Abstract
The consumption of probiotics has been associated with a wide range of health benefits for consumers. Products containing probiotics need to have effective delivery of the microorganisms for their consumption to translate into benefits to the consumer. In the last few years, the microencapsulation of probiotic microorganisms has gained interest as a method to improve the delivery of probiotics in the host as well as extending the shelf life of probiotic-containing products. The microencapsulation of probiotics presents several aspects to be considered, such as the type of probiotic microorganisms, the methods of encapsulation, and the coating materials. The aim of this review is to present an updated overview of the most recent and common coating materials used for the microencapsulation of probiotics, as well as the involved techniques and the results of research studies, providing a useful knowledge basis to identify challenges, opportunities, and future trends around coating materials involved in the probiotic microencapsulation.
1. Introduction
Probiotics are defined by the Food and Agriculture Organization of the United Nations (FAO) and the World Health Organization (WHO) as “living microorganisms which, when ingested in certain amounts, provide health benefits to the host” [1]. The consumption of probiotics positively influences the growth of targeted microorganisms in the host gastrointestinal tract, eliminates harmful bacteria or fungi, and boosts the naturally occurring defence actions of the host’s immune system. Additionally, it also helps in the treatment for irritable bowel syndrome (IBS), gastrointestinal dysbiosis, as for other intestinal disorders [2,3,4]. Most of the known probiotics are Generally Recognized As Safe (GRAS), including Lactobacillus and Bifidobacterium species, and certain yeast strains such as Saccharomyces boulardii, S. cerevisiae CNCM I-3856, and Lipomyces starkeyi VIT-MN03 [3,4,5,6]. Several mechanisms are proposed on how these microorganisms are beneficial to the host wellbeing. Some examples are: by the production of antimicrobial or antifungal peptides; by stimulating changes in the intestinal environment which make it unfavourable for other microorganisms, including pathogens; as well as by competing for nutrients and for attachment to intestinal epithelial cells [7,8,9]. Probiotic microorganisms are also required to have certain features, such as: genetic stability; resistance to the gastric environment (acid and bile tolerance); adhesion capability to a mucosal surface; good in vitro/in vivo growth properties; maintaining high viability at processing; survival during storage, among others. These features ensure the survivor of a large number of these beneficial microorganisms for the successful colonization of the host’s colon. Strict safety criteria are also compulsory, such as origin, the lack of pathogenicity and infectivity, or presence of virulence factors (toxicity, metabolic activity, and intrinsic properties, i.e., antibiotic resistance) [10,11].
Microencapsulation with edible coatings is often used to carrying a wide variety of products, such as: probiotics, flavours, fragrances, enzymes, antioxidants, antimicrobials, lipids, minerals, edible pigments, nucleic acids, etc. [12,13]. During the last decades, the microencapsulation has arisen as a trendy method for enhancing the survival of probiotic microorganisms. Probiotic microcapsules, when ingested, should result in more efficient probiotic delivery to the host gastrointestinal tract [14,15]. The term “microencapsulation” is defined as a process in which tiny particles or droplets of liquid or solid material are surrounded by a coating, or embedded in a homogeneous or heterogeneous film of polymeric matrix, to give small capsules with many useful properties [16,17]. According to the size, the capsules can be classified as: macro- (>5000 μm), micro- (0.2 to 5000μm), and nano-capsules (<0.2 μm) [18].
The protection efficiency provided by microencapsulation depends on many parameters, such as the probiotic microorganism strain, the method of microencapsulation, the coating material, among others. Microencapsulation has proven to reduce probiotic cell damage and enhance survival in simulated gastrointestinal fluid (SGIF) models. [14]. Coating material gives protection to the microorganism through the control of stress response mechanisms against gastric environment, which involve: moisture, solute migration, gas exchange, oxidative reaction rates, etc. It also offers protection from adverse external conditions as UV light and heat. Many technical approaches based on several physical and chemical principles have been explored for the microencapsulation of probiotic microorganism. Successful methods of microencapsulation include: spray drying; spray chilling; spray freeze drying; extrusion; electrospraying; layer-by-layer; fluidised bed drying; and other physicochemical techniques such as emulsification and coacervation. Furthermore, most of the coating materials currently used in the microencapsulation of probiotics mainly comprises proteins, polysaccharides, and lipids. These naturally occurring polymers, or their chemically modified versions, are often used alone or in blends to form the structural coatings [19]. A successful probiotic microencapsulation mainly depends on the compatibility of all the components, namely the type of microorganism, the method of microencapsulation and the coating material. Little changes in the composition of coating and/or core material, as well as in the physical and/or chemical treatments that the capsules are subjected to, make great differences in the final properties of the microcapsule [20]. In this regard, this review aims to give an overview of the most featured edible coating materials used until to date for the microencapsulation of probiotics. This work first summarises current methods of microencapsulation and then further presents a review of the commonly used coating materials, such as proteins, polysaccharides, and lipids.
2. Methods for Microencapsulation of Probiotics
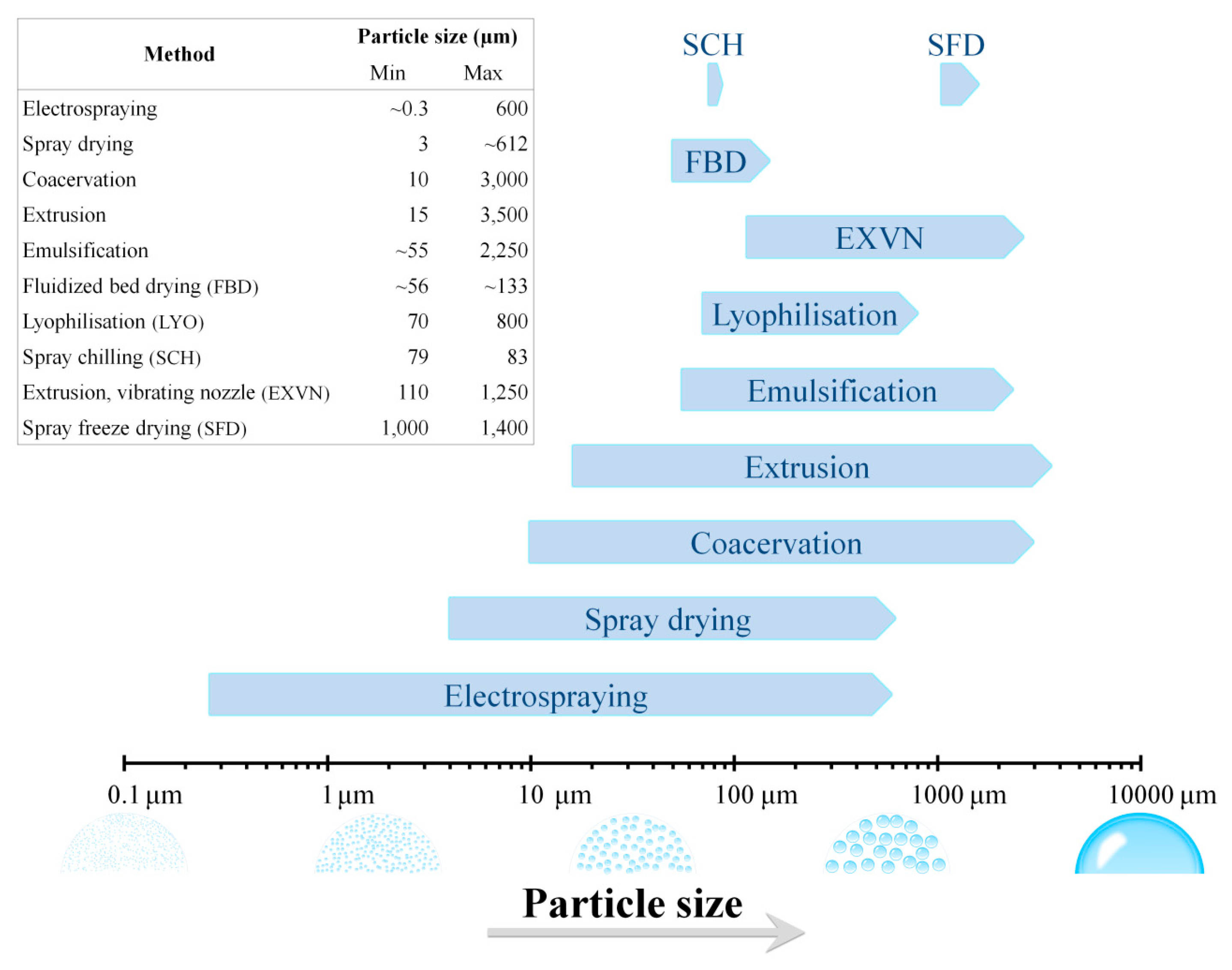
The physical and/or chemical properties of the edible microcapsule are largely defined by the method of encapsulation, the probiotic microorganism, and the coating admixtures. Modern and innovative methods for microencapsulation have been developed in the last decades, which led to creating a wide variety of functional probiotics microcapsules. Table 1 summarizes the different methods commonly employed for the microencapsulation of probiotics whereas Table 2 summarizes the permeability properties of films based on edible film-forming materials. However, it is important to point out that food industries more likely prefer cheaper processes. So, from the industrial perspective the main aspect of the method applied to manufacture is the cost; it should be cheap and also functional without compromise ethics or the quality of final product. The different methods for the microencapsulation of probiotic microorganisms involved several physical and chemical principles. Successful methods used in the microencapsulation of probiotics include: spray drying [21,22]; spray chilling (also called spray cooling or congealing) [23,24]; spray freeze drying [25,26]; lyophilisation [27,28]; electrospraying [29,30]; layer-by-layer [31,32]; fluidised bed drying [33,34]; extrusion [35,36] and its improved version: the vibrating nozzle technology [37,38]. Emulsification [39,40] and coacervation [41,42] are other important and often used physicochemical techniques. Although uniform particle size distribution may be preferred in many applications, these different microencapsulation methods produce microcapsules with a wide range of particle sizes. Figure 1 provides some basic guidelines to help decide which of the described microencapsulation methods could be most suitable for a particular, desired microcapsule size. Nonetheless, it should be considered that, in addition to the method, the particle size output of the microcapsules also varies as a function of the coating materials employed for the probiotic microencapsulation (Table 1).

Table 1.
Different works reporting the microencapsulation of probiotics. The table summarizes the probiotic microorganisms, the method of microencapsulation, and the coating material employed for the development of probiotic microcapsules, as well as the reported average particle size.1.

Table 2.
Different works reporting the water vapor, O2, and CO2 permeability of some edible films.
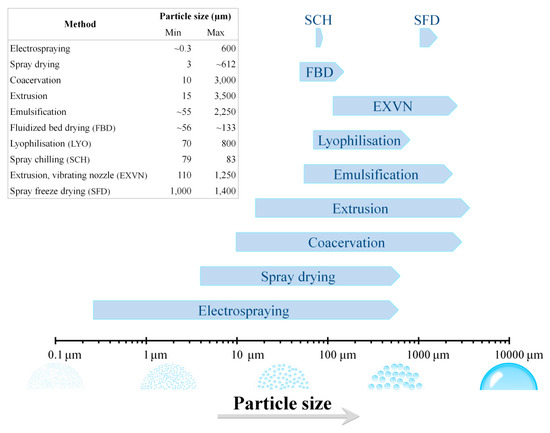
Figure 1.
Output particle size ranges of the different methods employed for the microencapsulation of probiotic microorganisms.
The various methods described below have the same aim, namely, coating living microorganisms with a protective layer. However, these methods have different basic principles. The specific operating conditions employed by the listed methods, as well as the encapsulation efficiency, are summarised in Table S1.
The spray drying and spray chilling methods for the microencapsulation of probiotics are similar in many ways; both methods involve dispersion of the core material via atomization into a chamber with an environmental condition that promotes the solidification of coating. Then, the formed microcapsules are separated from the humid air by a filter or by a cyclone in order to collect them in powder form. The temperature of the chamber for coating solidification is the main difference between these methods. During spray drying, hot air lead to the rapid evaporation of solvent in which the coating material is dissolved, while in spray chilling solidification is accomplished by atomising the hot molten mixture of core-coating materials in an environment which is cooled below the melting point of the coating material [43,44]. Freeze drying is also denominated as lyophilisation or cryodesiccation. It is a drying process in which the solvent medium is frozen and then sublimed (direct transition from the solid phase to the gas phase) under a reduced pressure environment. On the other hand, in the spray freeze drying technique, a liquid containing the coating and core materials is sprayed into a cryogenic chamber wherein the spray droplets are rapidly frozen. Then the frozen droplets are lyophilised to take away the solvent and generate dried particles [43,45].
Extrusion and emulsification are both basic techniques used to produce hydrocolloid microcapsules, which are also denominated droplet and two-phase system methods, respectively. The extrusion method consists in the preparation of a hydrocolloid solution containing the probiotic cells. Then, the suspension is forced through a small orifice, as a syringe needle, in a way that the resulting droplets freely drip into the hardening solution. The droplet scheme is an old, cheap, and common procedure to create microcapsules. However, in spite of the low cost of production, the most important disadvantage of this procedure is that the coating of microcapsules has slow solidification rates, which makes difficult to scale-up the process [46]. An evolving progress of the extrusion process is the vibrating nozzle method [47]. This method is based on a mechanical principle where a vibrational frequency, with defined amplitude, is applied to an extruded jet causing that the laminar jet break-up in defined-size droplets [48]. The droplet size depends on jet diameter, the velocity of the extruded fluid, viscosity, surface tension, and the frequency of disturbance. During the last decades, studies focused on the microencapsulation of probiotic have been using the vibrating technology [37,49,50,51].
The emulsification method is another cheap method for the probiotic microencapsulation process that, in contrast to extrusion, can be easily scaled-up. By mixing the probiotic cells and the coating polymer in aqueous and oil phases, a ‘solution-like’ is formed consisting of small droplets. Then, the water soluble polymer becomes insoluble after the addition of cross linking agents (e.g., calcium chloride) which lead to the formation of gel particles in the oil phase. Later, microcapsules can be recovered by filtration [52]. The size of the microcapsule in this process is mainly defined by the agitation rate of the mixture. Emulsifying agents (e.g., Tween 80 and lauryl sulphate) decrease the interfacial tension of the two immiscible phases producing a better homogenization and can be used for the preparation of smaller capsules [46,53].
Microencapsulation by coacervation basically consists of three steps performed under continuous agitation. The first step involves the formation of three immiscible chemical phases: the assembly liquid, the core medium, and the coating material. The second step consists of a phase of coating deposition, where the core material is dispersed in the polymer coating solution. The last step is the rigidification of coating, where the immiscible coating material becomes rigid, which generally involves warm, cross-connect, or desolvation methods. Furthermore, the coacervation method can be divided into simple and complex categories. Simple coacervation involves the addition of a strongly hydrophilic substance to a colloid solution to form two phases, while complex coacervation manipulates the acidic/basic nature of one or more colloid system to induce the production of microcapsules [18,54].
Among the different methods mentioned previously, the electrospraying, the layer-by-layer, and the fluidised bed drying are emerging methods in the field of the microencapsulation of probiotics. The electrospraying technology used for microencapsulation is based on the principle of electrohydrodynamics. Electrospraying is also known as electrohydrodynamic atomization. The process typically involves a high voltage electrical field which is applied to a capillary where a liquid, containing the core material, flows and it is sprayed towards a charged collector where the spherical droplets are deposited. The solidification occurs by different means, for example by chemical hardening or by solvent evaporation. This method was combined with other microencapsulation techniques to increase the efficiency of the microencapsulation process. Until now, the electrospray extrusion technique has been successfully used for probiotic microencapsulation [29,55].
