Properties of a Plasma-Nitrided Coating and a CrNx Coating on the Stainless Steel Bipolar Plate of PEMFC
Abstract
:1. Introduction
2. Experimental
2.1. Materials and Specimen Preparation
2.2. Deposition of the Coating
2.3. Electrochemical and Contact Resistance Tests
2.4. Surface Morphology Analysis
3. Results and Discussion
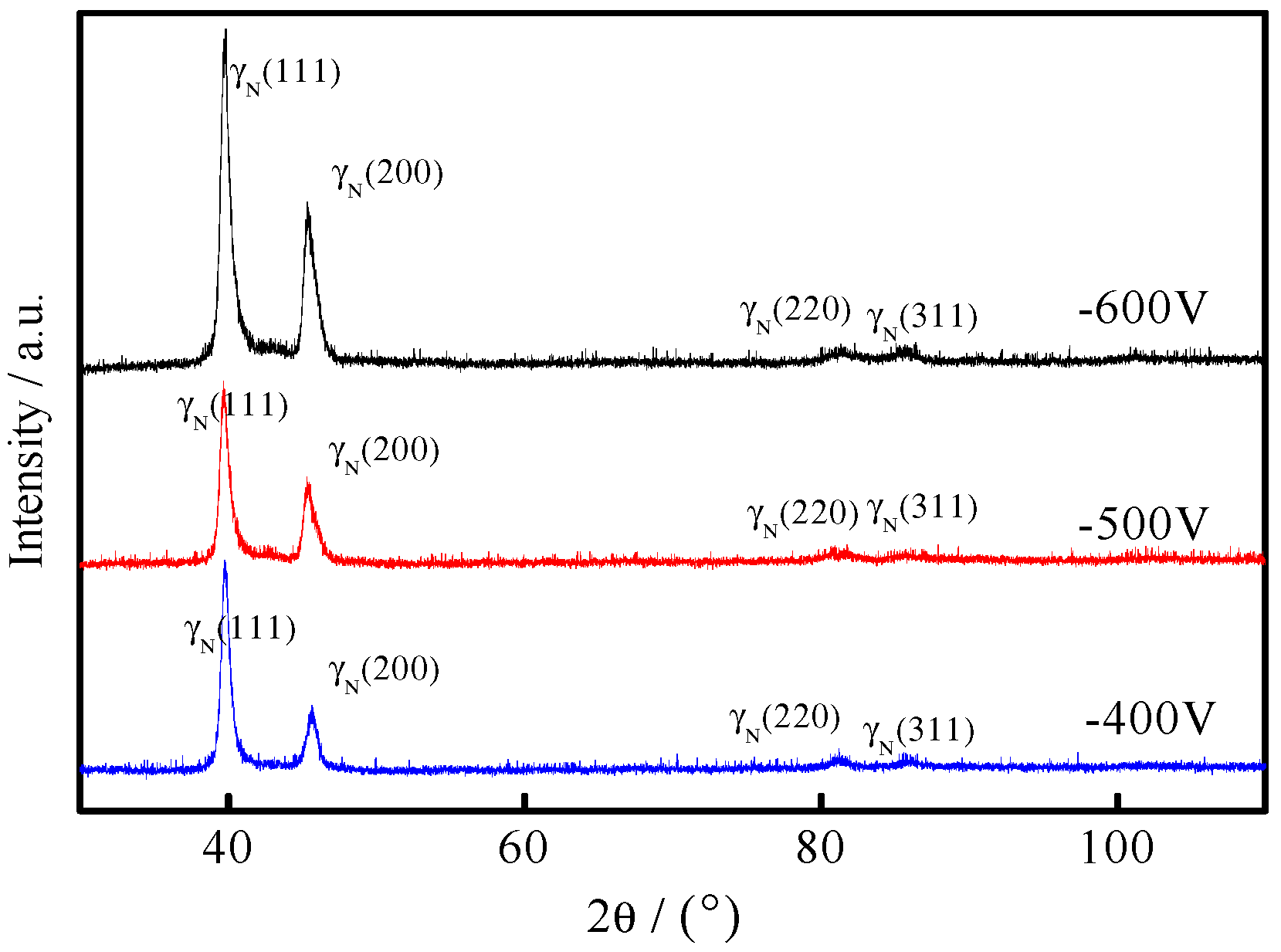
3.1. Phase Constitution of the Coating
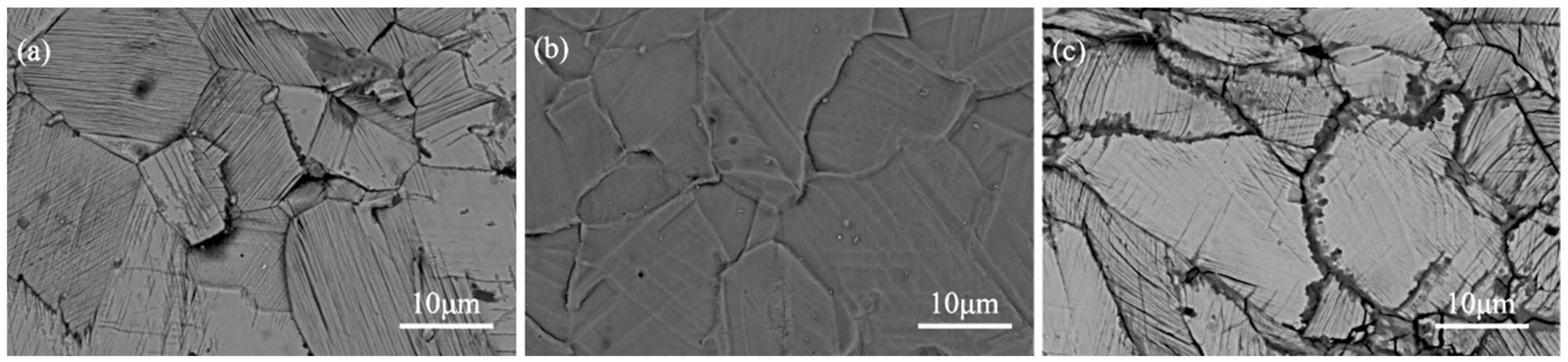
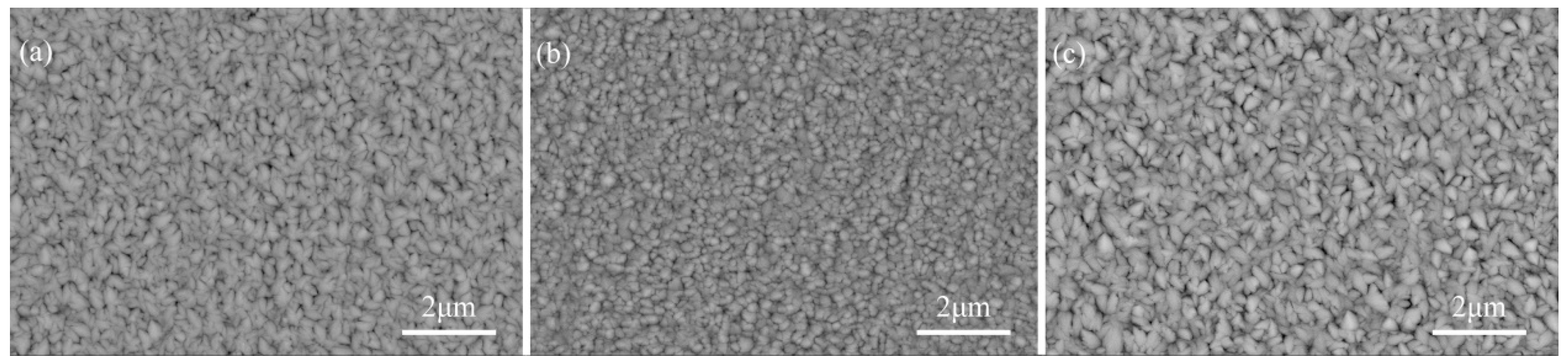
3.2. Morphology of the Coating
3.3. Electrochemical Measurements
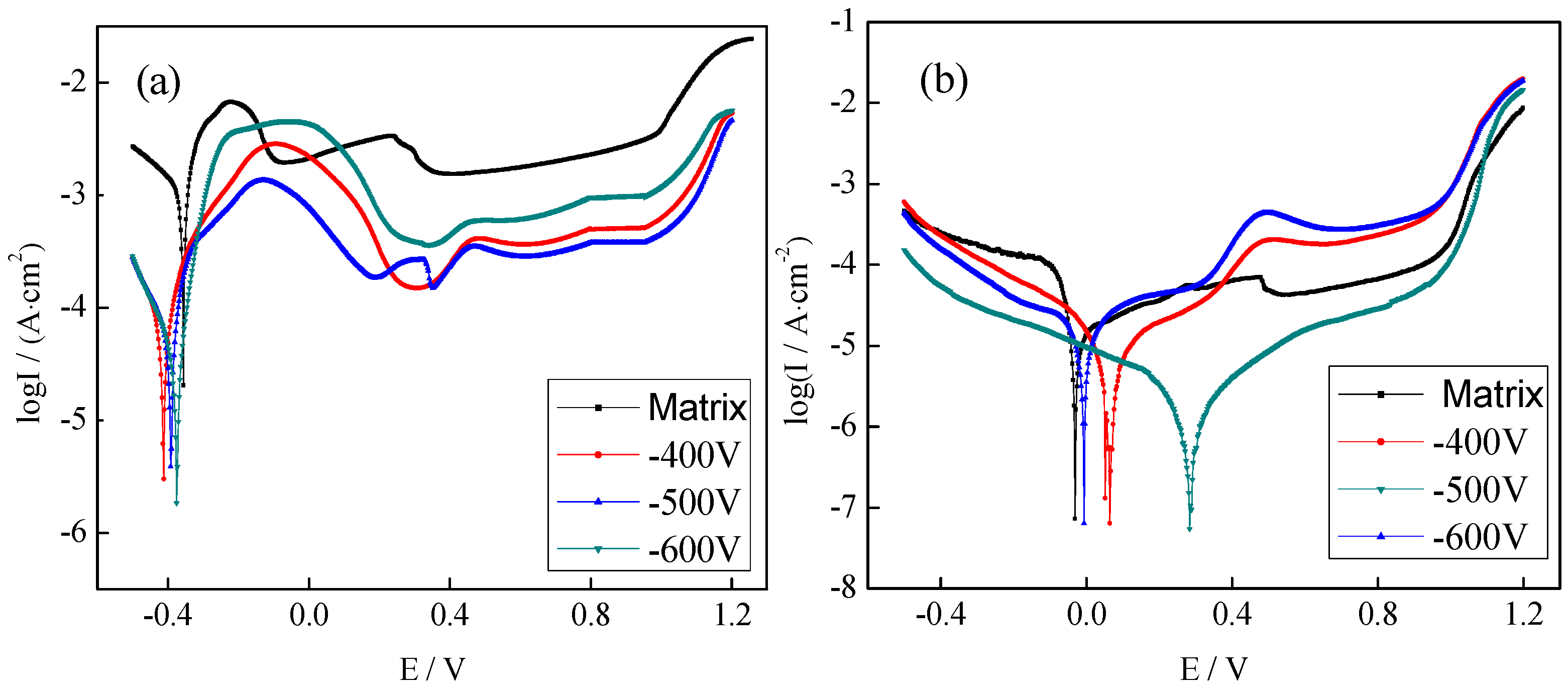
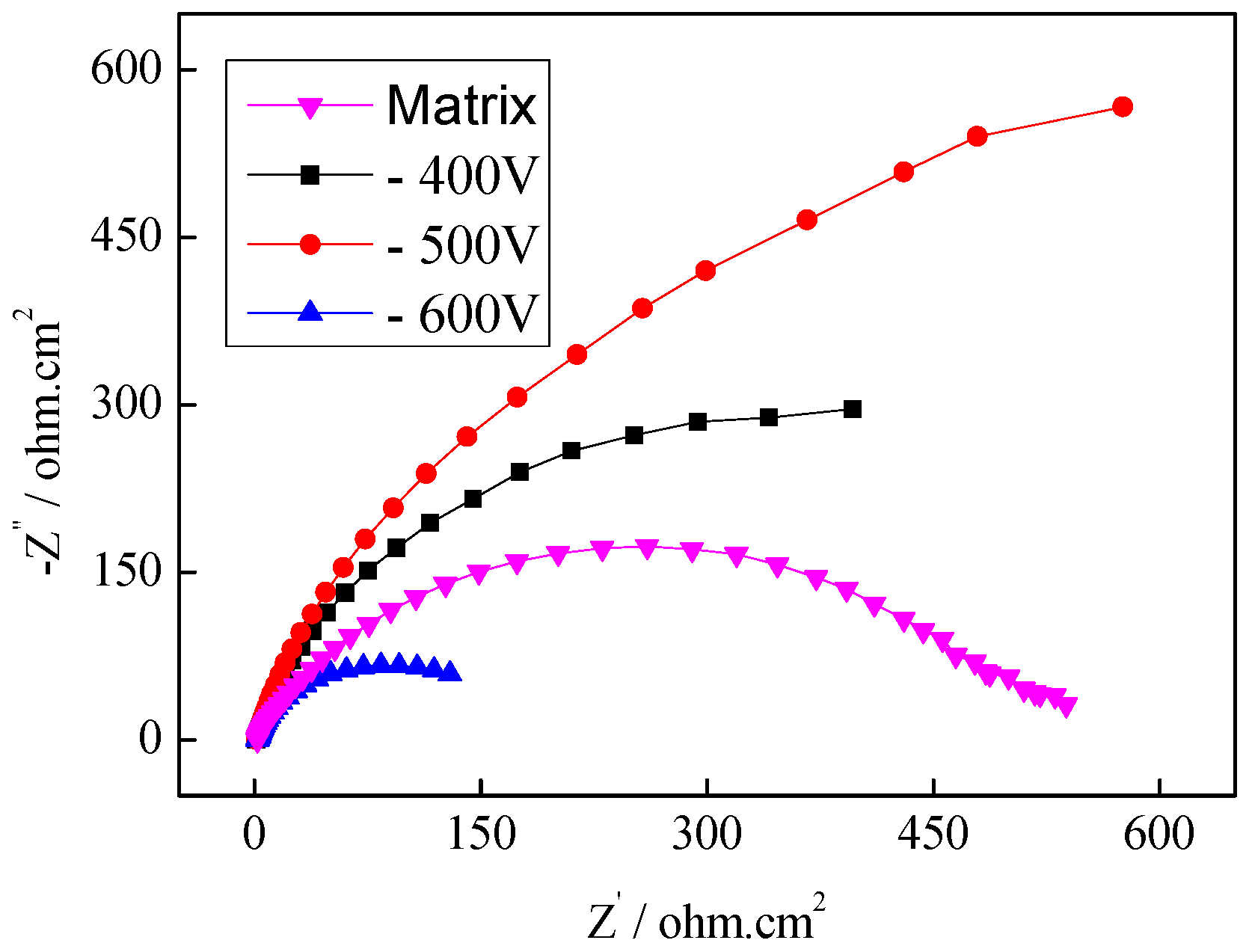
3.3.1. Electrochemical Measurements of the Plasma-Nitrided Coating
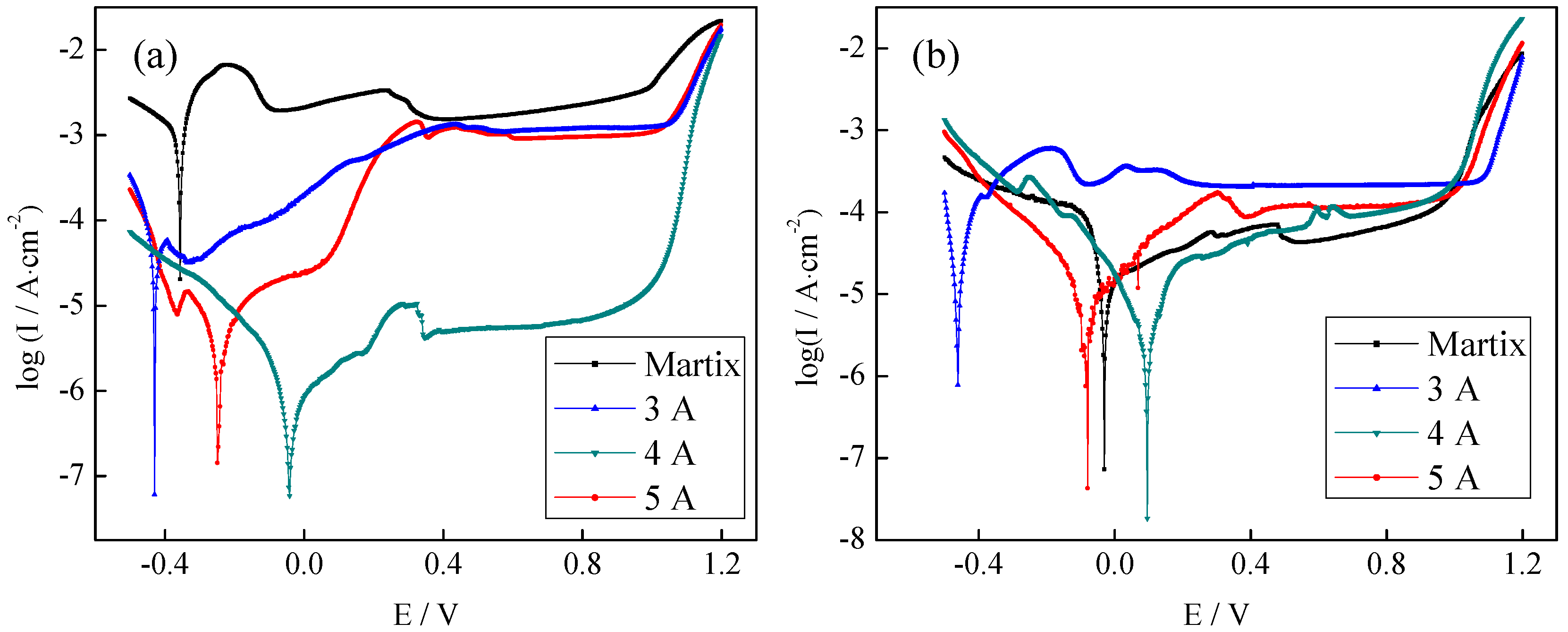
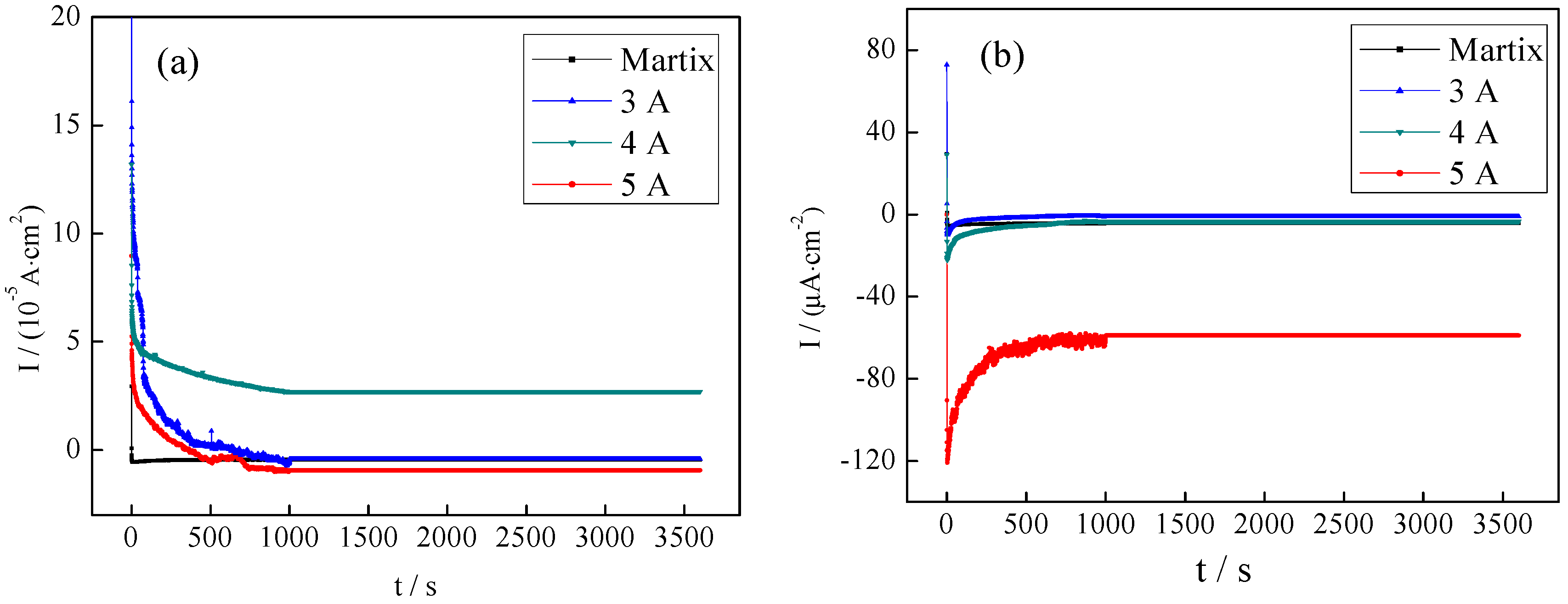
3.3.2. Electrochemical Measurements of the CrNx Coating
3.3.3. Corrosion Resistance of Coatings
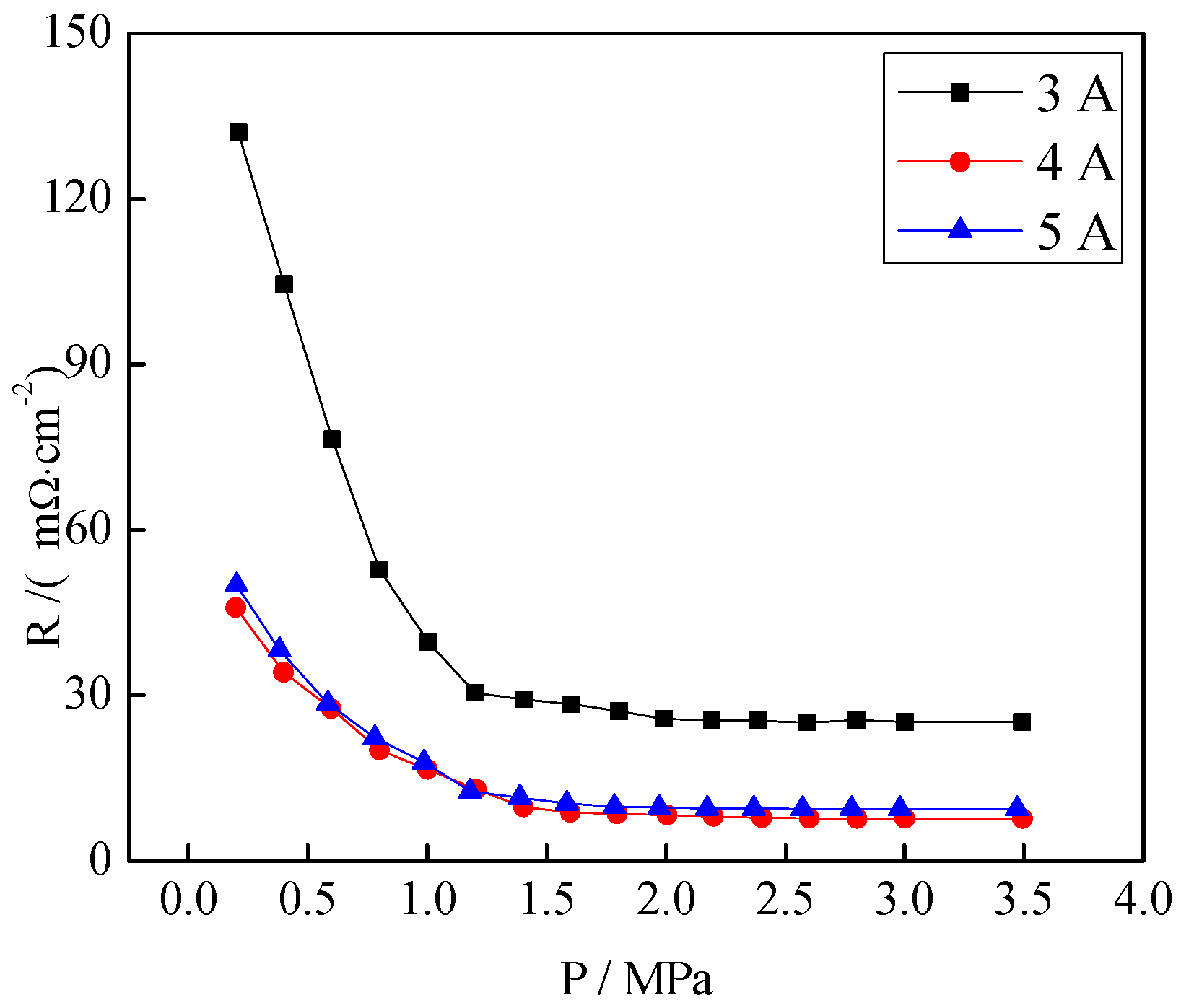
3.4. Contact Resistance of the CrNx Coating
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| PEMFC | Proton exchange membrane fuel cell |
| EIS | Electrochemical impedance spectroscopy |
| CHP | Combined heat and power |
| PVD | Physical vapor deposition |
| CVD | Chemical vapor deposition |
| SEM | Scanning electron microscope |
| ICR | Interfacial contact resistance |
| WE | Working electrode |
| CE | Counter electrode |
| RE | Reference electrode |
| EDS | Energy dispersive spectrometer |
| γN | Austenite phase formed by nitrogen atom penetrating into matrix |
| γ | Austenite phase |
| θ | Incidence angle |
| Ecorr/μA·cm−2 | Corrosion potential |
| Icorr/μA·cm−2 | Current density |
| Cr | Chromium |
| CrNx | Chromium nitride |
| H2 | Hydrogen gas |
| O2 | Oxygen gas |
| N2 | Nitrogen gas |
| Ar | Argon gas |
