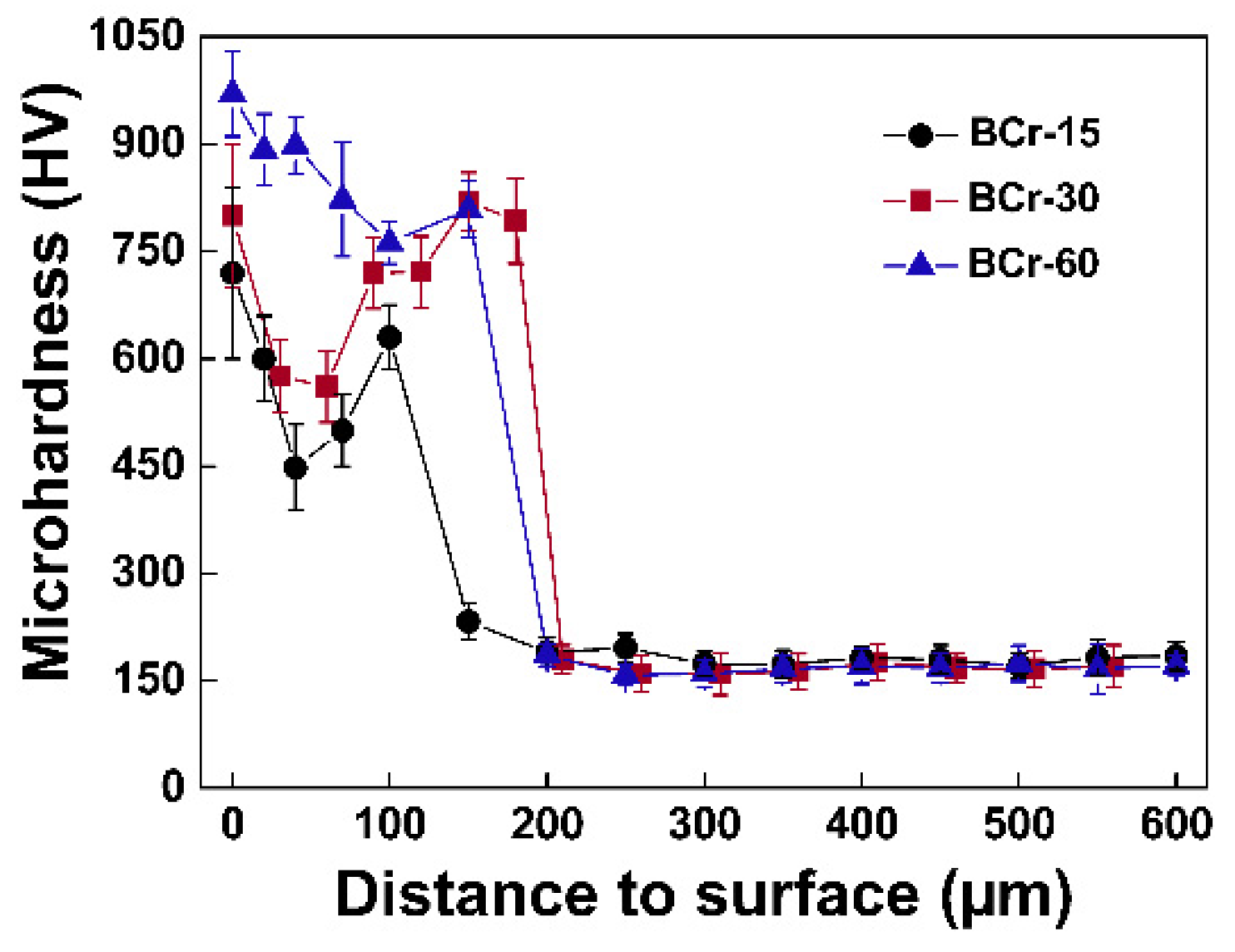
3.4.1. Microhardness
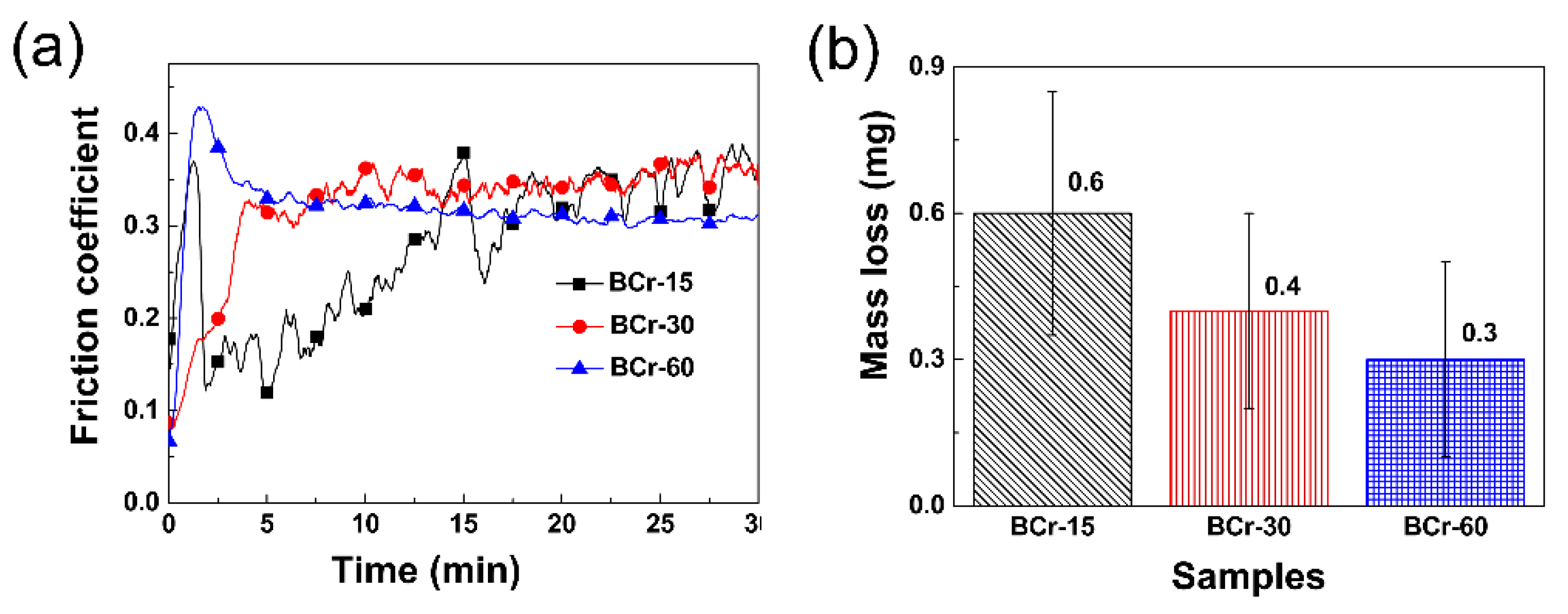
3.4.2. Friction-Related Wear Behavior
4. Conclusions
- As the chromizing time increases, the phases of the B-Cr duplex-alloyed coating vary significantly. The BCr-15 coating is composed of ferrite and borides (Fe2B, Cr2B, and CrFeB), while the BCr-30 coating contains ferrite, borides (Fe2B, Cr2B, and CrFeB), and a small amount of chromium carbide Cr7C3. Pure Cr, chromium boride Cr2B, τ-phase Fe23(C,B)6, and chromium carbides CrxCy (Cr7C3 and Cr23C6) are detected in the BCr-60 sample.
- In terms of microstructure, the B-Cr duplex-alloyed coatings mainly contain equiaxed grains of HC-pearlite and different second phases. As the chromizing time increases, the second phases change from the net-like Fe2B to the block-like Cr2B and CrxCy.
- Kinetics analysis reveals that the interface reaction leads to the rapid growth of the BCr coating at the early stage, and the diffusion controlled growth results in the slow growth at the late stage of chromizing.
- With the prolonging of chromizing time, the average microhardness of the coatings exhibits an increasing trend, and the wear resistance is significantly improved.