The layer-by-layer method (LbL) is based on the chemical electrostatic attraction of positively and negatively charged materials [56]. The preparation of microcapsules by LbL consists in the self-assembly of layers by electrostatic adsorption of materials with opposite charge to the surface of the core material. This method represents an efficient strategy to assemble multi-layered capsules by the sequential exposure of charged substrates on the core material surface. The process can be repeated until obtaining the required number of coating layers [57]. Finally, in the fluidised bed drying method the core material is fluidised in the gaseous phase and mixed with the coating material in the form of particles or fine droplets. The coating material is deposited on the surface of the core material and forms a layer due to electrostatic forces. Two studies have demonstrated the effectiveness of the fluidised bed drying method for probiotic microencapsulation [33,34].
3. Edible Coating Materials
The coating material is the barrier that contains the core medium, protecting against external conditions. It is also known as wall, shell, membrane, carrier material, external phase, or matrix material. The arrangement of the coating material on the surface of the core material is the main determinant of the functional properties of the microcapsule. Additionally, the coating material can arrange in one, two, or more coating layers containing the core material. Great challenges have been dealt with before for the development of edible probiotic microcapsules. In this regard, bioactive ingredients for coating material have been the subject matter of many studies for the last two decades [15,144]. An ideal coating material should have the following desirable characteristics: be chemically inert with the core material; be able to seal and contain the core material inside the capsule; capability to provide protection against unfavourable conditions; and be sustainable and cheap. To date, no ideal coating exist yet that fits for all purposes, mainly because the coating characteristics cannot be simultaneously improved. Thus, obtaining suitable coatings for microcapsules as a probiotic delivery system, implies to find a point of balance among desirable characteristics, such as protecting against the effects of moisture, acidity, pressure, gas interchange (O2/CO2), and/or thermal aggression. Nevertheless, for an appropriate selection of the coating material, the interdependence with the microencapsulation method and the probiotic microorganism should also be considered. A wide variety of natural or synthetic polymers are currently available which choice depends on the core composition to be coated, as well as the desired features in the final microcapsules [145,146]. In order to be used in the microencapsulation of probiotic cells, coating agents must also be edible film-forming materials. Edible coating materials based on bio-polymers are widely used and comprise: proteins (such as zein, soy protein, collagen, and gelatine), polysaccharides (such as cellulose derivatives, starch, alginate, and chitosan), and lipids (such as fats and waxes) [19].
The properties of edible film-forming materials are an important factor to consider when formulating an edible coating for the microencapsulation. Since probiotic cells are living microorganisms, they are sensitive to the presence of water or gases (O2/CO2). Conveniently, edible film-forming materials possess selective permeability (e.g., water vapour and gases) which allow control respiration exchange and microbial development. It has been shown that by combining edible film-forming materials, it is possible to improve or modulate the physical properties of the resulting edible film. The WVP and gas permeability are some of the most important properties of an edible film. The water vapour (WVP), oxygen (PO2) and carbon dioxide permeability (PCO2) of several edible film-forming materials have been studied, and some of the reported values are summarised in Table 2. However, when comparing these values, it is important to consider the different conditions that were employed for testing such films, i.e., the temperature and relative humidity (RH). For example, the effect of RH on permeability is significant; little differences in RH during testing can result in drastic changes in the permeability [147].
Due to their hydrophilic nature, polysaccharide- and protein-based films are generally rather poor water barriers and they generally have good gas barrier properties, particularly under low RH conditions. Increasing RH leads to a sharp increase in gas permeability. Most films based on pure polysaccharides tend to display lower WVP values than most protein-based films. However, at elevated RH, all protein-based films exhibit increased WVP values. Thus, the selectivity of these hydrophilic materials is sensitive to moisture variations. On the other hand, lipid-based films have extremely low WVP values and suitable gas barrier properties, which is a consequence of their hydrophobic, crystalline structures. Generally, if the degree of crystallinity of lipid is higher, the permeability of the film will be lower. Indeed, the presence of carbon-carbon double bonds, branching or the reduction in the length of the carbon chains disrupts the lipid crystalline structure which results in decreased oxygen permeability. As stated previously, the formulation of composites allows combining the properties of each component. The incorporation of lipids into polysaccharide- or protein-based films can reduce their WVP values and gas permeability. Accordingly, at high RH, the addition of lipidic compounds to the films results in a decrease in the water and gas permeability [147,148,149].
Coating materials should also provide other desirable traits for the food industry, such as: be economical, meet quality and food safety standards, have no chemical reactivity with core materials and possess rheological traits (such as viscous, elastic, and plastic properties) making it easier to work with at high concentrations during the encapsulation process [146,150]. They must have good solubility in commonly-used industrial solvents used in the microencapsulation process (e.g., water, ethanol, etc.). Coating materials also require other necessary features, for example: capability to seal and maintain the core material within its structure during processing or storage; be able to disperse or emulsify the active material and to stabilize the produced emulsion; be able to favour the desolventization in the microencapsulation process; provide maximum protection against environmental conditions; and be chemically inert with the encapsulated core material during processing and during the shelf life of the final product. In the following sections, we describe the most common coating materials that have been used lately for microencapsulation of probiotics. Proteins, polysaccharides, and lipids are among the most featured edible coating materials used to date in the microencapsulation of probiotics; and their use has improved the stability of probiotics during processing, storage, and simulated gastrointestinal conditions.
4. Proteins
Proteins are great materials for probiotics microencapsulation; however, they are frequently used in a mix with other coating agents. To date, few works have employed proteins as a solely coating agent [26,30,96,104]. Many proteins have been widely used as coating agents due to their properties that act as a good barrier against the O2 and CO2 permeability. Each protein owns a unique set of physicochemical properties. Its particular sequence of amino acids allows a wide variety of both intra- and intermolecular interactions and with other material participating in the formation of the edible matrix [15,19].
The proteins used as coating agents for probiotic microcapsules can be categorized, according to their nature, as vegetable or animal proteins. Examples of proteins from animal source are: gelatine, casein, whey protein concentrate (WPC), whey protein isolate (WPI), egg white, and caseinates. On the other hand, examples of proteins from vegetable source are: corn (zein), pea, wheat, and soy proteins. Particularly, some proteins are highlighted to be ideal for creating or improving coatings in accordance with specific microencapsulation methods. For example, gelatine is a large, fibrillar protein obtained through the partial hydrolysis of collagen [151]. The fact that gelatine is one of the oldest and multiple-purposes ingredients in the food industry makes it also one of the most studied proteins as coating agent in the preparation of microcapsules [152]. Moreover, the properties of gelatine and its ability to interact with a wide variety of polysaccharides allows its use as a coating material in a several microencapsulation methods, such as extrusion, complex coacervation, spray chilling, spray drying, and lyophilisation [23,42,67,100,153,154]. Another particular advantage of using gelatine as a coating material is its linear structure, which provides a better oxygen barrier than globular proteins. Nonetheless, the use of gelatine for probiotic microencapsulation has few disadvantages like the varying purity, which make unknown the exact molecular weight within preparations, or that it generally needs to be blended with other materials to achieve particular properties, such as viscosity, gel strength, or adhesiveness. Additionally, due to its animal origin, it results inappropriate in the creation of microcapsules within the bounds of the vegetarian or kosher trends [154,155,156].
Egg white (albumen), soy proteins, and whey proteins are some examples of globular proteins used in probiotic microencapsulation. These proteins have good emulsifying and gelling properties that are considered as ideal materials for microencapsulation through the coacervation process [154,157,158]. However, only a few studies using whey proteins have been carried out for probiotic coatings by coacervation [58,68]. Others techniques has been preferred over coacervation using globular proteins as coating materials. For example, a coating composed of egg albumen and stearic acid was employed to preserve Lactobacillus acidophilus by electrospraying and fluidised bed drying [34]. Similarly, a blend of alginate and soy protein isolate was used as a coating material for the microencapsulation by extrusion of L. plantarum [83] and the microencapsulation of L. acidophilus by spray drying was achieved using soy extract and maltodextrin as coating agents [22]. It is important to point out that soy protein isolates have recently attracted interest as a probiotic coating ingredient. Among other vegetable proteins available, soy proteins isolate represents a source of high quality proteins and a reliable alternative for vegetarians and people allergic to milk [159]. Additionally, soy proteins own several properties (e.g., gelation, emulsification) that make it suitable to use as coating material in the food industry [160]. The synergistic effects of the combination of soy proteins isolate and other edible materials enhanced the final properties of microcapsule coatings. Moreover, Wang et al. recently reported that the gelation properties of soy protein isolate are transformed by the presence of CaSO4, MgCl2, or MgSO4. Their studies provided insights into the structural behaviour of soy protein gels and would be useful toward future applications in the food industry including the microencapsulation process [161,162].
Milk proteins used as coating materials are available in native and processed forms. Many current works regarding the microencapsulation of probiotics described the use of caseins, whey proteins (WPC and WPI), and products containing both casein and whey proteins. Bovine milk contains ~3.5% protein, which composition is mainly casein and whey proteins. Casein is the most remarkable protein as it represents nearly 80% of the total protein content in milk. Hence, whey proteins are defined as any protein which keeps in solution after removal of casein from milk [163,164]. Caseins mainly comprise αS1-, αS2-, β-, and κ- caseins variants whereas whey proteins comprise β-lactoglobulin (β-LG), α-lactalbumin (α-LA), immunoglobulins, and serum albumins. Caseins and whey proteins present genetic variability that give them inherent properties as different molecular weight, isoelectric point, hydrophobicity, and so on [164,165]. At a pH of 4.6, caseins are insoluble, whereas whey proteins are soluble. Thus, the isoelectric precipitation is the main method to obtain caseins from dairy products. During the process known as curdling, the casein is precipitated, washed, and dried, resulting in the separation from whey proteins. When the water soluble derivatives of acid caseins react with alkali solutions, the resulting product is known as caseinate [166]. Sodium caseinate (SC) is the most common form of casein used as a coating material due to their physicochemical features that confer excellent surface active properties similar to caseins, as well as an increased resistance to heat denaturation. Indeed, caseinates are more preferred than whey proteins when using the spray drying method of dairy-based oil-in-water emulsions [164]. Additionally, the commercial enzyme transglutaminase (TGase) has been recognized to improve texture and sensory traits in dairy products. This enzyme forms inter- and intra-molecular isopeptide bonds between proteins by cross-linking of glutamine and lysine residues [167]. TGase is able to induce the gelation of caseinates under mild conditions, sufficient to achieve successful microencapsulation of heat-sensitive, living probiotics [65,66].
Whey proteins are commercially available as concentrates (WPC) and isolates (WPI) containing 35%–85% and >95% of protein, respectively. Their processing technologies for purification and separation are different and, in consequence, their compositions also differ. WPC is obtained by centrifugation followed by ultrafiltration/diafiltration and spray drying. On the other hand, for the manufacture of WPI additional steps such as ion exchange chromatography are carried out [168,169]. Due to these manufacturing differences, WPCs are characterized by a low fat and cholesterol content, and high levels of lactose and total lipids whereas WPIs have high protein content and low concentration of lactose and lipids [170].
The β-LG protein in bovine milk represents the highest amount of whey protein composition (50%–60%). Consequently, this typical globular protein (162 amino-acid residues) has been well characterized. The β-LG exhibits excellent thermogelification properties, a fact that determines the gelation properties of WPC [169]. The α-lactalbumin (α-LA) is a calcium metallo-protein consisting of 123 amino-acid residues. It is another major component (~20%) of whey proteins. Studies showed an increase in gelation ability and gel properties (strength, rigidity, or viscoelasticity) of β-LG when mixed with α-LA, suggesting a synergistic effect of these proteins in the gelation behaviour [171]. The calcium atom of α-LA can promote the formation of intermolecular ionic bonds, which makes this protein thermally stable and allow it to bind to other proteins [172]. Among several bio-properties attributed to α-LA, the digestion of α-LA releases small peptides that have shown antimicrobial activity against pathogenic bacteria, such as Escherichia coli, Klebsiella pneumoniae, Staphylococcus aureus, Staphylococcus epidermis, streptococci, and Candida albicans. Moreover, these small peptides also exhibited the capacity to prevent enteropathogenic Escherichia coli, Salmonella typhimurium, and Shigella flexneri from adhering to intestinal cells [172,173]. Whey proteins, in its different forms, were recently explored as coating materials for the microencapsulation of probiotics [28,30,41,58,72,95,97,102,103,104,105].
Finally, sweet whey (SW) is an example of product containing both casein and whey proteins. It is a concentrated, dried by-product of cheese production with a rich protein content that is mainly composed of β-LG, followed by α-LA, αS1-casein, lactophorin (a small phosphoglycoprotein), fatty acid binding proteins (FABP), bovine serum albumin (BSA), as well as immunoglobulins G heavy and light chain (IgG-HC and IgG-LC, respectively) [164,174]. Sweet whey was successfully used as coating material for the microencapsulation by spray drying of Bifidobacterium lactis [102].
5. Polysaccharides
Polysaccharides or polyglycans are bio-polymers consisting of monosaccharide blocks that possess hydroxyl groups, such groups may interact with water or other molecules via intra- and intermolecular hydrogen bonds. However, the behaviour of polysaccharides is also influenced by the nature of their monomers and their substituent groups, which allows a wide variation in their molecular and functional characteristics. The substituent groups may be unmodified (natural polysaccharides) or modified, also known as synthesized or seminatural polysaccharides. Regardless of its origin or composition, polysaccharides commonly employed as coating materials for probiotic encapsulation may be classified according to their magnitude of electric charge into five categories: anionic, cationic, non-ionic, amphoteric [175].
5.1. Anionic Polysaccharides
Anionic polysaccharides tend to be negative at pH values above their pKa value and neutral when well below their pKa value. Alginate, pectin, carrageenan, xanthan, gellan gum, and gum Arabic are natural anionic polysaccharides mostly used in probiotic microencapsulation; whereas commonly used modified anionic polysaccharides are: sodium carboxymethyl cellulose (CMC), also known as cellulose gum, and carboxymethyl chitin (CMCH), also called chitin liquid. Ionic species in the surrounding milieu may alter the electrical charge of polysaccharides. Monovalent or multivalent ions, such as Na+ or Ca2+, may interact to negatively charged groups on the bio-polymer chain, modifying overall charge characteristics. The gelation of anionic polysaccharides involves interaction to oppositely charged groups on the polymer chain. For example, the gelation of alginates and pectins with divalent metal ions, such as Ca2+ [176].
Alginate is an ingredient widely used as a coating material for the production of microcapsules by ionic gelation using extrusion and spray drying methods. However, pectin also showed similar capabilities and it is considered more resistant to acids and the gastrointestinal environment when compared to alginate [68]. Alginate is an unbranched heteropolysaccharide extracted from the cell wall of brown algae (Laminaria spp.). Its structure consists of ß-1,4 glycosidic bonds formed between the ß-D-mannuronic acid and α-L-guluronic acid residues with varying composition [177]. On the other hand, pectin is extracted from some fruits or fruit peels (e.g., apple pomace and citrus peels) and forms a coating structure via calcium gelification which is mainly composed of linear α-(1-4)-linked D-galacturonic acid segments, as well as of highly branched segments with other neutral sugars such as arabinose, galactose, and xylose [178]. In spite of their similarities, alginate is currently the coating material most extensively used for the microencapsulation of probiotic microorganisms (Nearly half from the summary of references presented in Table 1), in comparison to pectin [29,36,63,68,78].