| Rt | Charge transfer resistance |
References
- Mousa, G.; Golnaraghi, F.; DeVaal, J.; Young, A. Detecting proton exchange membrane fuel cell hydrogen leak using electrochemical impedance spectroscopy method. J. Power Sources 2014, 246, 110–116. [Google Scholar] [CrossRef]
- Holloway, L. One step solution for fifighting bacteria and growing bone. Sci. Transl. Med. 2019, 11, 5326. [Google Scholar] [CrossRef]
- Hu, L.; Cui, Y. Energy and environmental nanotechnology in conductive paper and textiles. Sci. Energy Environ. 2012, 5, 6423–6435. [Google Scholar] [CrossRef]
- Dastjerdi, R.; Montazer, M. A review on the application of inorganic nano-structured materials in the modifification of textiles: Focus on anti-microbial properties. J. Colloids Surf. Biointerfaces 2010, 79, 5–18. [Google Scholar] [CrossRef]
- Carmo, M.; Fritz, L.; Mergel, J. A comprehensive review on PEMFC water electrolysis. Sci. Hydrog. Energy 2013, 38, 4901–4934. [Google Scholar] [CrossRef]
- Taherian, R. A review of composite and metallic bipolar plates in proton exchange membrane fuel cell: Materials, fabrication, and material selection. Sci. Power Sources 2014, 265, 370–390. [Google Scholar] [CrossRef]
- Stenzel, O. The Physics of Thin Film Optical Spectra: An Introduction; Springer: Berlin, Germany, 2016. [Google Scholar]
- Bayrak, G.; Yilmaz, S. Crystallization kinetics of plasma-sprayed basalt coatings. J. Ceram. Int. 2006, 32, 441–446. [Google Scholar] [CrossRef]
- Wang, L.; Sun, J.; Li, P.; Jing, B.; Li, S.; Wen, Z.; Ji, S. Niobized AISI 304 stainless steel bipolar plate for proton exchange membrane fuel cell. Sci. Power Sources 2012, 208, 397–403. [Google Scholar] [CrossRef]
