The Structure, Morphology, and Mechanical Properties of Ta-Hf-C Coatings Deposited by Pulsed Direct Current Reactive Magnetron Sputtering
Abstract
:1. Introduction
2. Experimental Details
2.1. Deposition
2.2. Characterizations
3. Results and Discussion
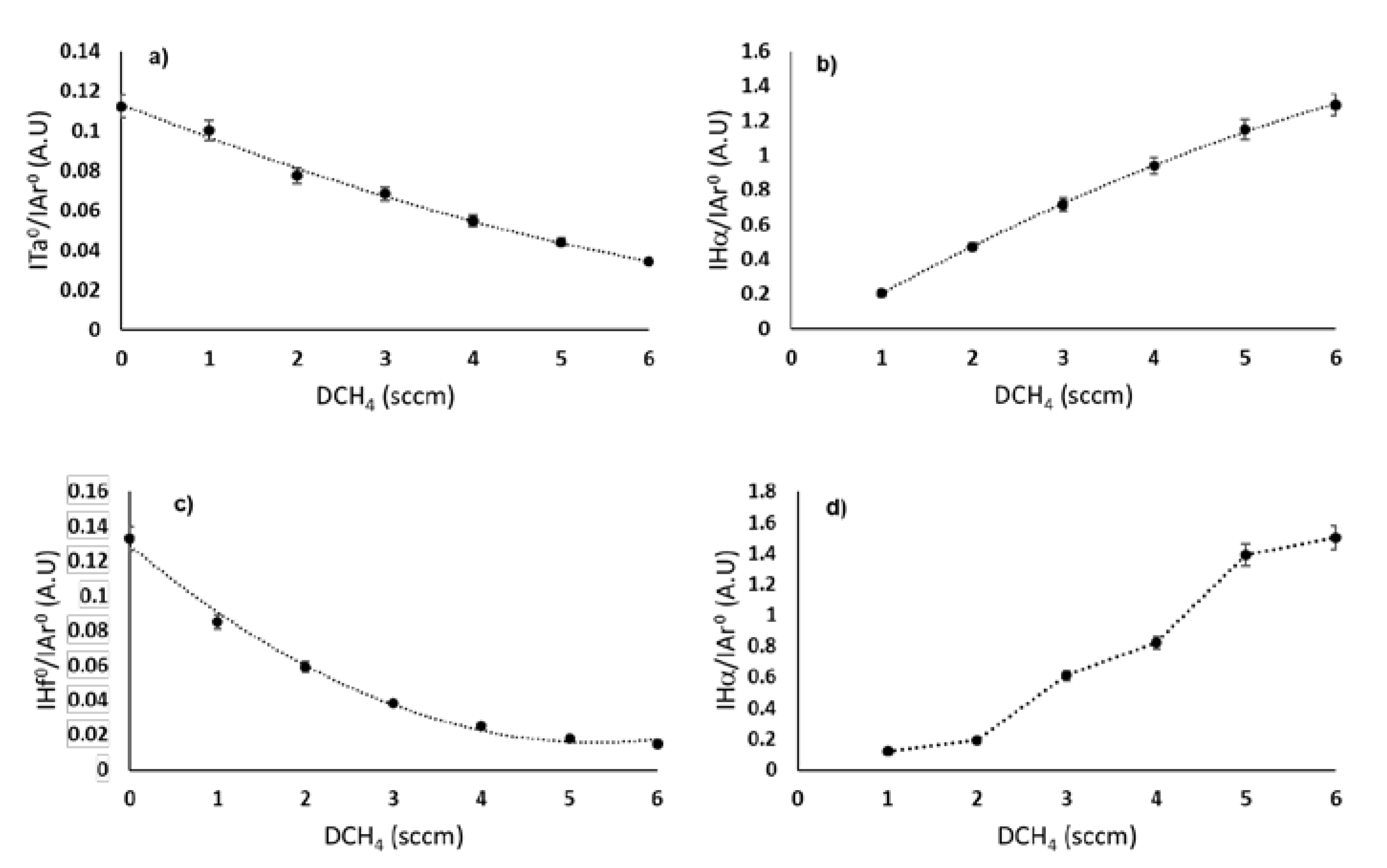
3.1. Reactive Discharge Characterization