Carrageenans are extracted from red seaweeds (Rhodophyta) and consist of different sulfated polysaccharide mixtures. Red seaweeds are able to produce three distinctive types of commercial carrageenans (κ-, ι-, and λ-carrageenan) that differ in their chemical structures and properties [179]. Importantly, only κ- (monosulfated) and ι-(bisulfated) carrageenan types possess anhydrous bridges that, under the presence of certain cations such as K+ and Ca2+, allow gelation to occur. On the other hand, λ-carrageenan (trisulfated) type is unable to form hydrogen bridges, consequently, it does not allow gelation to happen [180]. Thus, depending on type (κ-, ι-, and λ-), carrageenans have a wide varied of gel properties [181,182,183]. All types of carrageenan are soluble in hot water. The κ-carrageenan forms a hard, brittle gel which is melted by heating at low temperature, while ι-carrageenan is soluble in cold water forming soft, elastic gels. The ι-carrageenan has shown to be a promising coating material in microcapsules that contain flavours and aromatic compounds. Despite this, ι-carrageenan has not been widely investigated yet for its use in probiotic microencapsulation [39,179]. Currently, studies of probiotic microencapsulation using carrageenan as coating material have been mainly focused on κ- carrageenan. Furthermore, the gel properties of κ-carrageenan have been improved by mixing it with other coating materials, such as vegetable oils, calcium alginate, as well as several gums (e.g., xanthan, gellan, and locust bean gums). These mixtures were used for the successful microencapsulation of probiotic through emulsification [39,69,70,84,183].
Xanthan gum, gellan gum and gum Arabic are other anionic polysaccharides used in the microencapsulation of probiotics. Xanthan and gellan gums are bacterial extracellular polysaccharides produced by Xanthomonas campestris and Sphingomonas elodea, respectively. On the other hand, gum Arabic (also known as gum acacia) is a tree exudate from some plants of the Acacia family. Particularly, the Acacia senegal tree is mostly appreciated for commercial production of gum Arabic [178].
Xanthan gum (XG) is produced by several species of the family Xanthomonadaceae. However, currently XG is industrially produced from Xanthomonas campestris, a plant-associated bacterium. The chemical structure of XG consists of pentasaccharide repeating units, that consisting of a side-chain comprising one glucuronic acid residue between two mannose units, attached to every second glucose unit of a linear backbone of cellulose (β-(1-4)-linked glucose) [184]. The structure of XG also contains varying proportions of O-acetyl and pyruvyl residues which depend on the microbial specie, as well as on their fermentation conditions [185]. The acetyl and pyruvyl residues play a fundamental role due their protonation at pH > 4.5, which confers to XG a polyanionic charge characteristic. The interaction between the side chains of XG and the acetyl/pyruvyl residues leads to intramolecular crosslinking, promoting changes in folding arrangement in the XG structure. Thus, the XG features mainly depend on the milieu conditions, such as pH, the nature of the electrolyte, and the ionic strength [185]. Current studies have shown that the presence of acetyl or pyruvate on the outer mannoses of the xanthan gum influences on the stability of the helical (ordered) conformation [186,187,188]. In addition, the content of acetyl and pyruvate groups is an important parameter for practical applications, as it is directly related to the rheological properties of XG in aqueous solutions. For example, lower levels in pyruvyl substitutions confer low viscosity, while higher levels promote a gel behaviour [185,188]. Importantly, the acetyl and pyruvyl residues in the XG set the conditions for complexation with divalent cations (e.g., Ca2+ or Mg2+) [189]. XG has proven to be an excellent coating agent for the microencapsulation of probiotics, protecting probiotic cells against simulated gastrointestinal conditions and high temperatures [71,74,85,86,89,183,190,191]. Moreover, XG was successfully combined with other coating materials (such as: alginate, chitosan, gellan and β-cyclodextrin) in order to improve its coating properties in the microencapsulation of probiotics [85,86,89,191]. For example, the microencapsulation of Lactobacillus plantarum LAB12 using XG and alginate as coating materials, improved the cell viability of the probiotic microorganism in conditions simulating gastric juice and bile salts, when compared to free cells. In this regard, the addition of chitosan to the coating complex XG-alginate enhances L. plantarum LAB12 survival, providing a higher protection against the low pH and high temperatures [89,191]. Similarly, other studies using XG in combination with other encapsulant materials are: the XG-chitosan and XG-chitosan-XG complexes which improved survival of L. acidophilus in microcapsules added to dairy beverages [85] and the XG-gellan gum complex which improved cell survivor of Bifidobacterium lactis in microcapsules preserved in sodium phosphate buffer (pH 6.8) [86].
Gellan gum (GG) is an anionic bacterial polysaccharide with a lineal structure of tetrasaccharide repeating sequence that consists of two residues of β-D-glucose, one of β-D-glucuronate and one of α-L-rhamnose. The chemical structure of GG is naturally acylated, however, the deacylated form of GG is obtained by alkaline hydrolysis treatment. Variations in the content of acyl groups of GG confer distinctive properties, including their gelation behaviour. Currently, GG is available in two forms: high acyl (acylated) and low acyl (deacylated) commercially known as Gelrite and Kelcogel, respectively. Each GG type has individual properties. In the presence of gel-promoting cations, the high acyl GG forms soft and flexible hydrogels upon cooling at 65 °C, while the low acyl GG forms rigid and brittle hydrogels upon cooling at 40 °C [192]. The deacylated form of GG has been successfully used as a coating material for microencapsulation of probiotic. In addition to the XG-GG mixture cited before [86], the microencapsulation of L. casei using a sodium caseinate-GG mixture as coating agent, also enhanced the probiotic survival in simulated environments of gastric fluid and bile salts [64].
Gum Arabic or gum acacia (GA) is mainly composed of D-galactose, L-arabinose, L-rhamnose, D-glucuronic acid, and 4-O-methyl-D-glucuronic acid. The chemical structure of GA is quite complex, the backbone consists of 1-3-linked β-D-galactopyranosyl units with side chains of two to five (1–6) linked β-D-glucopyranosyl units, joined to the backbone by 1,6-linkages. Both main chain and side branches may contain α-L-rhamnopyranose, β-D-glucuronic acid, β-D-galactopyranose, and α-L-arabinofuranosyl units [193,194]. Remarkably, GA is covalently associated to a protein moiety that is rich in amino acid residues of hydroxyproline, proline, and serine. GA is highly soluble in water (up to 50% w/v) and also presents a relatively low viscosity in comparison to other exudate gums. These properties are attributed to its highly branched structure and its relatively low molecular weight. On the other hand, the protein moiety of GA provides the surface activity, foaming abilities, and emulsifying characteristics of this polysaccharide [178,195]. In this regard, combinations of gelatine-GA [67], whey protein isolate (WPI)-GA [58], and the individual mixture of seed, leaf, or pulp extracts of the miracle fruit (Synsepalum dulcificum) with GA [21], were used as coating materials for the microencapsulation of probiotics and these coatings successfully improved the survival of probiotic cells during processing, simulated gastrointestinal in vitro conditions, and upon storage, when compared to free cells.
Carboxymethyl cellulose (CMC) and carboxymethyl chitin (CMCH) are modified anionic polysaccharides, also called semi-synthetic anionic polysaccharides. CMC and CMCH are cellulose and chitin derivatives, respectively. Interestingly, cellulose and chitin are the first and the second most abundant natural polysaccharides on earth. The chemical structure of cellulose consists of a linear structure of β-1,4-linked D-glucose residues, while chitin is composed of β-1,4-linked units of the amino sugar N-acetyl-glucosamine [196,197,198]. CMC and CMCH are both broadly used in the food industry, including their use as coating material in the microencapsulation of probiotics. CMC is a water soluble derivative of cellulose. It is obtained by the reaction of cellulose with alkali and chloroacetic acid, resulting in the partial replacement of the hydroxyl groups of anhydrous glucose by carboxymethyl groups (–CH2-COOH) [199]. A study using CMC and chitosan as coating materials for the microencapsulation of L. acidophilus showed the ability of these materials to improve of probiotic viability during its simulated gastrointestinal transit [32]. In another study, blends of CMC and κ-carrageenan were used as coating materials for the microencapsulation of probiotic L. plantarum, and they showed suitability for the production of microcapsules for oral delivery of viable probiotics [84].
CMCH, also known as chitin liquid, is a water soluble anionic polysaccharide. CMCH is synthesised by replacing the hydroxyl groups of chitin with carboxymethyl groups [200]. CMCH has been widely used for different applications, such as drug delivery systems, antimicrobial, food, cosmetic, among others [197]. However, little has been done regarding its potential as a coating material for probiotic microencapsulation. In a recent study, sodium alginate and CMCH were used as a coating material for the microencapsulation of Bifidobacterium. When compared to free cells, microencapsulated probiotic showed an increased survival under simulated in vitro gastrointestinal conditions which represents an efficient mean to produce microcapsules as a probiotic delivery system [79].
5.2. Cationic Polysaccharides
The cationic polysaccharides are those that tend to be positive below their pKa value, while remaining neutral when well above their pKa value. Chitosan is the only naturally derived cationic polysaccharide [176]. Chitosan is mainly composed of (1,4)-linked 2-amino-2-deoxy-β-D-glucan, which is resulting from partial deacetylation of chitin. However, chitosan is also naturally occurring in insect exoskeletons and various bacterial parasites, but in too small quantities to be commercially exploited [175]. Despite the fact that chitosan has a broad antimicrobial spectrum it has been used in mix with other encapsulating agents for the microencapsulation of probiotics [201,202]. Chitosan in combination with materials like: alginate, starch, whey protein isolate, and xanthan gum; provided protection to several probiotic microorganisms under simulated in vitro gastrointestinal environments, which may be an effective way to deliver probiotic benefits to the consumer [77,78,80,81,85,90,91,92,94,98,153]. An outstanding example is the microencapsulation of Bacillus coagulants using alginate and chitosan as coating materials. Anselmo et al. demonstrated that these probiotic microcapsules enhance the survival of probiotics against simulated gastrointestinal conditions. They also demonstrated that the microencapsulated probiotics have a higher adherence to the mucosal surface of fresh porcine intestinal tissues and to the EpiIntestinalTM system (an isolated intestinal model that recreates physiological intestine structures), in comparison to free cells. Furthermore, they also used a mouse model to assess in vivo probiotic survival, where microencapsulated probiotic exhibited significant survival advantages when compared to free cells [31].
Other synthetic cationic polysaccharides were described previously, for example, those with cosmetic applications such as cationic hydroxyethylcellulose, cationic guar, and cationic hydroxypropylguar [175]. However, despite their potential and advantages as cationic materials, none of them has been reported yet as a coating material for probiotic microencapsulation.
5.3. Non-ionic Polysaccharides
Non-ionic polysaccharides are macromolecules that do not carry a formal charge. However, other neighbouring species and/or milieu conditions may influence their charge characteristics changing their regular solution behaviour. Natural, non-ionic polysaccharides such as starch, maltodextrins, cyclodextrins, and guar gum have been employed as coating materials for probiotic microencapsulation. Additionally, modified, non-ionic polysaccharides like cellulose ethers (e.g., hydroxypropyl methyl- and hydroxypropyl-cellulose) have also been used as coating materials [175,183].
Starch is a soft, white, tasteless powder. It is produced by plants and is mainly composed of two different polysaccharides of D-glucose: the linear and helical amylose and the highly branched amylopectin. Amylose consists of almost exclusively linear molecules with α-(1,4)-linked D-glucose units which render a helical structure, while amylopectin consists of a central chain of repeating α-(1,4)-linked D-glucose units, which is randomly decorated with side chains of α-(1,6)-linked D-glucose units [183]. The ratio of amylose/amylopectin contained in the starch varies depending on the source and defines its intrinsic characteristics. Starch of high amylose content is known to form strong and flexible films, probably due to the predominant amylose structure and its crystallization properties; a common example is the high amylose maize starch also known as resistant starch (RS) [203,204]. Starch films conveniently hold several properties that make it quite suitable as a coating material for probiotic microencapsulation. Starch films are: odourless, tasteless, colourless, non-toxic, and semipermeable to carbon dioxide, moisture, oxygen, as well as lipid and flavour components [203]. In this regard, a modified version of starch (octenyl-succinate starch) was assessed as a coating material for the microencapsulation of Bifidobacterium. Octenyl-succinate starch is a food additive also known as E1450, it was preferred due to its particular suitability for the spray drying method for microencapsulation. Such microencapsulation process failed to improve probiotic survival under acid conditions or when added to dry food preparations, in comparison to free cells. Despite that, the microencapsulation using the spray drying method and E1450 as coating material, was successfully optimized for the production of microcapsules containing viable Bifidobacterium probiotic cells [101]. Furthermore, starch was described as a potential prebiotic compound for the microencapsulation of diverse probiotics. In several studies, the microencapsulation of probiotics were conducted using RS mixed with other coating materials, such as alginate, chitosan, sodium caseinate, as well as hydrolysed or isolated whey protein (Table 1). In general, the addition of RS in the coating material provided an additional protection to the microcapsules against simulated gastrointestinal conditions and under different storage temperatures [61,66,81,82,91,103].
Maltodextrin is formally defined as “purified, nutritive mixtures of saccharide polymers obtained by partial hydrolysis of edible starch”. Maltodextrins can be produced from any starch and is chemically composed of D-glucose units connected in chains of variable length. The dextrose equivalent (DE) and the degree of polymerization (DP) are the two parameters that vary among maltodextrins and ultimately define their properties [205]. Conveniently, some of these properties are suitable for microencapsulation, such are: good solubility; film formation; moisture control; easy digestibility; easy spray-drying; capability to form gels, among others. In this regard, maltodextrin has been positively associated with others edible agents to improve drying properties of probiotic coatings during microencapsulation. For example, the microencapsulation by spray freeze drying of Lactobacillus paracasei using maltodextrin and trehalose as coating materials significantly improved both the probiotic survival and viability [25]. A recent study found similar results in the microencapsulation of L. acidophilus by spray drying using maltodextrin and soy extract as coating materials [22].
Cyclodextrins (CDs) are cyclic oligosaccharides produced by the cyclodextrin glycosyltransferase (CGTase), a bacterial enzyme that catalyses the starch degrading activity and the cyclization reaction of oligosaccharides. The CDs structure consist of α-(1,4)-linked glucose residues forming a closed circular molecule, usually containing six (α-CD), seven (β-CD), or eight (γ-CD) glucopyranose units [206]. Currently, β-CD and their derivatives are the most studied cyclic oligosaccharides. Many advantages have been reported regarding β-CD, such as: its safety and metabolism; the ability to remove cholesterol in many foods (e.g. eggs and dairy products); its consumption prevents the elevation of plasma cholesterol and triacylglycerols; among others [207]. Additionally, cross-linked β-CD microcapsules have been broadly used to provide controlled release of drugs [208,209]. However, only a few studies on β-CD as a coating material have been carried out for the microencapsulation of probiotics. Recently, the microencapsulation was described of Saccharomyces boulardii, L. acidophilus, and B. bifidum employing β-CD and gum Arabic as coating materials. In general, the microencapsulation of probiotics using β-CD enhanced survivability in simulated gastro-intestinal conditions and also provided heat resistance, when compared to free cells [24]. On the other hand, Farrez et al. [191] reported the use of β-CD together with alginate, xanthan gum, and chitosan as coating materials for the microencapsulation of L. plantarum LAB12. In addition to the improvements reported in [24], they also demonstrated that microcapsules containing probiotic and β-CD have a combined cholesterol-lowering capability.