- Ctibor, P.; Nevrlá, B.; Pala, Z.; Sedlácek, J.; Soumar, J.; Kubatík, T.; Neufuss, K.; Vilémová, M.; Medˇrický, J. Study on the plasma-sprayed amorphous diopside and annealed fine-grained crystalline diopside. J. Ceram. Int. 2015, 41, 10578–10586. [Google Scholar] [CrossRef]
- Ctibor, P.; Neufuss, K.; Pala, Z.; Kotlan, J.; Soumar, J. Dielectric and mechanical properties of plasma-sprayed olivine. J. Rom. Rep. Phys. 2015, 67, 600–616. [Google Scholar]
- Samadi, H.; Pershin, L.; Coyle, T.W. Effect of in-flight particle properties on deposition of air plasma-sprayed forsterite. Sci. Surf. Coating. 2010, 204, 3300–3306. [Google Scholar] [CrossRef]
- Xu, T.; He, X.; Chen, Z.; He, L.; Lu, M.; Ge, J.; Weng, J.; Mu, Y.; Duan, K. Effect of magnesium particle fraction on osteoinduction of hydroxyapatite sphere-based scaffolds. Sci. Mater. Chem. B 2019, 7, 5648–5660. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Wang, W.; He, Q. Research and development status of plasma physical vapor deposition equipment. J. Therm. Process. Technol. 2018, 22, 21–26. (In Chinese) [Google Scholar] [CrossRef]
- Bigi, A.; Falini, G.; Foresti, E.; Ripamonti, A.; Gazzano, M.; Roveri, N. Magnesium influence on hydroxyapatite crystallization. J. Inorg. Biochem. 1993, 49, 69–78. [Google Scholar] [CrossRef]
- Deng, Z.; Liu, M.; Mao, J.; Zhang, X.; Chen, W.; Chen, Z. Deposition mechanism based on plasma spray-physical vapor deposition. J. Inorg. Mater. 2017, 32, 1285–1291. (In Chinese) [Google Scholar] [CrossRef]
- Choi, I.S.; Park, J.C. The corrosion behavior of TiAlN coatings prepared by PVD in a hydrofluoric gas atmosphere. Sci. Surf. Coat. 2000, 131, 383–385. [Google Scholar] [CrossRef]