Author Contributions
Funding
Conflicts of Interest
References
- Zong, L.; Guo, N.; Li, R.; Yu, H. Effect of B content on microstructure and wear resistance of Fe-3Ti-4C hardfacing alloys produced by plasma-transferred arc welding. Coatings 2019, 9, 265. [Google Scholar] [CrossRef]
- Hu, J.; He, C.; Yang, X.; Li, H.; Xu, H.; Guo, N. Microstructure and tribological properties of self-lubricating FeS coating prepared by chemical bath deposition coating technique. Appl. Sci. 2019, 9, 4422. [Google Scholar] [CrossRef]
- Dehghanghadikolaei, A.; Fotovvati, B. Coating techniques for functional enhancement of metal implants for bone replacement: A review. Materials 2019, 12, 1795. [Google Scholar] [CrossRef]
- Beck-Broichsitter, M. Polymer-coated aperture plates for tailored atomization processes. Mater. Sci. Eng.: C. 2020, 110, 110666. [Google Scholar] [CrossRef]
- Fu, J.; Ma, S.; Zhu, X.; Xu, C.; Yan, Z.; Cheng, D.; Ma, C. Influence of solid lubricant WS2 on the tribological properties of plasma electrolytic oxidation coating of ZL109. Mater. Res. Express 2019, 6, 1265c8. [Google Scholar]
- Nicoll, A.R.; Gruner, H.; Prince, R.; Wuest, G. Thermal spray coatings for high temperature protection. Surf. Eng. 1985, 1, 59–71. [Google Scholar] [CrossRef]
- Ravi, K.; Deplancke, T.; Lame, O.; Ogawa, K.; Cavaillé, J.-Y.; Dalmas, F. Influence of nanoceramic interlayer on polymer consolidation during cold-spray coating formation. J. Mater. Process Tech. 2019, 273, 116254. [Google Scholar] [CrossRef]
- Dai, J.; Guan, H.; Chai, L.; Xiang, K.; Zhu, Y.; Qiu, R.; Guo, N.; Liu, Y. Comparative study of microstructural characteristics and hardness of β-quenched Zr702 and Zr–2.5Nb alloys. Materials 2019, 12, 3752. [Google Scholar] [CrossRef]
- Anees, S.M.; Dasari, A. A review on the environmental durability of intumescent coatings for steels. J. Mater. Sci. 2017, 53, 124–145. [Google Scholar] [CrossRef]
- Hu, J.; Zhang, Y.; Yang, X.; Li, H.; Xu, H.; Ma, C.; Dong, Q.; Guo, N.; Yao, Z. Effect of pack-chromizing temperature on microstructure and performance of AISI 5140 steel with Cr-coatings. Surf. Coat. Technol. 2018, 344, 656–663. [Google Scholar] [CrossRef]
- Hu, J.; Ma, C.; Yang, X.; Xu, H.; Guo, N.; Yu, H. Microstructure evolution during continuous cooling in AISI 5140 steel processed by induction heating chromizing. J. Mater. Eng. Perform. 2017, 26, 5530–5537. [Google Scholar] [CrossRef]
- Jiang, J.; Hu, J.; Yang, X.; Guo, N.; Xu, H.; Li, H.; Jin, Y.; Yu, H. Microstructure and annealing behavior of Cr-coatings deposited by double glow plasma on AISI 5140 steel. Results Phys. 2019, 15, 102674. [Google Scholar] [CrossRef]
- Hu, J.; Wang, J.; Jiang, J.; Yang, X.; Xu, H.; Li, H.; Guo, N. Effect of heating treatment on the microstructure and properties of Cr–Mo duplex-alloyed coating prepared by double glow plasma surface alloying. Coatings 2019, 9, 336. [Google Scholar] [CrossRef]
- Hu, J.; Zeng, J.; Yang, Y.; Yang, X.; Li, H.; Guo, N. Microstructures and wear resistance of boron-chromium duplex-alloyed coatings prepared by a two-step pack cementation process. Coatings 2019, 9, 529. [Google Scholar] [CrossRef]
- Lindner, T.; Löbel, M.; Sattler, B.; Lampke, T. Surface hardening of FCC phase high-entropy alloy system by powder-pack boriding. Surf. Coat. Technol. 2019, 371, 389–394. [Google Scholar] [CrossRef]
- Hu, J.; Jiang, J.; Li, H.; Yang, X.; Xu, H.; Jin, Y.; Ma, C.; Dong, Q.; Guo, N. Effect of annealing treatment on microstructure and properties of Cr-coatings deposited on AISI 5140 steel by brush-plating. Coatings 2018, 8, 193. [Google Scholar] [CrossRef]