Guar gum (GUG) is another natural, non-ionic polysaccharide also known as guaran. It is derived from the seeds of guar plant Cyanaposis tetragonolobus. GUG is chemically a galactomannan, which structure consists of a backbone of β-1,4-linked D- mannose units and with side chains of scattered single units of α-1,6-linked D-galactose. It has been largely described as a coating material for drug delivery; however it was recently described as a coating agent for probiotic microencapsulation. Ameeta et al. [87] described the microencapsulation of Lactobacillus acidophilus LA1 using fructo-oligosaccharide (FOS) or partially-hydrolysed GUG as co-encapsulating agents in alginate-starch microcapsules. The addition of FOS or GUG improved the survival of microencapsulated probiotic under simulated gastrointestinal environments and during heat processing. In another study, the microencapsulation of a mixed culture of three probiotic (L. acidophilus, L. rhamnosus, and B. longum) was conducted using GUG or XG as coating materials. Microcapsules were then successfully incorporated in cream biscuits with the aim to develop a functional probiotic food [210]. In this regard, microcapsules of Lactobacillus strains were prepared with GUG and alginate as coating agents for the supplementation of probiotics to milk chocolate drinks [35]. In addition to the improved probiotic viability during the product storage, both studies found that the incorporation of probiotic microcapsules did not affect the taste and flavour of the final products. On the other hand, a recent study also explored the use of GUG as a coating agent for the microencapsulation of probiotic yeast S. cerevisiae. In contrast to the previous studies, probiotic microcapsules were subsequently used for fish feed supplementation. Interestingly, the administration of microencapsulated probiotic was further shown to be beneficial to the fish host, as it improved rates of growth, feed conversion ratio, and stimulating the immune response [27].
In addition to carboxymethyl cellulose (CMC), other non-ionic cellulose ethers have also been able to participate as coating ingredients for probiotic microencapsulation. Common examples of non-ionic cellulose ethers are: methyl cellulose (MC); hydroxyethyl cellulose (HEC); hydroxypropyl cellulose (HPC); hydroxypropyl methyl cellulose (HPMC); and microcrystalline cellulose (MCC). Pop et al. (2015) described the use of alginate mixed with several non-ionic polysaccharides as co-encapsulating materials. The study was focused on investigating the microencapsulation of B. lactis 300B with the aim to obtain microcapsules with adequate physical/biochemical properties able to withstand probiotic viability and feasible to be scaled-up. Co-encapsulating agents used to develop the experimental coatings were hydroxypropyl methylcellulose (HPMC), sodium-carboxymethyl cellulose (Na-CMC), microcrystalline cellulose (MCC), two types of starch (BR-07 and BR-08), dextrin, and pullulan. They found that the probiotic survival is largely dependent on the encapsulating material mixture. Alginate-pullulan and alginate-HPMC were the two admixtures that provided high protection to the probiotic during the encapsulating process and after 15 days of storage [38]. On the other hand, the microencapsulation of B. lactis was also performed using non-ionic polysaccharides as coating materials via the layer-by-layer method. HPMC was used as the inner layer while the outer layer was based on a combination of HPC and poloxamer. Polaxamer is a non-ionic surfactant which consists of a poly(ethylene oxide)—poly(propylene oxide)—poly(ethyelene oxide) triblock copolymer (PEO-PPO-PEO). The coating mixture composed of HPMC/HPC/poloxamer is described as a smart polymer due to its ability to respond to changes in the milieu, such as the temperature. Its structure displays fast macroscopic changes depending on a lower critical solution temperature (LCST). Below the LCST the polymer is soluble, but as the temperature increases beyond the LCST the polymer turn into insoluble. The microencapsulated probiotic was added to powdered infant formula (PIF) and the probiotic survival was tested during reconstitution of PIF. The microcapsules were able to protect probiotic cells during the reconstitution of PIF at high temperature (70 °C) since coating may form an insoluble gel that protects entrapped cells. Additionally, the coating gel dissolves when cooling at 40 °C realising the probiotic cells [33].
5.4. Amphoteric Polysaccharides
Amphoteric polysaccharides are polymers that carry on both cationic and anionic charges on the same chain. They are normally synthesised using natural polysaccharides as building blocks. Some common examples are carboxymethyl chitosan (CMCS), N-[(2’-Hydroxy-2’,3´-dicarboxy)ethyl] chitosan, sulphated chitosan, and modified amphoteric starches, which have been used in the cosmetic industry [175]. CMCS is synthesized by replacing the amino and hydroxyl groups of chitosan with carboxymethyl groups. When compared to chitosan, CMCS possess valuable properties such as improved water solubility, moisture absorption, high viscosity, non-toxicity, biocompatibility, and good ability to form films and hydrogels [211,212]. Until now, only CMCS has been reported as a coating material for the microencapsulation of probiotics. The microencapsulation of Lactobacillus casei ATCC 393 was conducted in matrices of alginate, chitosan, and CMCS by the extrusion method. Microcapsules increased the survival of L. casei after cold-air-flow drying and under simulated gastrointestinal conditions [73].
6. Lipids
Lipids are a heterogeneous group of molecules, such as fats, fatty acid, waxes, and phospholipids, among others. Lipids can be used as edible coating materials as they work by primarily blocking moisture transport due to their relatively low polarity. However, their hydrophobic nature confers fragility to the formed coatings. For that reason, lipids need be blended with other coating agents such as proteins or polysaccharides, in order to improve their coating characteristics [213,214]. For example, polysaccharides or proteins confer selective permeability to gases (O2 or CO2) along with durability, structural cohesion, and integrity, whereas the addition of lipids to the mixture improves its water vapour resistance [121].
Most of the lipids employed in the microencapsulation of probiotics are fats. Depending on their source edible fats may be of animal or plant origin. For example, fats from animal source are fish oil, butter, or pork oil. On the other hand, examples of plant fats are sunflower, corn, or olive oils. Fats are typically found as mono-, di- or triglycerides, composed of fatty acids and glycerol. Thus, their properties largely depend on the fatty acid composition. Fatty acids are a group of molecules with a carboxylate hydrophilic head covalently linked to a hydrophobic tail with different numbers of carbon and hydrogen atoms that determine their molecular weight. Melting point is related to the molecular weight of the fatty acid, a greater molecular weight renders a higher melting point. Additionally, the presence of unsaturations in the hydrophobic chain also influences on the melting point; saturated fatty acids have higher melting points than unsaturated fatty acids. The melting point of fats is the main property used for microencapsulation, since thermal solidification is induced at temperatures below their melting point [213,214]. Vegetable fats have been extensively used as co-encapsulating materials in the microencapsulation of probiotics by emulsification [34,39,59,60,61,62,63,64,65,66,67,69,70,71,104,183] or by spry drying [24,103,104]. On the other hand, in a recent study, Silva et al. reported the microencapsulation of probiotics using vegetable oil as a sole coating material or covered with gelatine-gum Arabic. This type of microencapsulation protected probiotic cells under simulated gastrointestinal conditions and stress conditions (e.g., pH, temperature, sodium chloride, and sucrose), when compared to free cells [23]. Despite the reported success, the viability of the microencapsulated probiotic during storage still needs to be improved.
Waxes are lipid materials commonly used in the food industry. Some examples of naturally occurring waxes are beeswax, candelilla wax, and carnauba wax, whereas paraffin wax and oxidized polyethylene wax are examples of synthetic waxes. Natural waxes consist of a complex mixture of organic molecules like long alkyl chains, long-chain fatty acids, long-chain alcohols, ketones, aldehydes and fatty acid esters; they may also contain aromatic compounds. Beeswax is a common example of natural wax; it is secreted by honeybees and used for their comb construction. It mostly consists of higher monohydric alcohols (C24–C33), saturated hydrocarbons (C25–C33), and long-chain fatty acids C24–C34. Due to its composition, it has a melting point (MP) of 61−65 °C as well as a good solubility in other waxes and oils. Beeswax is a plastic material at room temperature, but at low temperatures, it turns into a brittle material. Candelilla wax is a secretion of the candelilla plant (Euphorbia cerifera, E. antisphylitica) with a MP of 65−68.8 °C. Its composition consists of mainly hydrocarbons (C29–C33), higher molecular weight esters, free fatty acids, and resins. Its degree of hardness is intermediate between beeswax and carnauba wax. Moreover, carnauba wax is an excretion from the leaves of the Tree of Life (Copernica cerifera) with a MP of 82.5−86 °C. Its composition consists of saturated fatty acid esters (C24–C32) and saturated long-chain alcohols. This wax has the highest melting point and specific gravity among the natural waxes. Due to this, it is used to increase melting point, stiffness, strength, and lustre of lipidic blends. On the other hand, two synthetic waxes commonly used in the food industry are paraffin wax and oxidised polyethylene wax. Both are petroleum-derived products that can be melted into liquid form and shaped into various forms like pellets, flakes or powder. Paraffin wax (MP ~50 °C) is composed of hydrocarbon fractions of generic formula CnH2n+2 ranging C18–C32. Oxidised polyethylene wax (MP 97−115 °C) is also known as food additive E914, it is defined as the polar reaction products from the mild oxidation of polyethylene [215,216].
The waxes described above are considered GRAS substances and have been largely used in the food industry, for example, as food additives or as protective coatings for fruits, vegetables and cheese. Despite that, waxes have been barely employed as coating materials for the microencapsulation of probiotics. For example, Mandal et al. [61] reported the use of beeswax, stearic acid or poly-L-lysine as external coating of probiotic microcapsules prepared with resistant starch and alginate. Microcapsules coated with beeswax and stearic acid showed an improved survival rate of the encapsulated probiotic cells of Lactobacillus casei under simulated gastrointestinal conditions. Particularly, the stearic acid coating gave the better protection and displayed a complete release of encapsulated probiotics in simulated colonic pH solution. In a similar study, Rao et al. [217] described the use of beeswax or stearic acid as external coating of probiotic microcapsules prepared with cellulose-acetate-phthalate (CAP). Conversely, they found that microcapsules coated with beeswax displayed the highest survival of Bifidobacterium pseudolongum after sequential incubation in simulated gastric juice followed by intestinal juice.
Phospholipids are a large class of lipids commonly used in the food industry, which have the ability to form emulsions, micelles and liposomes. These lipids contain phosphorus and play an important structural and metabolic role in living cells. Phospholipids are more complex than simple lipids (fats and waxes). Examples of phospholipids are phosphatidic acid (phosphatidate) (PA), phosphatidylethanolamine (cephalin) (PE), phosphatidylcholine (lecithin) (PC), and phosphatidylserine (PS). Their general structure is composed of a phosphate group esterified to a glycerol molecule which may have one or two esterified fatty acids. The phosphate group is also esterified to an alcohol which provides an electric charge and a hydrophilic character to the molecule. The fatty acid tails provide a neutral charge and a hydrophobic character. These characteristics give an amphipathic nature to the phospholipids which is essential for the formation of biological membranes [218]. In this regard, phospholipids are the primary components of liposomes; when phospholipids are dispersed in water the molecules aggregate forming a characteristic bilayer which is a consequence of the interaction between the hydrophobic fatty acid chains and the hydrophilic water environment. Such interactions promote the formation of closed, sealed vesicles also known as liposomes [217]. The liposome entrapment is another microencapsulation technique employed in the food industry [145,219]. Liposomes have been largely employed as delivery systems of bioactive compounds, e.g., drugs, vitamins, enzymes, etc. Even though liposomes showed great potential for encapsulation and controlled release of nutritional compounds, their application in foods has yet to be fully exploited [53,220]. For example, to date the microencapsulation of probiotics by liposome entrapment has not been reported, which may be due to the cost associated to the process and the materials, as well as for the large size of probiotic microorganisms [221]. Nevertheless, the microencapsulation of probiotics using the liposome entrapment technique represents an opportunity to test such technique, the potential raw materials and the behaviour of loaded liposomes in the gastrointestinal tract. The resistance of liposomes to gastric and intestinal juices as well as the probiotic delivery efficacy once in the intestinal environment are also topics that need to be investigated.
7. Conclusions
Among the many strategies used for the preservation of probiotics, microencapsulation has emerged as a promising approach to enhance the effectiveness of consuming probiotic microorganisms. Microencapsulation ensures a highest rate of survival of probiotic microorganisms from the production, storage and delivery into the gastrointestinal system of the consumer. The design of probiotic microcapsules must consider the interrelationship between the encapsulation process, the type of probiotic microorganism and the coating materials. Currently, a broad variety of edible materials based on bio-polymers are available as coating agents for the microencapsulation of probiotics. Coating materials based on polysaccharides are the most extensively explored. Alginate and starches are the polysaccharides most commonly used with virtually all the reported microencapsulation methods (Table 1). In contrast, proteins, lipids and semi-synthetic polysaccharides have not yet fully been exploited as coating agents for the encapsulation of probiotics, even though many of these are described as polymeric compounds and are currently in use for the microencapsulation of various compounds of importance in the food and pharmaceutical industry. The different forms of whey proteins are the most frequent protein-based material which has also been employed in almost all the reported encapsulation methods, except for the layer-by-layer and spray chilling methods. There are many protein-based materials that still can be exploited, such as vegetable proteins, egg albumin or gelatine, which are cheap and easy-available materials. On the other hand, little has been done regarding the use of lipids as coating materials for the microencapsulation of probiotics. Reports have been mainly limited to the use of vegetable fats as co-encapsulating agents employing the emulsification and spray drying methods. Due to their relative availability and permeability properties, waxes, mono- and di-acylglycerols, or phospholipids, are some of the promising lipid materials that still can be explored either as pure or composite coating materials.
In spite of the recent advances in this field, new approaches regarding the microencapsulation of probiotics represent a field of opportunities to be investigated. For example, the liposome entrapment is a microencapsulation method that has been widely used to encapsulate bioactive compounds, however, to date the microencapsulation of probiotics using such technique has not been reported, which represents a field of research opportunities to be investigated in the future. Nonetheless, the research towards new combinations of current coating materials and microencapsulation processes, or the development/discovery of new edible film-forming materials, are two important challenges that still need to be undertaken for further progress in the field of probiotic microencapsulation.
Finally, despite the fact that many techniques, coating materials, and probiotic microorganism have been investigated, few works have focused on in vivo assessment of the viability and biological properties for probiotic microencapsulated [91,140,156]. Hence, another important topic to be addressed is if the survival advantages of microencapsulated probiotics translate into animal or human models.
Supplementary Materials
The following are available online at https://www.mdpi.com/2079-6412/10/3/197/s1, Table S1: Different works reporting the microencapsulation of probiotics.
Author Contributions
R.L.S.-O. conceptualized and visualized this manuscript; R.L.S.-O. and A.d.l.C.P.-C. conceived and wrote the initial draft of this manuscript; A.G.-T., D.O., N.G.-S., and A.C.P.-C. collaborated finalizing the manuscript for submission. All authors collaborated collecting information and discussing the collected information. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding. The APC was funded by all authors.
Acknowledgments
The authors acknowledge the National Council of Science and Technology from Mexico (CONACyT). Angel de la Cruz Pech-Canul was supported by CONACyT through the “Cátedras CONACyT para Jóvenes Investigadores” Programme (Projects #609).
Conflicts of Interest
The authors declare no conflict of interest.