- Popa, A.; Stan, G.; Husanu, A.; Pasuk, I.; Popescu, I.; Popescu, A.; Mihailescu, I. Multi-layer haemocompatible diamond-like carbon coatings obtained by combined radio frequency plasma enhanced chemical vapor deposition and magnetron sputtering. Sci. Mater. 2013, 24, 2695–2707. [Google Scholar] [CrossRef]
- Li, Y.; Xu, H.; Qiu, J.; Xu, J.; Wang, L. Plasma nitrided technology of AISI 316L austenitic stainless steel hollow cathode discharge ion source. J. China Surf. Eng. 2014, 27, 25–30. (In Chinese) [Google Scholar] [CrossRef]
- Guo, Y.; Teng, Y.; Gao, J.; Zhang, X.; Huang, X.; Xie, Z.; Zhou, Y. Effect of pulse bias on surface structure and tribological properties of low temperature plasma nitrided stainless steel. J. Vac. Sci. Technol. 2017, 37, 902–908. (In Chinese) [Google Scholar] [CrossRef]
- Ji, C.; Shi, H.; Cui, X. Study on Microstructure and Properties of Duplex Stainless Steel Formed by Surface Nitridation of SUS430. J. Therm. Process Technol. 2017, 46, 179–181. (In Chinese) [Google Scholar] [CrossRef]
- Wang, J.; Sun, J.; Zhang, F. Performance of stainless steel bipolar plate after low temperature plasma nitrided. J. Mater. Prot. 2010, 43, 65–67. (In Chinese) [Google Scholar] [CrossRef]
- Pan, C.; Zhou, Z.; Yu, X. Coatings as the useful drug delivery system for the prevention of implant-related infections. Sci. Orthop. Surg. Res. 2018, 13, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nam, N.; Jo, D.; Kim, J.; Yoon, D. Corrosion protection of CrN/TiN multi-coating for bipolar plate of polymer electrolyte membrane fuel cell. J. Solid Films 2011, 519, 6787–6791. [Google Scholar] [CrossRef]
- Mani, S.; Srinivasan, A.; Rajendran, N. Effect of nitrides on the corrosion behaviour of 316L SS bipolar plates for proton exchange membrane fuel cell (PEMFC). Sci. Hydrog. Energy 2015, 40, 3359–3369. [Google Scholar] [CrossRef]