- Hosmani, S.S.; Kuppusami, P.; Goyal, R.K. Chromizing, Carburizing, and Duplex Surface Treatment. In An Introduction to Surface Alloying of Metals; Springer: New Delhi, India, 2014; pp. 89–101. [Google Scholar]
- Lin, C.-K.; Hsu, C.-H.; Cheng, Y.-H.; Ou, K.-L.; Lee, S.-L. A study on the corrosion and erosion behavior of electroless nickel and TiAlN/ZrN duplex coatings on ductile iron. Appl. Surf. Sci. 2015, 324, 13–19. [Google Scholar] [CrossRef]
- Luo, X.; Wang, Y.Q.; Yang, Y.Q.; Zhang, M.X.; Huang, B.; Liu, S.; Jin, N. Effect of C/Mo duplex coating on the interface and tensile strength of SiCf/Ti-21Al-29Nb composites. J. Alloys Compd. 2017, 721, 653–660. [Google Scholar] [CrossRef]
- Rahmani, S.; Omrani, A.; Shabanpanah, S. Structure, surface properties and corrosion resistance of St 37 steel coated with PS/Ni-Ba-B duplex coatings. Surf. Coat. Technol. 2019, 373, 1–6. [Google Scholar] [CrossRef]
- Hu, J.; Ma, C.; Xu, H.; Guo, N.; Hou, T. Development of a Composite Technique for Preconditioning of 41Cr4 Steel Used as Gear Material: Examination of Its Microstructural Characteristics and Properties. Sci. Technol. Nucl. Install. 2016, 2016, 1–6. [Google Scholar] [CrossRef]
- Zhang, F.; Yan, M. Microstructure and wear resistance of in situ formed duplex coating fabricated by plasma nitriding Ti Coated 2024 Al Alloy. J. Mater. Sci. Technol. 2014, 30, 1278–1283. [Google Scholar] [CrossRef]
- Yilbas, B.S.; Kumar, A.; Bhushan, B. Single- and two-layer coatings of metal blends onto carbon steel: Mechanical, wear, and friction characterizations. JOM 2013, 66, 37–45. [Google Scholar] [CrossRef]
- Aghaie-Khafri, M.; Mohamadpour Nazar Abady, M. A study of chromo-boronizing on DIN 1.2714 steel by duplex surface treatment. JOM 2012, 64, 694–701. [Google Scholar] [CrossRef]
- Kheyrodin, M.; Habibolahzadeh, A.; Mousavi, S.Y.B. Wear and corrosion behaviors of duplex surface treated 316L austenitic stainless steel via combination of boriding and chromizing. Prot. Met. Phys. Chem. Surf. 2017, 53, 105–111. [Google Scholar] [CrossRef]
- Günen, A.; Kanca, E.; Çakir, H.; Karakaş, M.S.; Gök, M.S.; Küçük, Y.; Demir, M. Effect of borotitanizing on microstructure and wear behavior of Inconel 625. Surf. Coat. Technol. 2017, 311, 374–382. [Google Scholar] [CrossRef]
- Pourasad, J.; Ehsani, N.; Valefi, Z. Oxidation resistance of a SiC–ZrB2 coating prepared by a novel pack cementation on SiC-coated graphite. J. Mater. Sci. 2016, 52, 1639–1646. [Google Scholar] [CrossRef]
- Balusamy, T.; Sankara Narayanan, T.S.N.; Ravichandran, K. Effect of surface mechanical attrition treatment (SMAT) on boronizing of EN8 steel. Surf. Coat. Technol. 2012, 213, 221–228. [Google Scholar] [CrossRef]
- Lin, N.; Guo, J.; Xie, F.; Zou, J.; Tian, W.; Yao, X.; Zhang, H.; Tang, B. Comparison of surface fractal dimensions of chromizing coating and P110 steel for corrosion resistance estimation. Appl. Surf. Sci. 2014, 311, 330–338. [Google Scholar] [CrossRef]
- Xie, F.; Zhang, G.; Pan, J. Characterizing AISI 1045 steel surface duplex-treated by alternating current field enhanced pack aluminizing and nitriding. Appl. Surf. Sci. 2018, 431, 44–47. [Google Scholar] [CrossRef]
- Campos-Silva, I.; Ortiz-Domínguez, M.; Bravo-Bárcenas, O.; Doñu-Ruiz, M.A.; Bravo-Bárcenas, D.; Tapia-Quintero, C.; Jiménez-Reyes, M.Y. Formation and kinetics of FeB/Fe2B layers and diffusion zone at the surface of AISI 316 borided steels. Surf. Coat. Technol. 2010, 205, 403–412. [Google Scholar] [CrossRef]