References
- FAO/WHO. Probiotics in Food. In Health and Nutritional Properties and Guidelines for Evaluation; FAO/WHO: Rome, Italy, 2006; p. 56. [Google Scholar]
- Dunne, C. Adaptation of bacteria to the intestinal niche: Probiotics and gut disorder. Inflamm. Bowel Dis. 2001, 7, 136–145. [Google Scholar] [CrossRef]
- Gu, Y.; Zhou, G.; Liu, X.; Wang, B.; Cao, H. Saccharomyces Boulardii, a Yeast Probiotic, Regulates Serotonin Transporter in the Intestine. Gastroenterology 2019, 156, 26. [Google Scholar] [CrossRef]
- Gu, Y.; Zhou, G.; Qin, X.; Huang, S.; Wang, B.; Cao, H. The Potential Role of Gut Mycobiome in Irritable Bowel Syndrome. Front. Microbiol. 2019, 10, 1894. [Google Scholar] [CrossRef]
- Cayzeele-Decherf, A.; Pélerin, F.; Leuillet, S.; Douillard, B.; Housez, B.; Cazaubiel, M.; Jacobson, G.K.; Jüsten, P.; Desreumaux, P. Saccharomyces cerevisiae CNCM I-3856 in irritable bowel syndrome: An individual subject meta-analysis. World J. Gastroenterol. 2017, 23, 336–344. [Google Scholar] [CrossRef] [PubMed]
- Ragavan, M.L.; Das, N. Optimization of exopolysaccharide production by probiotic yeast Lipomyces starkeyi VIT-MN03 using response surface methodology and its applications. Ann. Microbiol. 2019, 69, 515–530. [Google Scholar] [CrossRef]
- Sanders, M.E.; Benson, A.; Lebeer, S.; Merenstein, D.J.; Klaenhammer, T.R. Shared mechanisms among probiotic taxa: Implications for general probiotic claims. Curr. Opin. Biotechnol. 2018, 49, 207–216. [Google Scholar] [CrossRef] [PubMed]
- Jacouton, E.; Michel, M.-L.; Torres-Maravilla, E.; Chain, F.; Langella, P.; Humaran, L.G.B. Elucidating the Immune-Related Mechanisms by Which Probiotic Strain Lactobacillus casei BL23 Displays Anti-tumoral Properties. Front. Microbiol. 2019, 9, 9. [Google Scholar] [CrossRef]
- Lebeer, S.; A Bron, P.; Marco, M.L.; Van Pijkeren, J.-P.; Motherway, M.O.; Hill, C.; Pot, B.; Roos, S.; Klaenhammer, T. Identification of probiotic effector molecules: Present state and future perspectives. Curr. Opin. Biotechnol. 2018, 49, 217–223. [Google Scholar] [CrossRef]
- Corcoran, B.M.; Stanton, C.; Fitzgerald, G.F.; Ross, R.P. Survival of Probiotic Lactobacilli in Acidic Environments Is Enhanced in the Presence of Metabolizable Sugars. Appl. Environ. Microbiol. 2005, 71, 3060–3067. [Google Scholar] [CrossRef]
- Tripathi, M.; Giri, S. Probiotic functional foods: Survival of probiotics during processing and storage. J. Funct. Foods 2014, 9, 225–241. [Google Scholar] [CrossRef]
- McClements, D.J. Requirements for food ingredient and nutraceutical delivery systems. In Encapsulation Technologies and Delivery Systems for Food Ingredients and Nutraceuticals; Garti, N., McClements, D.J., Eds.; Woodhead Publishing: Sawston, UK, 2012; pp. 3–18. [Google Scholar] [CrossRef]
- Monnard, P.-A.; Oberholzer, T.; Luisi, P. Entrapment of nucleic acids in liposomes. Biochim. Biophys. Acta (BBA) Biomembr. 1997, 1329, 39–50. [Google Scholar] [CrossRef]
- Shori, A.B. Microencapsulation Improved Probiotics Survival during Gastric Transit. HAYATI J. Biosci. 2017, 24, 1–5. [Google Scholar] [CrossRef]
- Pavli, F.; Tassou, C.; Nychas, G.-J.E.; Chorianopoulos, N. Probiotic Incorporation in Edible Films and Coatings: Bioactive Solution for Functional Foods. Int. J. Mol. Sci. 2018, 19, 150. [Google Scholar] [CrossRef]
- Gharsallaoui, A.; Roudaut, G.; Chambin, O.; Voilley, A.; Saurel, R. Applications of spray-drying in microencapsulation of food ingredients: An overview. Food Res. Int. 2007, 40, 1107–1121. [Google Scholar] [CrossRef]
- Yang, W.; Wang, L.; Ban, Z.; Yan, J.; Lu, H.; Zhang, X.; Wu, Q.; Aghdam, M.S.; Luo, Z.; Li, L. Efficient microencapsulation of Syringa essential oil; the valuable potential on quality maintenance and storage behavior of peach. Food Hydrocoll. 2019, 95, 177–185. [Google Scholar] [CrossRef]
- Ayoub, A.; Sood, M.; Singh, J.; Bandral, J.; Gupta, N.; Bhat, A. Microencapsulation and its applications in food industry. J. Pharmacogn. Phytochem. 2019, 8, 32–37. [Google Scholar]
- Quirós-Sauceda, A.E.; Ayala-Zavala, J.; Olivas, G.; González-Aguilar, G.A. Edible coatings as encapsulating matrices for bioactive compounds: A review. J. Food Sci. Technol. 2014, 51, 1674–1685. [Google Scholar] [CrossRef]
- Janjarasskul, T.; Krochta, J. Edible Packaging Materials. Annu. Rev. Food Sci. Technol. 2010, 1, 415–448. [Google Scholar] [CrossRef] [PubMed]
- Fazilah, N.F.; Hamidon, N.H.; Bin Ariff, A.; Khayat, M.E.; Wasoh, H.; Halim, M. Microencapsulation of Lactococcus lactis Gh1 with Gum Arabic and Synsepalum dulcificum via Spray Drying for Potential Inclusion in Functional Yogurt. Molecules 2019, 24, 1422. [Google Scholar] [CrossRef] [PubMed]
- Menezes, L.A.A.; De Almeida, C.A.M.; Mattarugo, N.M.D.S.; Ferri, E.A.V.; Bittencourt, P.R.S.; Colla, E.; Drunkler, D.A. Soy extract and maltodextrin as microencapsulating agents for Lactobacillus acidophilus: A model approach. J. Microencapsul. 2018, 35, 705–719. [Google Scholar] [CrossRef]
- Silva, M.; Tulini, F.L.; Matos, F.E.; Oliveira, M.G.; Thomazini, M.; Favaro-Trindade, C.S. Application of spray chilling and electrostatic interaction to produce lipid microparticles loaded with probiotics as an alternative to improve resistance under stress conditions. Food Hydrocoll. 2018, 83, 109–117. [Google Scholar] [CrossRef]
- Arslan-Tontul, S.; Erbas, M. Single and double layered microencapsulation of probiotics by spray drying and spray chilling. LWT 2017, 81, 160–169. [Google Scholar] [CrossRef]
- Semyonov, D.; Ramon, O.; Kaplun, Z.; Levin-Brener, L.; Gurevich, N.; Shimoni, E. Microencapsulation of Lactobacillus paracasei by spray freeze drying. Food Res. Int. 2010, 43, 193–202. [Google Scholar] [CrossRef]
- Dolly, P.; Anishaparvin, A.; Joseph, G.S.; Anandharamakrishnan, C. Microencapsulation of Lactobacillus plantarum (mtcc 5422) by spray-freeze-drying method and evaluation of survival in simulated gastrointestinal conditions. J. Microencapsul. 2011, 28, 568–574. [Google Scholar] [CrossRef] [PubMed]
- Boonanuntanasarn, S.; Ditthab, K.; Jangprai, A.; Nakharuthai, C. Effects of Microencapsulated Saccharomyces cerevisiae on Growth, Hematological Indices, Blood Chemical, and Immune Parameters and Intestinal Morphology in Striped Catfish, Pangasianodon hypophthalmus. Probiotics Antimicrob. Proteins 2018, 11, 427–437. [Google Scholar] [CrossRef] [PubMed]
- Rajam, R.; Kumar, S.B.; Prabhasankar, P.; Anandharamakrishnan, C. Microencapsulation of Lactobacillus plantarum MTCC 5422 in fructooligosaccharide and whey protein wall systems and its impact on noodle quality. J. Food Sci. Technol. 2014, 52, 4029–4041. [Google Scholar] [CrossRef] [PubMed]
- Coghetto, C.C.; Brinques, G.B.; Siqueira, N.; Pletsch, J.; Soares, R.M.; Ayub, M.A.Z. Electrospraying microencapsulation of Lactobacillus plantarum enhances cell viability under refrigeration storage and simulated gastric and intestinal fluids. J. Funct. Foods 2016, 24, 316–326. [Google Scholar] [CrossRef]
- Gómez-Mascaraque, L.G.; Morfin, R.C.; Pérez-Masiá, R.; Sánchez, G.; López-Rubio, A. Optimization of electrospraying conditions for the microencapsulation of probiotics and evaluation of their resistance during storage and in-vitro digestion. LWT 2016, 69, 438–446. [Google Scholar] [CrossRef]
- Anselmo, A.C.; McHugh, K.J.; Webster, J.; Langer, R.; Jaklenec, A. Layer-by-Layer Encapsulation of Probiotics for Delivery to the Microbiome. Adv. Mater. 2016, 28, 9486–9490. [Google Scholar] [CrossRef]
- Priya, A.J.; Vijayalakshmi, S.P.; Raichur, A. Enhanced Survival of Probiotic Lactobacillus acidophilusby Encapsulation with Nanostructured Polyelectrolyte Layers through Layer-by-Layer Approach. J. Agric. Food Chem. 2011, 59, 11838–11845. [Google Scholar] [CrossRef]
- Penhasi, A. Microencapsulation of probiotic bacteria using thermo-sensitive sol-gel polymers for powdered infant formula. J. Microencapsul. 2015, 32, 372–380. [Google Scholar] [CrossRef] [PubMed]
- Pitigraisorn, P.; Srichaisupakit, K.; Wongpadungkiat, N.; Wongsasulak, S. Encapsulation of Lactobacillus acidophilus in moist-heat-resistant multilayered microcapsules. J. Food Eng. 2017, 192, 11–18. [Google Scholar] [CrossRef]
- Deshpande, H.; Kharat, V.; Katke, S. Studies on Process Standardization and Sensory Evaluation of Probiotic Chocolate. Int. J. Curr. Microbiol. Appl. Sci. 2019, 8, 1527–1534. [Google Scholar] [CrossRef]
- Lee, S.; Kirkland, R.; Grunewald, Z.I.; Sun, Q.; Wicker, L.; De La Serre, C.B. Beneficial Effects of Non-Encapsulated or Encapsulated Probiotic Supplementation on Microbiota Composition, Intestinal Barrier Functions, Inflammatory Profiles, and Glucose Tolerance in High Fat Fed Rats. Nutrients 2019, 11, 1975. [Google Scholar] [CrossRef] [PubMed]
- De Prisco, A.; Maresca, D.; Ongeng, D.; Mauriello, G. Microencapsulation by vibrating technology of the probiotic strain Lactobacillus reuteri DSM 17938 to enhance its survival in foods and in gastrointestinal environment. LWT 2015, 61, 452–462. [Google Scholar] [CrossRef]
- Pop, O.; Brandau, T.; Schwinn, J.; Vodnar, D.C.; Socaciu, C. The influence of different polymers on viability of Bifidobacterium lactis 300b during encapsulation, freeze-drying and storage. J. Food Sci. Technol. 2014, 52, 4146–4155. [Google Scholar] [CrossRef]
- Setijawati, D.; Nursyam, H.; Salis, H. Carrageenan: The difference between PNG and KCL gel precipitation method as Lactobacillus acidophilus encapsulation material. IOP Conf. Ser. Earth Environ. Sci. 2018, 137, 12073. [Google Scholar] [CrossRef]
- Ding, W.; Shah, N. Effect of Various Encapsulating Materials on the Stability of Probiotic Bacteria. J. Food Sci. 2009, 74, M100–M107. [Google Scholar] [CrossRef]
- Bosnea, L.; Moschakis, T.; Nigam, P.; Biliaderis, C. Growth adaptation of probiotics in biopolymer-based coacervate structures to enhance cell viability. LWT 2017, 77, 282–289. [Google Scholar] [CrossRef]
- Da Silva, T.M.; Barin, J.S.; Lopes, E.J.; Cichoski, A.J.; Flores, E.M.D.M.; Silva, C.D.B.D.; De Menezes, C.R. Development, characterization and viability study of probiotic microcapsules produced by complex coacervation followed by freeze-drying. Ciência Rural 2019, 49, 49. [Google Scholar] [CrossRef]
- Fang, Z.; Bhandari, B. Spray drying, freeze drying and related processes for food ingredient and nutraceutical encapsulation. In Encapsulation Technologies and Delivery Systems for Food Ingredients and Nutraceuticals; Elsevier BV: Amsterdam, The Netherlands, 2012; pp. 73–109. [Google Scholar]
- Oxley, J. Spray cooling and spray chilling for food ingredient and nutraceutical encapsulation. In Encapsulation Technologies and Delivery Systems for Food Ingredients and Nutraceuticals; Elsevier BV: Amsterdam, The Netherlands, 2012; pp. 110–130. [Google Scholar]
- Ali, M.E.; Lamprecht, A. Spray freeze drying as an alternative technique for lyophilization of polymeric and lipid-based nanoparticles. Int. J. Pharm. 2017, 516, 170–177. [Google Scholar] [CrossRef] [PubMed]
- Mortazavian, A.; Razavi, S.H.; Ehsani, M.R.; Sohrabvandi, S. Principles and methods of microencapsulation of probiotic microorganisms. Iran. J. Biotechnol. 2007, 5, 1–18. [Google Scholar]
- Whelehan, M.; Marison, I.W. Microencapsulation using vibrating technology. J. Microencapsul. 2011, 28, 669–688. [Google Scholar] [CrossRef] [PubMed]
- Rayleigh, L. On the capillary phenomena of jets. Proc. R. Soc. Lond. 1879, 29, 71–97. [Google Scholar]
- Olivares, A.; Silva, P.; Altamirano, C. Microencapsulation of probiotics by efficient vibration technology. J. Microencapsul. 2017, 34, 667–674. [Google Scholar] [CrossRef] [PubMed]
- Mazzitelli, S.; Tosi, A.; Balestra, C.; Nastruzzi, C.; Luca, G.; Mancuso, F.; Calafiore, R.; Calvitti, M. Production and Characterization of Alginate Microcapsules Produced by a Vibrational Encapsulation Device. J. Biomater. Appl. 2008, 23, 123–145. [Google Scholar] [CrossRef]
- Shi, L.-E.; Li, Z.-H.; Li, D.-T.; Xu, M.; Chen, H.-Y.; Zhang, Z.; Tang, Z.-X. Encapsulation of probiotic Lactobacillus bulgaricus in alginate–milk microspheres and evaluation of the survival in simulated gastrointestinal conditions. J. Food Eng. 2013, 117, 99–104. [Google Scholar] [CrossRef]
- Krasaekoopt, W.; Bhandari, B.; Deeth, H. Evaluation of encapsulation techniques of probiotics for yoghurt. Int. Dairy J. 2003, 13, 3–13. [Google Scholar] [CrossRef]