- Chen, S.; Yuan, L.; Li, Q.; Li, J.; Zhu, X.; Jiang, Y.; Sha, O.; Yang, X.; Xin, J.H.; Wang, J.; et al. Durable antibacterial and nonfouling cotton textiles with enhanced comfort via zwitterionic sulfopropylbetaine coating. Sci. Small. 2016, 12, 3516–3521. [Google Scholar] [CrossRef]
- Oladijo, O.P.; Mathabatha, M.H.; Ntsoane, T.P. Characterization and corrosion behaviour of plasma sprayed Zn–Sn alloy coating on mild steel. Sci. Surf. Coat. Technol. 2018, 352, 654–661. [Google Scholar] [CrossRef]
- Stan, G.E.; Marcov, D.A.; Pasuk, I.; Miculescu, F.; Pina, S.; Tulyaganov, D.U.; Ferreira, J.M.F. Bioactive glass thin films depositd by magnetron sputtering technique: The role of working pressure. Sci. Appl. Surf. 2010, 256, 7102–7110. [Google Scholar] [CrossRef]
- Stenzel, O.; Wilbrandt, S.; Kaiser, N.; Schmitz, C.; Turowski, M.; Ristau, D.; Awakowicz, P.; Brinkmann, R.P.; Musch, T.; Rolfes, I.; et al. Plasma and optical thin film technologies. J. Proc. SPIE 2011, 8168, 81680L. [Google Scholar]
- Evans, C.C.; Bradley, J.D.B.; Parsy, F.; Phillips, K.C.; Senaratne, R.; Martí-Panameño, E.A.; Mazur, E. Thermally Managed Z-Scan Measurements of Titanium Dioxide Thin Films; Photonics West: San Francisco, CA, USA, 2011. [Google Scholar]
- Zhang, M.; Lin, G.; Wu, B.; Shao, Z. Composition optimization of arc ion plated CrNx films on 316L stainless steel as bipolar plates for polymer electrolyte membrane fuel cells. Sci. Power Sources 2012, 205, 318–323. [Google Scholar] [CrossRef]
- Ageorges, H.; Medarhri, Z.; Ctibor, P.; Fauchais, P. Plasma-sprayed basalt/chromium oxide coatings. Sci. High Temp. Mater. 2007, 11, 71–81. [Google Scholar] [CrossRef]
- Han, C.; Mohanty, B.; Choi, H.; Cho, Y. Surface scaling evolution and dielectric properties of sputter-deposited low loss Mg2SiO4 thin films. Sci. Surf. Coat. 2013, 231, 229–233. [Google Scholar] [CrossRef]