- Medvedovski, E.; Jiang, J.R.; Robertson, M. Tribological properties of boride based thermal diffusion coatings. Adv. Appl. Ceram. 2014, 113, 427–437. [Google Scholar] [CrossRef]
- Streiff, R.; Cerclier, O.; Boone, D.H. Structure and hot corrosion behavior of platinum-modified aluminide coatings. Surf. Coat. Technol. 1987, 32, 111–126. [Google Scholar] [CrossRef]
- Kurt, B.; Günen, A.; Kanca, Y.; Koç, V.; Gök, M.S.; Kırar, E.; Askerov, K. Properties and Tribologic Behavior of Titanium Carbide Coatings on AISI D2 Steel Deposited by Thermoreactive Diffusion. JOM 2018, 70, 2650–2659. [Google Scholar] [CrossRef]
- Samadi, V.; Habibolahzade, A. Evaluation of microstructures and wear properties of duplex boride coatings. Mater. Sci. Technol. 2013, 26, 41–46. [Google Scholar] [CrossRef]
- Biesuz, M.; Sglavo, V.M. Chromium and vanadium carbide and nitride coatings obtained by TRD techniques on UNI 42CrMoS4 (AISI 4140) steel. Surf. Coat. Technol. 2016, 286, 319–326. [Google Scholar] [CrossRef]
- Castillejo, F.E.; Marulanda, D.M.; Olaya, J.J.; Alfonso, J.E. Wear and corrosion resistance of niobium–chromium carbide coatings on AISI D2 produced through TRD. Surf. Coat. Technol. 2014, 254, 104–111. [Google Scholar] [CrossRef]
- Bai, C.-Y.; Lee, J.-L.; Wen, T.-M.; Hou, K.-H.; Wu, M.-S.; Ger, M.-D. The characteristics of chromized 1020 steel with electrical discharge machining and Ni electroplating pretreatments. Appl. Surf. Sci. 2011, 257, 3529–3537. [Google Scholar] [CrossRef]
- Castillejo, F.; Olaya, J.; Alfonso, J. Wear and Corrosion Resistance of Chromium–Vanadium Carbide Coatings Produced via Thermo-Reactive Deposition. Coatings 2019, 9, 215. [Google Scholar] [CrossRef]
- Tong, D.; Gu, J.; Yang, F. Numerical simulation on induction heat treatment process of a shaft part: Involving induction hardening and tempering. J. Mater. Process. Technol. 2018, 262, 277–289. [Google Scholar] [CrossRef]
- Campos-Silva, I.; Flores-Jiménez, M.; Bravo-Bárcenas, D.; Balmori-Ramírez, H.; Andraca-Adame, J.; Martínez-Trinidad, J.; Meda-Campaña, J.A. Evolution of boride layers during a diffusion annealing process. Surf. Coat. Technol. 2017, 309, 155–163. [Google Scholar] [CrossRef]
- Guo, N.; Liu, Q.; Xin, Y.; Luan, B.; Zhou, Z. The application of back-scattered electron imaging for characterization of pearlitic steels. Sci. China: Technol. Sci. 2011, 54, 2368–2372. [Google Scholar] [CrossRef]
- Ma, S.; Xing, J.; Yi, D.; Fu, H.; Zhang, J.; Li, Y.; Zhang, Z.; Liu, G.; Zhu, B. Effects of chromium addition on corrosion resistance of Fe–3.5B alloy in liquid zinc. Surf. Coat. Technol. 2011, 205, 4902–4909. [Google Scholar] [CrossRef]
- Lentz, J.; Röttger, A.; Theisen, W. Solidification and phase formation of alloys in the hypoeutectic region of the Fe–C–B system. Acta Mater. 2015, 99, 119–129. [Google Scholar] [CrossRef]
- Ma, S.; Xing, J.; Fu, H.; Gao, Y.; Zhang, J. Microstructure and crystallography of borides and secondary precipitation in 18 wt.% Cr–4wt.% Ni–1 wt.% Mo–3.5 wt.% B–0.27 wt.% C steel. Acta Mater. 2012, 60, 831–843. [Google Scholar] [CrossRef]
- Dong, Z.; Zhou, T.; Liu, J.; Zhang, X.; Shen, B.; Hu, W.; Liu, L. Effects of pack chromizing on the microstructure and anticorrosion properties of 316L stainless steel. Surf. Coat. Technol. 2019, 366, 86–96. [Google Scholar] [CrossRef]
- Lentz, J.; Röttger, A.; Theisen, W. Mechanism of the Fe3(B,C) and Fe23(C,B)6 solid-state transformation in the hypoeutectic region of the Fe-C-B system. Acta Mater. 2016, 119, 80–91. [Google Scholar] [CrossRef]