- Garti, N.; Aserin, A. Micelles and microemulsions as food ingredient and nutraceutical delivery systems. In Encapsulation Technologies and Delivery Systems for Food Ingredients and Nutraceuticals; Elsevier BV: Amsterdam, The Netherlands, 2012; pp. 211–251. [Google Scholar]
- Singh, M.; Hemant, K.; Ram, M.; Shivakumar, H. Microencapsulation: A promising technique for controlled drug delivery. Res. Pharm. Sci. 2010, 5, 65–77. [Google Scholar]
- Jaworek, A. Electrohydrodynamic microencapsulation technology. In Encapsulations; Elsevier BV: Amsterdam, The Netherlands, 2016; pp. 1–45. [Google Scholar]
- Yan, J.; Luo, Z.; Ban, Z.; Lu, H.; Li, D.; Yang, D.; Aghdam, M.S.; Li, L. The effect of the layer-by-layer (LBL) edible coating on strawberry quality and metabolites during storage. Postharvest Boil. Technol. 2019, 147, 29–38. [Google Scholar] [CrossRef]
- Liu, T.; Wang, Y.; Zhong, W.; Li, B.; Mequanint, K.; Luo, G.; Xing, M. Biomedical Applications of Layer-by-Layer Self-Assembly for Cell Encapsulation: Current Status and Future Perspectives. Adv. Heal. Mater. 2018, 8, 1800939. [Google Scholar] [CrossRef] [PubMed]
- Bosnea, L.A.; Moschakis, T.; Biliaderis, C.G. Complex Coacervation as a Novel Microencapsulation Technique to Improve Viability of Probiotics under Different Stresses. Food Bioprocess Technol. 2014, 7, 2767–2781. [Google Scholar] [CrossRef]
- Mokhtari, S.; Jafari, S.M.; Khomeiri, M.; Maghsoudlou, Y.; Ghorbani, M. The cell wall compound of Saccharomyces cerevisiae as a novel wall material for encapsulation of probiotics. Food Res. Int. 2017, 96, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Holkem, A.; Raddatz, G.C.; Barin, J.S.; Flores, E.M.M.; Müller, E.; Codevilla, C.F.; Jacob-Lopes, E.; Grosso, C.R.F.; De Menezes, C.R. Production of microcapsules containing Bifidobacterium BB-12 by emulsification/internal gelation. LWT 2017, 76, 216–221. [Google Scholar] [CrossRef]
- Mandal, S.; Hati, S.; Puniya, A.K.; Khamrui, K.; Singh, K. Enhancement of survival of alginate-encapsulated Lactobacillus casei NCDC 298. J. Sci. Food Agric. 2014, 94, 1994–2001. [Google Scholar] [CrossRef]
- Mandal, S.; Puniya, A.; Singh, K. Effect of alginate concentrations on survival of microencapsulated Lactobacillus casei NCDC-298. Int. Dairy J. 2006, 16, 1190–1195. [Google Scholar] [CrossRef]
- Gaudreau, H.; Champagne, C.P.; Remondetto, G.E.; Gomaa, A.; Subirade, M. Co-encapsulation of Lactobacillus helveticus cells and green tea extract: Influence on cell survival in simulated gastrointestinal conditions. J. Funct. Foods 2016, 26, 451–459. [Google Scholar] [CrossRef]
- Nag, A.; Han, K.-S.; Singh, H. Microencapsulation of probiotic bacteria using pH-induced gelation of sodium caseinate and gellan gum. Int. Dairy J. 2011, 21, 247–253. [Google Scholar] [CrossRef]
- Heidebach, T.; Foerst, P.; Kulozik, U. Transglutaminase-induced caseinate gelation for the microencapsulation of probiotic cells. Int. Dairy J. 2009, 19, 77–84. [Google Scholar] [CrossRef]
- Heidebach, T.; Foerst, P.; Kulozik, U. Influence of casein-based microencapsulation on freeze-drying and storage of probiotic cells. J. Food Eng. 2010, 98, 309–316. [Google Scholar] [CrossRef]
- Paula, D.D.A.; Martins, E.M.F.; Costa, N.D.A.; De Oliveira, P.M.; De Oliveira, E.B.; Ramos, A.M. Use of gelatin and gum arabic for microencapsulation of probiotic cells from Lactobacillus plantarum by a dual process combining double emulsification followed by complex coacervation. Int. J. Boil. Macromol. 2019, 133, 722–731. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, M.C.E.; Chaves, K.S.; Gebara, C.; Infante, F.N.; Grosso, C.R.; Gigante, M.L. Effect of microencapsulation of Lactobacillus acidophilus LA-5 on physicochemical, sensory and microbiological characteristics of stirred probiotic yoghurt. Food Res. Int. 2014, 66, 424–431. [Google Scholar] [CrossRef]
- Zer, B.; Uzun, Y.S.; Kirmaci, H.A.; Ozer, B. Effect of Microencapsulation on Viability of Lactobacillus acidophilus LA-5 and Bifidobacterium bifidum BB-12 During Kasar Cheese Ripening. Int. J. Dairy Technol. 2008, 61, 237–244. [Google Scholar] [CrossRef]
- Ozer, B.; Kirmaci, H.A.; Şenel, E.; Atamer, M.; Hayaloglu, A. Improving the viability of Bifidobacterium bifidum BB-12 and Lactobacillus acidophilus LA-5 in white-brined cheese by microencapsulation. Int. Dairy J. 2009, 19, 22–29. [Google Scholar] [CrossRef]
- Valero-Cases, E.; Frutos, M.J.; Fernandez, M.J.F. Effect of different types of encapsulation on the survival of Lactobacillus plantarum during storage with inulin and in vitro digestion. LWT 2015, 64, 824–828. [Google Scholar] [CrossRef]
- Samedi, L.; Charles, A.L. Viability of 4 Probiotic Bacteria Microencapsulated with Arrowroot Starch in the Simulated Gastrointestinal Tract (GIT) and Yoghurt. Foods 2019, 8, 175. [Google Scholar] [CrossRef] [PubMed]
- Li, X.Y.; Chen, X.; Sun, Z.W.; Park, H.J.; Cha, D.-S. Preparation of alginate/chitosan/carboxymethyl chitosan complex microcapsules and application in Lactobacillus casei ATCC 393. Carbohydr. Polym. 2011, 83, 1479–1485. [Google Scholar] [CrossRef]
- Albertini, B.; Vitali, B.; Passerini, N.; Cruciani, F.; Di Sabatino, M.; Rodriguez, L.; Brigidi, P. Development of microparticulate systems for intestinal delivery of Lactobacillus acidophilus and Bifidobacterium lactis. Eur. J. Pharm. Sci. 2010, 40, 359–366. [Google Scholar] [CrossRef]
- Krasaekoopt, W.; Bhandari, B.; Deeth, H. The influence of coating materials on some properties of alginate beads and survivability of microencapsulated probiotic bacteria. Int. Dairy J. 2004, 14, 737–743. [Google Scholar] [CrossRef]
- Qaziyani, S.D.; Pourfarzad, A.; Gheibi, S.; Nasiraie, L.R. Effect of encapsulation and wall material on the probiotic survival and physicochemical properties of synbiotic chewing gum: Study with univariate and multivariate analyses. Heliyon 2019, 5, e02144. [Google Scholar] [CrossRef]
- Trabelsi, I.; Bejar, W.; Ayadi, D.; Chouayekh, H.; Kammoun, R.; Bejar, S.; Ben Salah, R. Encapsulation in alginate and alginate coated-chitosan improved the survival of newly probiotic in oxgall and gastric juice. Int. J. Boil. Macromol. 2013, 61, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Nualkaekul, S.; Lenton, D.; Cook, M.; Khutoryanskiy, V.V.; Charalampopoulos, D. Chitosan coated alginate beads for the survival of microencapsulated Lactobacillus plantarum in pomegranate juice. Carbohydr. Polym. 2012, 90, 1281–1287. [Google Scholar] [CrossRef] [PubMed]
- Ying-Zi, J.; Huan-Xin, W.; Peng, H.; Xiao-Yan, C. The Application and Immobilization of Bifidobacterium with Carboxymethyl Chitin and Calcium Alginate (Original article in Chinese). Sci. Technol. Eng. 2018, 18, 37–43. [Google Scholar]
- Iyer, C.; Phillips, M.; Kailasapathy, K. Release studies of Lactobacillus casei strain Shirota from chitosan-coated alginate-starch microcapsules in ex vivo porcine gastrointestinal contents. Lett. Appl. Microbiol. 2005, 41, 493–497. [Google Scholar] [CrossRef] [PubMed]
- And, C.I.; Kailasapathy, K. Effect of Co-encapsulation of Probiotics with Prebiotics on Increasing the Viability of Encapsulated Bacteria under In Vitro Acidic and Bile Salt Conditions and in Yogurt. J. Food Sci. 2005, 70, M18–M23. [Google Scholar] [CrossRef]
- Mirzaei, H.; Pourjafar, H.; Homayouni, A. Effect of calcium alginate and resistant starch microencapsulation on the survival rate of Lactobacillus acidophilus La5 and sensory properties in Iranian white brined cheese. Food Chem. 2012, 132, 1966–1970. [Google Scholar] [CrossRef]
- Praepanitchai, O.-A.; Noomhorm, A.; Anal, A.K. Survival and Behavior of Encapsulated Probiotics (Lactobacillus plantarum) in Calcium-Alginate-Soy Protein Isolate-Based Hydrogel Beads in Different Processing Conditions (pH and Temperature) and in Pasteurized Mango Juice. BioMed Res. Int. 2019, 2019, 9768152–9768158. [Google Scholar] [CrossRef]
- Dafe, A.; Etemadi, H.; Zarredar, H.; Mahdavinia, G.R. Development of novel carboxymethyl cellulose/k-carrageenan blends as an enteric delivery vehicle for probiotic bacteria. Int. J. Boil. Macromol. 2017, 97, 299–307. [Google Scholar] [CrossRef]
- Shu, G.; He, Y.; Chen, L.; Song, Y.; Cao, J.; Chen, H. Effect of Xanthan⁻Chitosan Microencapsulation on the Survival of Lactobacillus acidophilus in Simulated Gastrointestinal Fluid and Dairy Beverage. Polymers 2018, 10, 588. [Google Scholar] [CrossRef]
- McMaster, L.D.; Kokott, S.A. Micro-encapsulation of Bifidobacterium lactis for incorporation into soft foods. World J. Microbiol. Biotechnol. 2005, 21, 723–728. [Google Scholar] [CrossRef]
- Ameeta, S.; Thompkinson, D.K.; Kumar, M.H.; Latha, S. Prebiotics in the microencapsulating matrix enhance the viability of probiotic Lactobacillus acidophilus LA1. Int. J. Fermented Foods 2013, 2, 12. [Google Scholar]
- Gbassi, G.; Vandamme, T.; Ennahar, S.; Marchioni, E. Microencapsulation of Lactobacillus plantarum spp in an alginate matrix coated with whey proteins☆. Int. J. Food Microbiol. 2009, 129, 103–105. [Google Scholar] [CrossRef] [PubMed]
- Fareez, I.M.; Lim, S.M.; Mishra, R.K.; Ramasamy, K. Chitosan coated alginate–xanthan gum bead enhanced pH and thermotolerance of Lactobacillus plantarum LAB12. Int. J. Boil. Macromol. 2015, 72, 1419–1428. [Google Scholar] [CrossRef] [PubMed]
- Chavarri, M.; Maranon, I.; Ares, R.; Ibanez, F.C.; Marzo, F.; Villarán, M.D.C. Microencapsulation of a probiotic and prebiotic in alginate-chitosan capsules improves survival in simulated gastro-intestinal conditions. Int. J. Food Microbiol. 2010, 142, 185–189. [Google Scholar] [CrossRef]
- Etchepare, M.D.A.; Raddatz, G.C.; Flores, E.M.M.; Zepka, L.Q.; Jacob-Lopes, E.; Barin, J.S.; Grosso, C.R.F.; De Menezes, C.R. Effect of resistant starch and chitosan on survival of Lactobacillus acidophilus microencapsulated with sodium alginate. LWT 2016, 65, 511–517. [Google Scholar] [CrossRef]
- Ragavan, M.L.; Das, N. Process optimization for microencapsulation of probiotic yeasts. Front. Boil. 2018, 13, 197–207. [Google Scholar] [CrossRef]
- Falco, C.Y.; Amadei, F.; Dhayal, S.K.; Cárdenas, M.; Tanaka, M.; Risbo, J. Hybrid coating of alginate microbeads based on protein-biopolymer multilayers for encapsulation of probiotics. Biotechnol. Prog. 2019, 35, e2806. [Google Scholar] [CrossRef]
- Yeung, T.W.; Üçok, E.F.; Tiani, K.A.; McClements, D.J.; Sela, D.A. Microencapsulation in Alginate and Chitosan Microgels to Enhance Viability of Bifidobacterium longum for Oral Delivery. Front. Microbiol. 2016, 7, 1946. [Google Scholar] [CrossRef]
- Hébrard, G.; Hoffart, V.; Beyssac, E.; Cardot, J.-M.; Alric, M.; Subirade, M. Coated whey protein/alginate microparticles as oral controlled delivery systems for probiotic yeast. J. Microencapsul. 2010, 27, 292–302. [Google Scholar] [CrossRef]
- Doherty, S.; Gee, V.; Ross, R.P.; Stanton, C.; Fitzgerald, G.; Brodkorb, A. Development and characterisation of whey protein micro-beads as potential matrices for probiotic protection. Food Hydrocoll. 2011, 25, 1604–1617. [Google Scholar] [CrossRef]
- Rajam, R.; Karthik, P.; Parthasarathi, S.; Joseph, G.; Anandharamakrishnan, C. Effect of whey protein – alginate wall systems on survival of microencapsulated Lactobacillus plantarum in simulated gastrointestinal conditions. J. Funct. Foods 2012, 4, 891–898. [Google Scholar] [CrossRef]
- Malmo, C.; La Storia, A.; Mauriello, G. Microencapsulation of Lactobacillus reuteri DSM 17938 Cells Coated in Alginate Beads with Chitosan by Spray Drying to Use as a Probiotic Cell in a Chocolate Soufflé. Food Bioprocess Technol. 2011, 6, 795–805. [Google Scholar] [CrossRef]
- Favaro-Trindade, C.S.; Grosso, C.R.F. Microencapsulation of L. acidophilus (La-05) and B. lactis (Bb-12) and evaluation of their survival at the pH values of the stomach and in bile. J. Microencapsul. 2002, 19, 485–494. [Google Scholar] [CrossRef] [PubMed]
- Flores-Belmont, I.A.; Palou, E.; López-Malo, A.; Jiménez-Munguía, M.-T. Simple and double microencapsulation of Lactobacillus acidophilus with chitosan using spray drying. Int. J. Food Stud. 2015, 4, 188–200. [Google Scholar] [CrossRef]
- O’Riordan, K.; Andrews, D.; Buckle, K.; Conway, P. Evaluation of microencapsulation of a Bifidobacterium strain with starch as an approach to prolonging viability during storage. J. Appl. Microbiol. 2001, 91, 1059–1066. [Google Scholar] [CrossRef]