- Xie, Y.; Zhai, W.; Chen, L.; Chang, J.; Zheng, X.; Ding, C. Preparation and in vitro evaluation of plasma-sprayed Mg2SiO4 coating on titanium alloy. J. Acta Biomater. 2009, 5, 2331–2337. [Google Scholar] [CrossRef]
- Xue, W.; Liu, X.; Zheng, X.; Ding, C. Plasma-sprayed diopside coatings for biomedical applications. Sci. Surf. Coat. 2004, 185, 340–345. [Google Scholar] [CrossRef]
- Salimijazi, H.; Hossejni, M.; Mostaghimi, J.; Pershin, L.; Coyle, T.; Samadi, H.; Shafyei, A. Plasma-sprayed coating using mullite and mixed alumina/silica powders. J. Spray Technol. 2012, 21, 825–830. [Google Scholar] [CrossRef]
- Ercenk, E.; Sen, U.; Yilmaz, S. Structural characterization of plasma-sprayed basalt–SiC glass–ceramic coatings. J. Ceram. Int. 2011, 37, 883–889. [Google Scholar] [CrossRef]
- Bouhifd, M.A.; Andrault, D.; Fiquet, G.; Richet, P. Thermal expansion of forsterite up to the melting point. Sci. Mater. 1996, 23, 1143–1146. [Google Scholar] [CrossRef]
- Fu, T.; Zhao, C.; Luo, H.; Zeng, Q.; Zhang, Y.; Liu, C. Structure and properties of 304 austenitic stainless steel by low temperature salt bath nitrocarburizing. J. Met. Heat Treat. 2011, 36, 98–101. (In Chinese) [Google Scholar] [CrossRef]
- Rietveld, H. A profile refinement method for nuclear and magnetic structures. J. Appl. Crystallogr. 1969, 2, 65–71. [Google Scholar] [CrossRef]
- Brady, M.P.; Weisbrod, K.; Paulauskas, I.; Buchanan, R.A.; More, K.L.; Wang, H.; Wilson, M.; Garzon, F.; Walker, L.R. Preferential thermal nitridation to form pinhole free Cr-nitrides to protect proton exchange membrane fuel cell metallic bipolar plates. Sci. Mater. 2004, 50, 1017–1022. [Google Scholar]