- Campos-Silva, I.; Ortiz-Domínguez, M.; Keddam, M.; López-Perrusquia, N.; Carmona-Vargas, A.; Elías-Espinosa, M. Kinetics of the formation of Fe2B layers in gray cast iron: Effects of boron concentration and boride incubation time. Appl. Surf. Sci. 2009, 255, 9290–9295. [Google Scholar] [CrossRef]
- Scheuer, C.J.; Cardoso, R.P.; Mafra, M.; Brunatto, S.F. AISI 420 martensitic stainless steel low-temperature plasma assisted carburizing kinetics. Surf. Coat. Technol. 2013, 214, 30–37. [Google Scholar] [CrossRef]
- Doñu Ruiz, M.A.; López Perrusquia, N.; Sánchez Huerta, D.; Torres San Miguel, C.R.; Urriolagoitia Calderón, G.M.; Cerillo Moreno, E.A.; Cortes Suarez, J.V. Growth kinetics of boride coatings formed at the surface AISI M2 during dehydrated paste pack boriding. Thin Solid Films 2015, 596, 147–154. [Google Scholar] [CrossRef]
- Ghadi, A.; Soltanieh, M.; Saghafian, H.; Yang, Z.G. Growth kinetics and microstructure of composite coatings on H13 by thermal reactive diffusion. Surf. Coat. Technol. 2017, 325, 318–326. [Google Scholar] [CrossRef]
- Rai, A.K.; Vijayashanthi, N.; Tripathy, H.; Hajra, R.N.; Raju, S.; Murugesan, S.; Saroja, S. Investigation of diffusional interaction between P91 grade ferritic steel and Fe-15 wt.%B alloy and study of kinetics of boride formation at high temperature. J. Nucl. Mater. 2017, 495, 58–66. [Google Scholar] [CrossRef]
- Zhang, G.-H.; Chae, J.-Y.; Kim, K.-H.; Suh, D.W. Effects of Mn, Si and Cr addition on the dissolution and coarsening of pearlitic cementite during intercritical austenitization in Fe-1mass%C alloy. Mater. Charact. 2013, 81, 56–67. [Google Scholar] [CrossRef]
- Lentz, J.; Röttger, A.; Theisen, W. Hardness and modulus of Fe2B, Fe3(C,B), and Fe23(C,B)6 borides and carboborides in the Fe-C-B system. Mater. Charact. 2018, 135, 192–202. [Google Scholar] [CrossRef]
- Moon, J.; Ha, H.-Y.; Park, S.-J.; Lee, T.-H.; Jang, J.H.; Lee, C.-H.; Han, H.N.; Hong, H.-U. Effect of Mo and Cr additions on the microstructure, mechanical properties and pitting corrosion resistance of austenitic Fe-30Mn-10.5Al-1.1C lightweight steels. J. Alloys Compd. 2019, 775, 1136–1146. [Google Scholar] [CrossRef]
- Fernández-Abia, A.I.; Barreiro, J.; Fernández-Larrinoa, J.; de Lacalle, L.N.L.; Fernández-Valdivielso, A.; Pereira, O.M. Behaviour of PVD coatings in the turning of austenitic stainless steels. Procedia Eng. 2013, 63, 133–141. [Google Scholar] [CrossRef]
- Rodríguez-Barrero, S.; Fernández-Larrinoa, J.; Azkona, I.; López de Lacalle, L.N.; Polvorosa, R. Enhanced performance of nanostructured coatings for drilling by droplet elimination. Mater. Manuf. Process 2014, 31, 593–602. [Google Scholar] [CrossRef]



© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zeng, J.; Hu, J.; Yang, X.; Xu, H.; Li, H.; Guo, N. Evolution of the Microstructure and Properties of Pre-Boronized Coatings During Pack-Cementation Chromizing. Coatings 2020, 10, 159. https://doi.org/10.3390/coatings10020159
Zeng J, Hu J, Yang X, Xu H, Li H, Guo N. Evolution of the Microstructure and Properties of Pre-Boronized Coatings During Pack-Cementation Chromizing. Coatings. 2020; 10(2):159. https://doi.org/10.3390/coatings10020159
Chicago/Turabian StyleZeng, Jing, Jianjun Hu, Xian Yang, Hongbing Xu, Hui Li, and Ning Guo. 2020. "Evolution of the Microstructure and Properties of Pre-Boronized Coatings During Pack-Cementation Chromizing" Coatings 10, no. 2: 159. https://doi.org/10.3390/coatings10020159
APA StyleZeng, J., Hu, J., Yang, X., Xu, H., Li, H., & Guo, N. (2020). Evolution of the Microstructure and Properties of Pre-Boronized Coatings During Pack-Cementation Chromizing. Coatings, 10(2), 159. https://doi.org/10.3390/coatings10020159