- Pinto, S.; Verruck, S.; Vieira, C.R.; Prudencio, E.S.; Amante, E.R.; Amboni, R.D.D.M.C. Influence of microencapsulation with sweet whey and prebiotics on the survival of Bifidobacterium-BB-12 under simulated gastrointestinal conditions and heat treatments. LWT 2015, 64, 1004–1009. [Google Scholar] [CrossRef]
- Ying, D.; Sanguansri, L.; Weerakkody, R.; Bull, M.; Singh, T.; Augustin, M.A. Effect of encapsulant matrix on stability of microencapsulated probiotics. J. Funct. Foods 2016, 25, 447–458. [Google Scholar] [CrossRef]
- Zou, Q.; Liu, X.; Zhao, J.; Tian, F.; Zhang, H.; Zhang, H.; Chen, W. Microencapsulation of Bifidobacterium bifidum F-35 in Whey Protein-Based Microcapsules by Transglutaminase-Induced Gelation. J. Food Sci. 2012, 77, 270. [Google Scholar] [CrossRef]
- Rajam, R.; Anandharamakrishnan, C. Spray freeze drying method for microencapsulation of Lactobacillus plantarum. J. Food Eng. 2015, 166, 95–103. [Google Scholar] [CrossRef]
- Ömür, Ş.; Ocak, B. Development and Characterization of Collagen Hydrolysate Films Incorporated with Melaleuca Alternifolia (Tea Tree) Essential Oil. In Proceedings of the International Agriculture, Environment and Health Congress, Aydin, Turkey, 26–28 October 2018. [Google Scholar]
- Park, H.J.; Chinnan, M.S. Gas and water vapor barrier properties of edible films from protein and cellulosic materials. J. Food Eng. 1995, 25, 497–507. [Google Scholar] [CrossRef]
- Ryu, S.; Rhim, J.; Roh, H.; Kim, S. Preparation and Physical Properties of Zein-Coated High-Amylose Corn Starch Film. LWT 2002, 35, 680–686. [Google Scholar] [CrossRef]
- Handa, A.; Gennadios, A.; Hanna, M.; Weller, C.; Kuroda, N. Physical and Molecular Properties of Egg-white Lipid Films. J. Food Sci. 1999, 64, 860–864. [Google Scholar] [CrossRef]
- Ket-On, A.; Pongmongkol, N.; Somwangthanaroj, A.; Janjarasskul, T.; Tananuwong, K. Properties and storage stability of whey protein edible film with spice powders. J. Food Sci. Technol. 2016, 53, 2933–2942. [Google Scholar] [CrossRef] [PubMed]
- Sothornvit, R.; Krochta, J. Water Vapor Permeability and Solubility of Films from Hydrolyzed Whey Protein. J. Food Sci. 2000, 65, 700–703. [Google Scholar] [CrossRef]
- Avena-Bustillos, R.; Olsen, C.; Olson, D.; Chiou, B.; Yee, E.; Bechtel, P.; McHugh, T. Water Vapor Permeability of Mammalian and Fish Gelatin Films. J. Food Sci. 2006, 71, 202. [Google Scholar] [CrossRef]
- Avena-Bustillos, R.; Krochta, J. Water Vapor Permeability of Caseinate-Based Edible Films as Affected by pH, Calcium Crosslinking and Lipid Content. J. Food Sci. 1993, 58, 904–907. [Google Scholar] [CrossRef]
- Khwaldia, K. Water Vapor Barrier and Mechanical Properties of Paper-Sodium Caseinate and Paper-Sodium Caseinate-Paraffin Wax Films. J. Food Biochem. 2010, 34, 998–1013. [Google Scholar] [CrossRef]
- Banerjee, R.; Chen, H. Functional Properties of Edible Films Using Whey Protein Concentrate. J. Dairy Sci. 1995, 78, 1673–1683. [Google Scholar] [CrossRef]
- Gennadios, A.; Brandenburg, A.H.; Park, J.W.; Weller, C.L.; Testin, R.F. Water vapor permeability of wheat gluten and soy protein isolate films. Ind. Crop. Prod. 1994, 2, 189–195. [Google Scholar] [CrossRef]
- Cho, S.Y.; Rhee, C. Mechanical properties and water vapor permeability of edible films made from fractionated soy proteins with ultrafiltration. LWT 2004, 37, 833–839. [Google Scholar] [CrossRef]
- Cho, S.Y.; Park, J.-W.; Batt, H.P.; Thomas, R.L. Edible films made from membrane processed soy protein concentrates. LWT 2007, 40, 418–423. [Google Scholar] [CrossRef]
- Anker, M.; Berntsen, J.; Hermansson, A.-M.; Stading, M. Improved water vapor barrier of whey protein films by addition of an acetylated monoglyceride. Innov. Food Sci. Emerg. Technol. 2002, 3, 81–92. [Google Scholar] [CrossRef]
- Mauer, L.; Smith, D.; Labuza, T. Water vapor permeability, mechanical, and structural properties of edible β-casein films. Int. Dairy J. 2000, 10, 353–358. [Google Scholar] [CrossRef]
- Vargas, M.; Pastor, C.; Chiralt, A.; McClements, D.J.; González-Martínez, C. Recent Advances in Edible Coatings for Fresh and Minimally Processed Fruits. Crit. Rev. Food Sci. Nutr. 2008, 48, 496–511. [Google Scholar] [CrossRef]
- Chakravartula, S.S.N.; Soccio, M.; Lotti, N.; Balestra, F.; Rosa, M.D.; Siracusa, V. Characterization of Composite Edible Films Based on Pectin/Alginate/Whey Protein Concentrate. Mater. 2019, 12, 2454. [Google Scholar] [CrossRef]
- Rankin, J.; Wolff, I.; Davis, H.; Rist, C. Permeability of Amylose Film to Moisture Vapor, Selected Organic Vapors, and the Common Gases. Ind. Eng. Chem. Chem. Eng. Data Ser. 1958, 3, 120–123. [Google Scholar] [CrossRef]
- Nogueira, G.; Fakhouri, F.; Velasco, J.I.; De Oliveira, R.A. Active Edible Films Based on Arrowroot Starch with Microparticles of Blackberry Pulp Obtained by Freeze-Drying for Food Packaging. Polymers 2019, 11, 1382. [Google Scholar] [CrossRef]
- Razzaq, H.A.; Pezzuto, M.; Santagata, G.; Silvestre, C.; Cimmino, S.; Larsen, N.; Duraccio, D. Barley β-glucan-protein based bioplastic film with enhanced physicochemical properties for packaging. Food Hydrocoll. 2016, 58, 276–283. [Google Scholar] [CrossRef]
- Dashipour, A.; Razavilar, V.; Hosseini, H.; Alibadi, S.S.; German, J.B.; Ghanati, K.; Khakpour, M.; Khaksar, R. Antioxidant and antimicrobial carboxymethyl cellulose films containing Zataria multiflora essential oil. Int. J. Boil. Macromol. 2015, 72, 606–613. [Google Scholar] [CrossRef]
- Wu, J.; Zhong, F.; Li, Y.; Shoemaker, C.; Xia, W. Preparation and characterization of pullulan–chitosan and pullulan–carboxymethyl chitosan blended films. Food Hydrocoll. 2013, 30, 82–91. [Google Scholar] [CrossRef]
- Fakhouri, F.; Tanada-Palmu, P.S.; Grosso, C.R. Characterization of composite biofilms of wheat gluten and cellulose acetate phthalate. Braz. J. Chem. Eng. 2004, 21, 261–264. [Google Scholar] [CrossRef]
- Plotto, A. Coatings for Fresh Fruits and Vegetables; Informa UK Limited: London, UK, 2011; pp. 185–242. [Google Scholar]
- Yang, L.; Paulson, A. Mechanical and water vapour barrier properties of edible gellan films. Food Res. Int. 2000, 33, 563–570. [Google Scholar] [CrossRef]
- Bifani, V.; Ramírez, C.; Ihl, M.; Rubilar, M.; García, A.; Zaritzky, N. Effects of murta (Ugni molinae Turcz) extract on gas and water vapor permeability of carboxymethylcellulose-based edible films. LWT 2007, 40, 1473–1481. [Google Scholar] [CrossRef]
- Liu, F.; Chang, W.; Chen, M.; Xu, F.; Ma, J.; Zhong, F. Film-forming properties of guar gum, tara gum and locust bean gum. Food Hydrocoll. 2020, 98, 105007. [Google Scholar] [CrossRef]
- Bertuzzi, M.; Vidaurre, E.C.; Armada, M.; Gottifredi, J. Water vapor permeability of edible starch based films. J. Food Eng. 2007, 80, 972–978. [Google Scholar] [CrossRef]
- Park, H.; Weller, C.; Vergano, P.; Testin, R. Permeability and Mechanical Properties of Cellulose-Based Edible Films. J. Food Sci. 1993, 58, 1361–1364. [Google Scholar] [CrossRef]
- Hagenmaier, R.D.; Shaw, P.E. Moisture permeability of edible films made with fatty acid and hydroxypropyl methyl cellulose. J. Agric. Food Chem. 1990, 38, 1799–1803. [Google Scholar] [CrossRef]
- Grandtner, G.; Cavrot, J.-P.; Bandur, G.; Rusnac, L.; Krausz, P.; Granet, R.; Joly, N.; Martin, P. Synthesis of fructooligosaccharide-based plastic films starting from inulin. e-Polymers 2005, 5, 1–7. [Google Scholar] [CrossRef]
- Hanlon, J.F. Institute of Packaging Professionals. Handbook of Package Engineering, 2nd ed.; Technomic Pub. Co.: Lancaster, PA, USA, 1992. [Google Scholar]
- Harnkarnsujarit, N.; Li, Y. Structure-property modification of microcrystalline cellulose film using agar and propylene glycol alginate. J. Appl. Polym. Sci. 2017, 134, 45533. [Google Scholar] [CrossRef]
- Zarabadi, M.H.; Kadivar, M.; Keramat, J. Production and evaluation the properties of laminated oat protein film and electrospun nylon. J. Food Process. Preserv. 2017, 42, e13513. [Google Scholar] [CrossRef]
- Sandhu, K.S.; Sharma, L.; Kaur, M.; Kaur, R. Physical, structural and thermal properties of composite edible films prepared from pearl millet starch and carrageenan gum: Process optimization using response surface methodology. Int. J. Boil. Macromol. 2019, 143, 704–713. [Google Scholar] [CrossRef] [PubMed]
- Gnanasambandam, R.; Hettiarachchy, N.; Coleman, M. Mechanical and Barrier Properties of Rice Bran Films. J. Food Sci. 1997, 62, 395–398. [Google Scholar] [CrossRef]
- Biduski, B.; Da Silva, F.T.; Silva, W.M.F.; El Halal, S.L.M.; Pinto, V.Z.; Dias, A.R.G.; Zavareze, E. Impact of acid and oxidative modifications, single or dual, of sorghum starch on biodegradable films. Food Chem. 2017, 214, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Peltzer, M.A.; Salvay, A.G.; Delgado, J.F.; De La Osa, O.; Wagner, J.R. Use of Residual Yeast Cell Wall for New Biobased Materials Production: Effect of Plasticization on Film Properties. Food Bioprocess Technol. 2018, 11, 1995–2007. [Google Scholar] [CrossRef]
- Ramos, P.E.; Cerqueira, M.A.; Coimbra, M.A.; Vicente, A.A. Physiological protection of probiotic microcapsules by coatings. Crit. Rev. Food Sci. Nutr. 2017, 58, 1864–1877. [Google Scholar] [CrossRef]
- Jeyakumari, A.; Zynudheen, A.A.; Parvathy, U. Microencapsulation of Bioactive Food Ingredients and Controlled Release A Review. MOJ Food Process. Technol. 2016, 2. [Google Scholar] [CrossRef]
- Achinna, P.; Kuna, A. Microencapsulation technology: A review. J. Res. ANGRAU 2010, 38, 86–102. [Google Scholar]
- Tzia, C.; Tasios, L.; Spiliotaki, T.; Chranioti, C.; Giannou, V.; Varzakas, T. Edible Coatings and Films to Preserve Quality of Fresh Fruits and Vegetables. In Engineering Aspects of Membrane Separation and Application in Food Processing; Informa UK Limited: London, UK, 2015; Volume 20152939, pp. 531–570. [Google Scholar]
- Vieira, M.G.A.; Da Silva, M.A.; Santos, L.; Beppu, M.M. Natural-based plasticizers and biopolymer films: A review. Eur. Polym. J. 2011, 47, 254–263. [Google Scholar] [CrossRef]
- Lacroix, M. Mechanical and Permeability Properties of Edible Films and Coatings for Food and Pharmaceutical Applications. In Edible Films and Coatings for Food Applications; Springer Science and Business Media LLC: Berlin/Heidelberg, Germany, 2009; pp. 347–366. [Google Scholar]
- Desai, K.G.; Park, H.J. Recent Developments in Microencapsulation of Food Ingredients. Dry. Technol. 2005, 23, 1361–1394. [Google Scholar] [CrossRef]
- Morrison, N.A.; Clark, R.C.; Chen, Y.L.; Talashek, T.; Sworn, G. Gelatin alternatives for the food industry. In Progress in Colloid and Polymer Science; Springer Science and Business Media LLC: Berlin/Heidelberg, Germany, 2007; Volume 114, pp. 127–131. [Google Scholar]
- Gómez-Guillen, M.C.; Giménez, B.; López-Caballero, M.; Montero, M. Functional and bioactive properties of collagen and gelatin from alternative sources: A review. Food Hydrocoll. 2011, 25, 1813–1827. [Google Scholar] [CrossRef]
- Nualkaekul, S.; Cook, M.; Khutoryanskiy, V.V.; Charalampopoulos, D. Influence of encapsulation and coating materials on the survival of Lactobacillus plantarum and Bifidobacterium longum in fruit juices. Food Res. Int. 2013, 53, 304–311. [Google Scholar] [CrossRef]
- Yan, C.; Zhang, W. Coacervation Processes. In Microencapsulation in the Food Industry; Elsevier BV: Amsterdam, The Netherlands, 2014; pp. 125–137. [Google Scholar]
- Thies, C. Microencapsulation of Flavors by Complex Coacervation. In Encapsulation and Controlled Release Technologies in Food Systems; Wiley: Hoboken, NJ, USA, 2007; pp. 149–170. [Google Scholar]
- Meng, Y.; Cloutier, S. Gelatin and Other Proteins for Microencapsulation. In Microencapsulation in the Food Industry; Elsevier BV: Amsterdam, The Netherlands, 2014; pp. 227–239. [Google Scholar]
- Weinbreck, F.; De Vries, R.; Schrooyen, P.; De Kruif, C.G. Complex Coacervation of Whey Proteins and Gum Arabic. Biomacromolecules 2003, 4, 293–303. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.-X.; Huang, G.-Q.; Wang, S.; Sun, Y.-T. Microencapsulation of capsanthin by soybean protein isolate-chitosan coacervation and microcapsule stability evaluation. J. Appl. Polym. Sci. 2013, 131, 131. [Google Scholar] [CrossRef]
- Rizzo, G.; Baroni, L. Soy, Soy Foods and Their Role in Vegetarian Diets. Nutrients 2018, 10, 43. [Google Scholar] [CrossRef] [PubMed]
- Nishinari, K.; Fang, Y.-P.; Guo, S.; Phillips, G. Soy proteins: A review on composition, aggregation and emulsification. Food Hydrocoll. 2014, 39, 301–318. [Google Scholar] [CrossRef]