- Dundar, F.; Dur, E.; Mahabunphachai, S.; Koc, M. Corrosion resistance characteristics of stamped and hydroformed proton exchange membrane fuel cell metallic bipolar plates. Sci. J. Power Sources 2010, 195, 3546–3552. [Google Scholar] [CrossRef]
- Ma, L.; Warthesen, S.; Shores, D.A. Evaluation of materials for bipolar plates in PEMFCs. Sci. New Mater. Electrochem. 2000, 3, 221–228. [Google Scholar]
- Brady, M.P.; Wang, H.; Yang, B.; Turner, J.A.; Bordignon, M.; Molins, R.; Abd Elhamid, M.; Lipp, L.; Walker, L.R. Growth of Cr-nitrides on commercial Ni–Cr and Fe–Cr base alloys to protect PEMFC bipolar plates. Sci. Hydrog. Energy 2007, 32, 3778–3788. [Google Scholar] [CrossRef]
- Liu, J.; Chen, F.; Chen, Y.; Zhang, D. Plasma nitrided titanium as a bipolar plate for proton exchange membrane fuel cell. Sci. Power Sources 2009, 187, 500–504. [Google Scholar] [CrossRef]
- Tian, R. Chromium nitride/Cr coated 316L stainless steel as bipolar plate for proton exchange membrane fuel cell. Sci. Electrochem. Acta. 2011, 48, 1735–1741. [Google Scholar] [CrossRef]
- Wu, B.; Fu, Y.; Xu, J.; Lin, G.; Hou, M. Chromium nitride films on stainless steel as bipolar plate for proton exchange membrane fuel cell. Sci. Power Sources 2009, 194, 976–980. [Google Scholar] [CrossRef]



| Component | C | Si | Mn | P | Cr | Ni | Mo | Fe |
|---|---|---|---|---|---|---|---|---|
| Content (%) | ≤0.08 | ≤1 | 2–3 | ≤0.035 | 16–18.5 | 10–14 | 2–3 | balance |
| Sample Tape | a-Lattice Constant (Å) | b-Lattice Constant (Å) | c-Lattice Constant (Å) | Average Crystallite Size (Å) |
|---|---|---|---|---|
| −400 V | 6.9623 ± 0.006 | 6.9623 ± 0.006 | 9.9381 ± 0.005 | 417.20 |
| −500 V | 6.97597 ± 0.002 | 6.9759 ± 0.002 | 9.96191 ± 0.004 | 419.84 |