- Wang, X.; Luo, K.; Liu, S.; Adhikari, B.; Chen, J. Improvement of gelation properties of soy protein isolate emulsion induced by calcium cooperated with magnesium. J. Food Eng. 2019, 244, 32–39. [Google Scholar] [CrossRef]
- Wang, X.; Zeng, M.; Qin, F.; Adhikari, B.; He, Z.; Chen, J. Enhanced CaSO4-induced gelation properties of soy protein isolate emulsion by pre-aggregation. Food Chem. 2018, 242, 459–465. [Google Scholar] [CrossRef]
- Mulvihill, D.M.; Donovan, M. Whey proteins and their thermal denaturation—A review. Irish J. Food Sci. Technol. 1987, 11, 43–75. [Google Scholar]
- Augustin, M.A.; Oliver, C.M. Use of Milk Proteins for Encapsulation of Food Ingredients. In Microencapsulation in the Food Industry; Elsevier BV: Amsterdam, The Netherlands, 2014; pp. 211–226. [Google Scholar]
- Visker, M.H.P.W.; Heck, J.; Van Valenberg, H.; Van Arendonk, J.; Bovenhuis, H. Short communication: A new bovine milk-protein variant: α-Lactalbumin variant D. J. Dairy Sci. 2012, 95, 2165–2169. [Google Scholar] [CrossRef]
- Sarode, A.; Sawale, P.; Khedkar, C.; Kalyankar, S.; Pawshe, R. Casein and Caseinate: Methods of Manufacture. In Encyclopedia of Food and Health; Elsevier BV: Amsterdam, The Netherlands, 2016; pp. 676–682. [Google Scholar]
- DeJong, G.; Koppelman, S.J. Transglutaminase Catalyzed Reactions: Impact on Food Applications. J. Food Sci. 2002, 67, 2798–2806. [Google Scholar] [CrossRef]
- Gangurde, H.; Patil, P.; Chordiya, M.; Baste, N. Whey protein. Sch. Res. J. 2011, 1, 69. [Google Scholar] [CrossRef]
- O’Mahony, J.; Fox, P. Milk: An Overview. In Milk Proteins; Elsevier BV: Amsterdam, The Netherlands, 2014; pp. 19–73. [Google Scholar]
- Morr, C.V.; Ha, E.Y.W. Whey protein concentrates and isolates: Processing and functional properties. Crit. Rev. Food Sci. Nutr. 1993, 33, 431–476. [Google Scholar] [CrossRef] [PubMed]
- Rojas, S.A.; Kung, B.; Senaratne, V.; Dalgleish, D.G.; Flores, A. Gelation of commercial fractions of β-lactoglobulin and α-lactalbumin. Int. Dairy J. 1997, 7, 79–85. [Google Scholar] [CrossRef]
- Santos, M.B.; Da Costa, N.R.; Garcia-Rojas, E.E. Interpolymeric Complexes Formed Between Whey Proteins and Biopolymers: Delivery Systems of Bioactive Ingredients. Compr. Rev. Food Sci. Food Saf. 2018, 17, 792–805. [Google Scholar] [CrossRef]
- Layman, D.; Lönnerdal, B.; Fernstrom, J.D. Applications for α-lactalbumin in human nutrition. Nutr. Rev. 2018, 76, 444–460. [Google Scholar] [CrossRef] [PubMed]
- Larsen, L.B.; Wedholm-Pallas, A.; Lindmark-Månsson, H.; Andrén, A. Different proteomic profiles of sweet whey and rennet casein obtained after preparation from raw versus heat-treated skimmed milk. Dairy Sci. Technol. 2010, 90, 641–656. [Google Scholar] [CrossRef][Green Version]
- Gruber, J. Polysaccharide-Based Polymers in Cosmetics. In Principles of Polymer Science and Technology in Cosmetics and Personal Care; Desmond Goddard, J.V.G.E., Ed.; CRC Press: London, UK, 1999; pp. 325–389. [Google Scholar] [CrossRef]
- Matalanis, A.; Jones, O.G.; McClements, D.J. Structured biopolymer-based delivery systems for encapsulation, protection, and release of lipophilic compounds. Food Hydrocoll. 2011, 25, 1865–1880. [Google Scholar] [CrossRef]
- Kwiecień, I.; Kwiecień, M. Application of Polysaccharide-Based Hydrogels as Probiotic Delivery Systems. Gels 2018, 4, 47. [Google Scholar] [CrossRef]
- Burnside, E. Hydrocolloids and Gums as Encapsulating Agents. In Microencapsulation in the Food Industry; Elsevier BV: Amsterdam, The Netherlands, 2014; pp. 241–252. [Google Scholar]
- Chakraborty, S. Carrageenan for encapsulation and immobilization of flavor, fragrance, probiotics, and enzymes: A review. J. Carbohydr. Chem. 2017, 36, 1–19. [Google Scholar] [CrossRef]
- Yuguchi, Y.; Thuy, T.T.T.; Urakawa, H.; Kajiwara, K. Structural characteristics of carrageenan gels: Temperature and concentration dependence. Food Hydrocoll. 2002, 16, 515–522. [Google Scholar] [CrossRef]
- Tavassoli-Kafrani, E.; Shekarchizadeh, H.; Masoudpour-Behabadi, M. Development of edible films and coatings from alginates and carrageenans. Carbohydr. Polym. 2016, 137, 360–374. [Google Scholar] [CrossRef] [PubMed]
- Saha, D.; Bhattacharya, S. Hydrocolloids as thickening and gelling agents in food: A critical review. J. Food Sci. Technol. 2010, 47, 587–597. [Google Scholar] [CrossRef] [PubMed]
- Wandrey, C.; Bartkowiak, A.; Harding, S. Materials for encapsulation. In Encapsulation Technologies for Active Food Ingredients and Food Processing, 1st ed.; Zuidam, N.J., Nedovic, V., Eds.; Springer-Verlag: New York, NY, USA, 2009; pp. 31–100. [Google Scholar] [CrossRef]
- Jansson, P.-E.; Kenne, L.; Lindberg, B. Structure of the extracellular polysaccharide from xanthomonas campestris. Carbohydr. Res. 1975, 45, 275–282. [Google Scholar] [CrossRef]
- Petri, D.F. Xanthan gum: A versatile biopolymer for biomedical and technological applications. J. Appl. Polym. Sci. 2015, 132, 132. [Google Scholar] [CrossRef]
- Kool, M.M.; Gruppen, H.; Sworn, G.; Schols, H.A. The influence of the six constituent xanthan repeating units on the order–disorder transition of xanthan. Carbohydr. Polym. 2014, 104, 94–100. [Google Scholar] [CrossRef] [PubMed]
- Kool, M.M.; Schols, H.A.; Delahaije, R.; Sworn, G.; Wierenga, P.A.; Gruppen, H. The influence of the primary and secondary xanthan structure on the enzymatic hydrolysis of the xanthan backbone. Carbohydr. Polym. 2013, 97, 368–375. [Google Scholar] [CrossRef]
- Wu, M.; Qu, J.; Shen, Y.; Dai, X.; Wei, W.; Shi, Z.; Li, G.; Ma, T. Gel properties of xanthan containing a single repeating unit with saturated pyruvate produced by an engineered Xanthomonas campestris CGMCC 15155. Food Hydrocoll. 2019, 87, 747–757. [Google Scholar] [CrossRef]
- Bergmann, D.; Furth, G.; Mayer, C. Binding of bivalent cations by xanthan in aqueous solution. Int. J. Boil. Macromol. 2008, 43, 245–251. [Google Scholar] [CrossRef]
- Cook, M.; Tzortzis, G.; Charalampopoulos, D.; Khutoryanskiy, V.V. Microencapsulation of probiotics for gastrointestinal delivery. J. Control. Release 2012, 162, 56–67. [Google Scholar] [CrossRef]
- Fareez, I.M.; Lim, S.M.; Mishra, R.K.; Ramasamy, K. Microencapsulation of Lactobacillus SP. Using Chitosan-Alginate-Xanthan Gum-?-Cyclodextrin and Characterization of its Cholesterol Reducing Potential and Resistance Against pH, Temperature and Storage. J. Food Process. Eng. 2016, 40, e12458. [Google Scholar] [CrossRef]
- Zia, K.M.; Tabasum, S.; Khan, M.F.; Akram, N.; Akhter, N.; Noreen, A.; Zuber, M. Recent trends on gellan gum blends with natural and synthetic polymers: A review. Int. J. Boil. Macromol. 2018, 109, 1068–1087. [Google Scholar] [CrossRef] [PubMed]
- Nussinovitch, A. Hydrocolloids in Flavor Encapsulation. In Water-Soluble Polymer Applications in Foods; Nussinovitch, A., Ed.; Blackwell Science: Oxford, UK, 2003; pp. 31–113. [Google Scholar] [CrossRef]
- Karlton-Senaye, B.; Ibrahim, S. Impact of gums on the growth of probiotics. Agro Food Ind. Hi-Tech 2013, 24, 10–14. [Google Scholar]
- Izydorczyk, M.; Cui, S.; Wang, Q. Polysaccharide Gums: Structures, Functional Properties, and Applications. Food Carbohydr. Chem. Phys. Prop. Appl. 2005. [Google Scholar] [CrossRef]
- Wallick, D. Cellulose Polymers in Microencapsulation of Food Additives. In Microencapsulation in the Food Industry; Elsevier BV: Amsterdam, The Netherlands, 2014; pp. 181–193. [Google Scholar]
- Narayanan, D.; Kumar, A.S.; Chennazhi, K.P. Versatile carboxymethyl chitin and chitosan nanomaterials: A review. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2014, 6, 574–598. [Google Scholar] [CrossRef]
- Elieh-Ali-Komi, D.; Hamblin, M.R. Chitin and Chitosan: Production and Application of Versatile Biomedical Nanomaterials. Int. J. Adv. Res. 2016, 4, 411–427. [Google Scholar]
- Heinze, T.; El Seoud, O.A.; Koschella, A. Etherification of Cellulose. In Springer Series on Polymer and Composite Materials; Springer Science and Business Media LLC: Berlin/Heidelberg, Germany, 2018; pp. 429–477. [Google Scholar]
- Liu, H.; Yang, Q.; Zhang, L.; Zhuo, R.; Jiang, X. Synthesis of carboxymethyl chitin in aqueous solution and its thermo- and pH-sensitive behaviors. Carbohydr. Polym. 2016, 137, 600–607. [Google Scholar] [CrossRef] [PubMed]
- Sahariah, P.; Másson, M. Antimicrobial Chitosan and Chitosan Derivatives: A Review of the Structure–Activity Relationship. Biomacromolecules 2017, 18, 3846–3868. [Google Scholar] [CrossRef]
- Călinoiu, L.-F.; Ștefănescu, B.E.; Pop, I.; Muntean, L.; Vodnar, D.C. Chitosan Coating Applications in Probiotic Microencapsulation. Coatings 2019, 9, 194. [Google Scholar] [CrossRef]
- Shah, U.; Naqash, F.; Gani, A.; Masoodi, F.A. Art and Science behind Modified Starch Edible Films and Coatings: A Review. Compr. Rev. Food Sci. Food Saf. 2016, 15, 568–580. [Google Scholar] [CrossRef]
- And, Z.L.; Han, J. Film-forming Characteristics of Starches. J. Food Sci. 2005, 70, 31. [Google Scholar] [CrossRef]
- BeMiller, J.N. Starches: Conversions, modifications, and uses. In Carbohydrate Chemistry for Food Scientists, 3rd ed.; BeMiller, J.N., Ed.; AACC International Press: Amsterdam, The Netherlands, 2019; pp. 191–221. [Google Scholar] [CrossRef]
- Van Der Veen, B.A.; Van Alebeek, G.-J.W.M.; Uitdehaag, J.C.M.; Dijkstra, B.W.; Dijkhuizen, L. The three transglycosylation reactions catalyzed by cyclodextrin glycosyltransferase from Bacillus circulans (strain 251) proceed via different kinetic mechanisms. JBIC J. Boil. Inorg. Chem. 2000, 267, 658–665. [Google Scholar] [CrossRef] [PubMed]
- Di Cagno, M.P. The Potential of Cyclodextrins as Novel Active Pharmaceutical Ingredients: A Short Overview. Molecules 2016, 22, 1. [Google Scholar] [CrossRef] [PubMed]
- Pariot, N.; Edwards-Lévy, F.; Andry, M.-C.; Levy, M.-C. Cross-linked β-cyclodextrin microcapsules: Preparation and properties. Int. J. Pharm. 2000, 211, 19–27. [Google Scholar] [CrossRef]
- Gidwani, B.; Vyas, A. A Comprehensive Review on Cyclodextrin-Based Carriers for Delivery of Chemotherapeutic Cytotoxic Anticancer Drugs. BioMed Res. Int. 2015, 2015, 1–15. [Google Scholar] [CrossRef]
- Muzzafar, A.; Sharma, V. Microencapsulation of probiotics for incorporation in cream biscuits. J. Food Meas. Charact. 2018, 12, 2193–2201. [Google Scholar] [CrossRef]
- Synthesis of Carboxymethyl Chitosan and its Rheological Behaviour in Pharmaceutical and Cosmetic Emulsions. J. Appl. Pharm. Sci. 2017, 7, 70–78.
- Zhao, L.; Zhu, B.; Jia, Y.; Hou, W.; Su, C. Preparation of Biocompatible Carboxymethyl Chitosan Nanoparticles for Delivery of Antibiotic Drug. BioMed Res. Int. 2013, 2013, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Shit, S.C.; Shah, P. Edible Polymers: Challenges and Opportunities. J. Polym. 2014, 2014, 1–13. [Google Scholar] [CrossRef]
- Aydin, F.; Kahve, H.; Ardic, M. Lipid-based edible films. J. Sci. Eng. Res. 2017, 4, 86–92. [Google Scholar]
- Wolfmeier, U.; Schmidt, H.; Michalczyk, G.; Payer, W.; Dietsche, W.; Boehlke, K.; Hohner, G.; Wildgruber, J.; Heinrichs, F.-L. Waxes. In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley: Hoboken, NJ, USA, 2000. [Google Scholar]
- Hall, D.J. Edible coatings from lipids, waxes, and resins. In Edible Coatings and Films to Improve Food Quality, 2nd ed.; Baldwin, E.A., Hagenmaier, R., Bai, J., Eds.; CRC Press: Boca Raton, FL, USA, 2011; pp. 79–101. [Google Scholar] [CrossRef]
- Rao, A.; Shiwnarain, N.; Maharaj, I. Survival of Microencapsulated Bifidobacterium pseudolongum in Simulated Gastric and Intestinal Juices. Can. Inst. Food Sci. Technol. J. 1989, 22, 345–349. [Google Scholar] [CrossRef]
- Xie, M. Phospholipids. In Encyclopedia of Food Chemistry; Melton, L., Shahidi, F., Varelis, P., Eds.; Academic Press: Oxford, UK, 2019; pp. 214–217. [Google Scholar] [CrossRef]
- Jackson, L. Microencapsulation and the food Industry. Lebensm. Wiss. Technol. 1991, 24, 289–297. [Google Scholar]
- Singh, H.; Thompson, A.; Liu, W.; Corredig, M. Liposomes as food ingredients and nutraceutical delivery systems. In Encapsulation Technologies and Delivery Systems for Food Ingredients and Nutraceuticals; Elsevier BV: Amsterdam, The Netherlands, 2012; pp. 287–318. [Google Scholar]
- Sarao, L.K.; Arora, M.; M, A. Probiotics, prebiotics, and microencapsulation: A review. Crit. Rev. Food Sci. Nutr. 2015, 57, 344–371. [Google Scholar] [CrossRef] [PubMed]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).