| −600 V | 6.96761 ± 0.015 | 6.9676 ± 0.015 | 9.90159 ± 0.019 | 416.30 |
| 3 A | 4.58119 ± 0.001 | 4.5811 ± 0.001 | 4.58119 ± 0.001 | 89.89 |
| 4 A | 4.8113 ± 0.002 | 4.8113 ± 0.002 | 4.5841 ± 0.015 | 96.15 |
| 5 A | 4.81079 ± 0.007 | 4.8107 ± 0.007 | 4.56199 ± 0.033 | 89.40 |
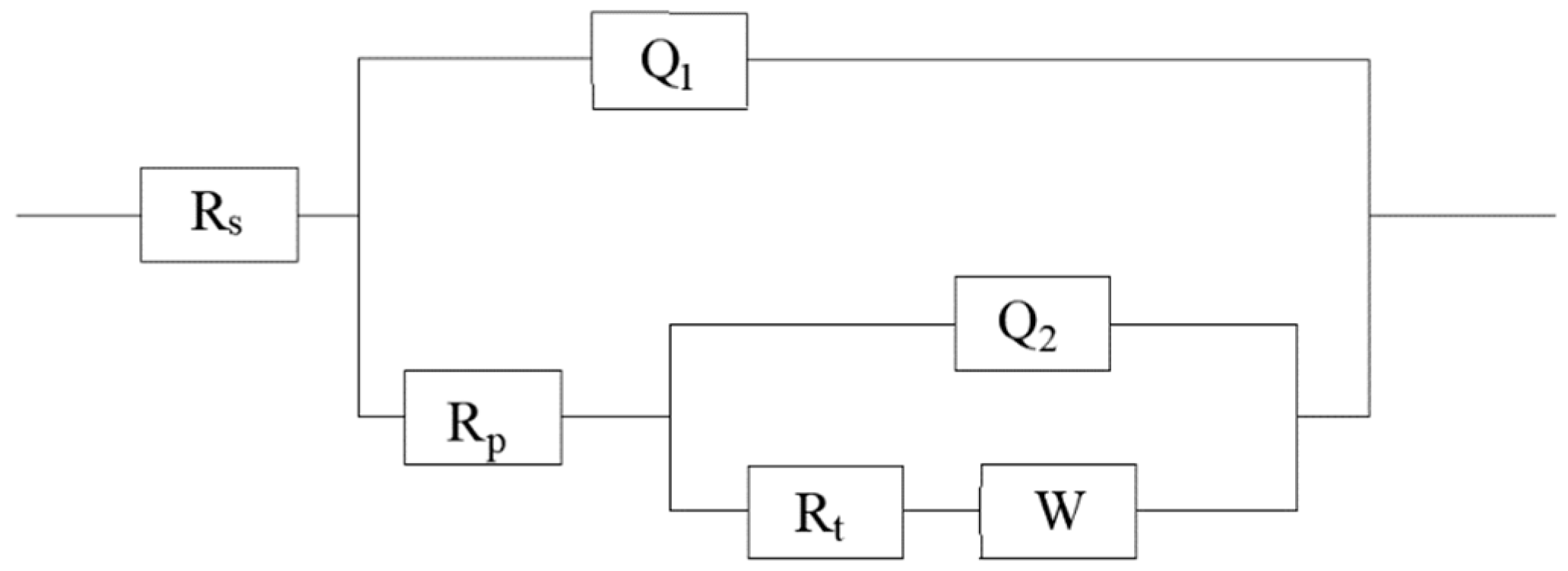
| Filament Current/A | Rs/Ω·cm2 | Q1/μF·cm2 | n1 | Rp/Ω·cm2 | Q2/μF·cm2 | n2 | Rt/Ω·cm-2 | W/mΩ·cm2 |
|---|---|---|---|---|---|---|---|---|
| Matrix | 1.858 | – | – | – | 304.3 | – | 504.3 | – |
| 3 A | 1.000 | 0.7672 | 0.9275 | 2.631 | 33.74 | 0.8735 | 448.5 | 5.797 |
| 4 A | 2.671 | 3.272 | 0.8912 | 169.7 | 39.51 | 0.9710 | 114.6 | 9.831 |
| 5 A | 1.033 | 1.058 | 0.8428 | 2.082 | 53.76 | 0.9665 | 251.2 | 4.765 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, M.; Kang, S.; Lu, J.; Yan, X.; Chen, T.; Wang, Z. Properties of a Plasma-Nitrided Coating and a CrNx Coating on the Stainless Steel Bipolar Plate of PEMFC. Coatings 2020, 10, 183. https://doi.org/10.3390/coatings10020183
Xu M, Kang S, Lu J, Yan X, Chen T, Wang Z. Properties of a Plasma-Nitrided Coating and a CrNx Coating on the Stainless Steel Bipolar Plate of PEMFC. Coatings. 2020; 10(2):183. https://doi.org/10.3390/coatings10020183
Chicago/Turabian StyleXu, Meiling, Shumei Kang, Jinlin Lu, Xinyong Yan, Tingting Chen, and Zimeng Wang. 2020. "Properties of a Plasma-Nitrided Coating and a CrNx Coating on the Stainless Steel Bipolar Plate of PEMFC" Coatings 10, no. 2: 183. https://doi.org/10.3390/coatings10020183
APA StyleXu, M., Kang, S., Lu, J., Yan, X., Chen, T., & Wang, Z. (2020). Properties of a Plasma-Nitrided Coating and a CrNx Coating on the Stainless Steel Bipolar Plate of PEMFC. Coatings, 10(2), 183. https://doi.org/10.3390/coatings10020183