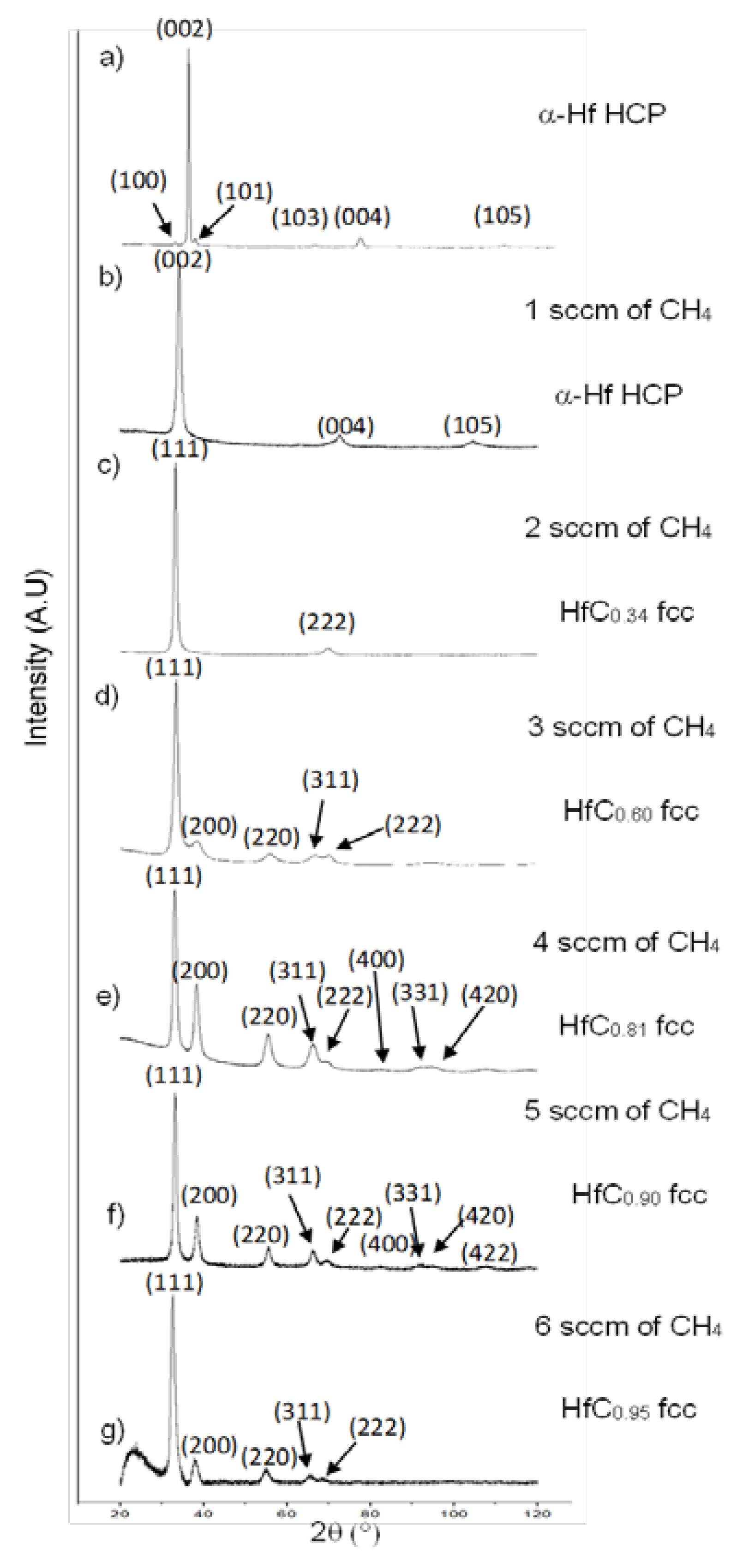
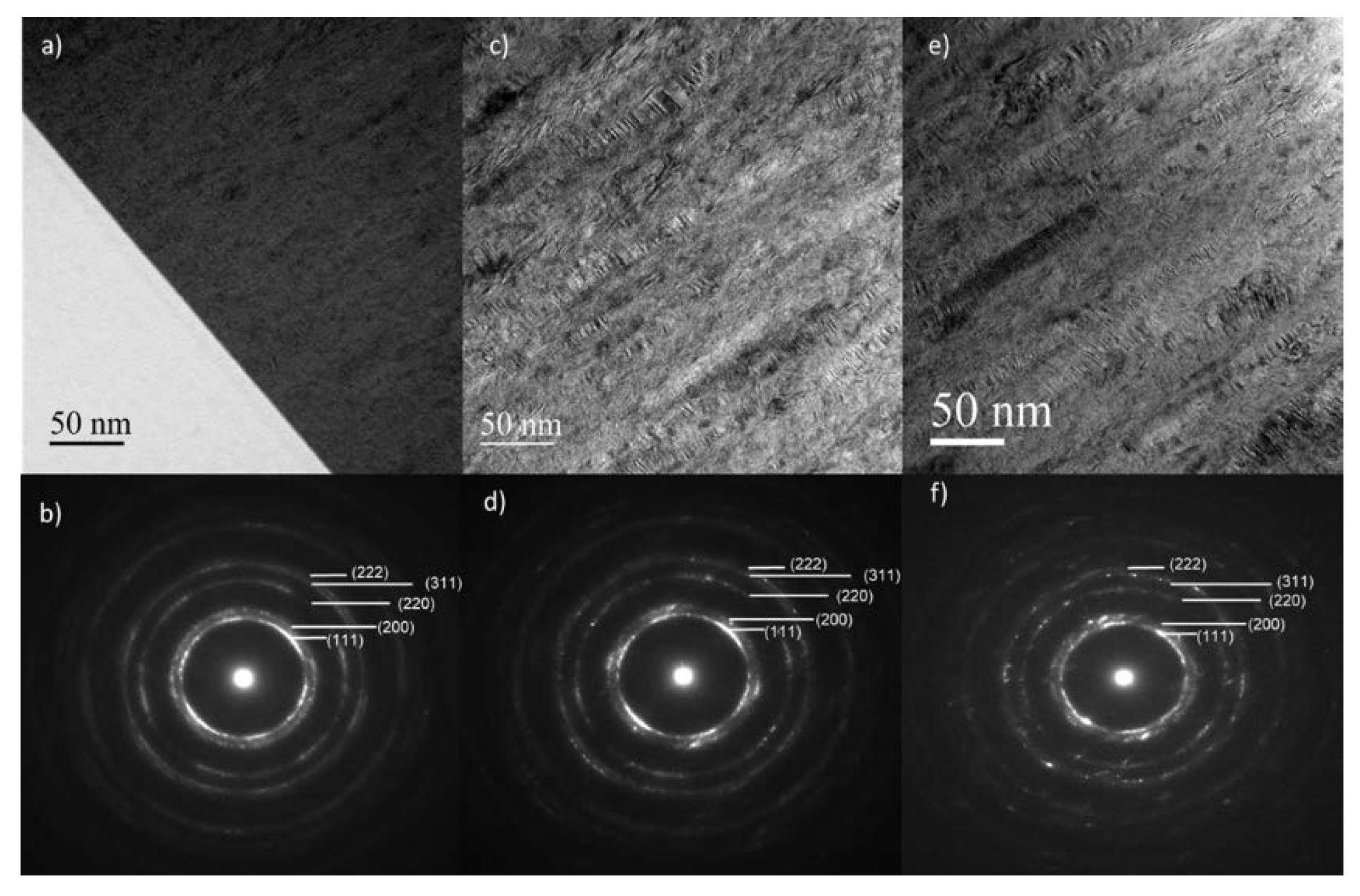
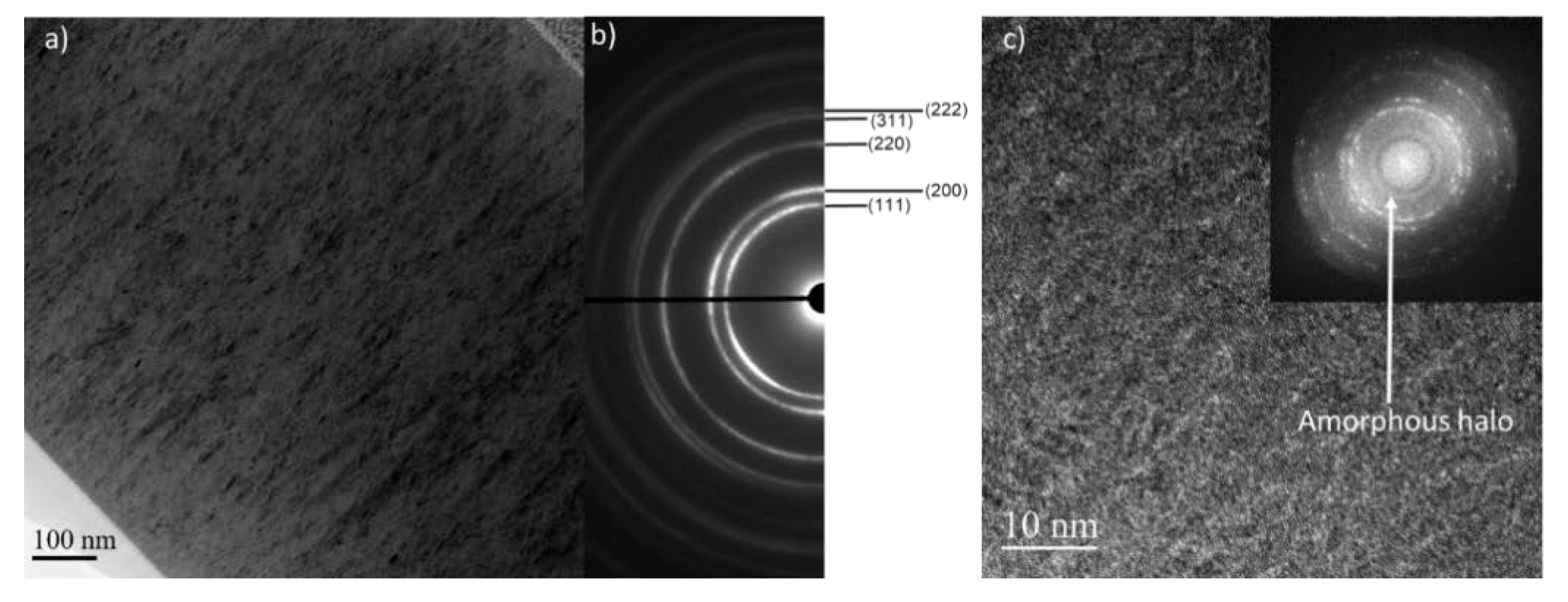
3.2. Composition, Structure, and Microstructure
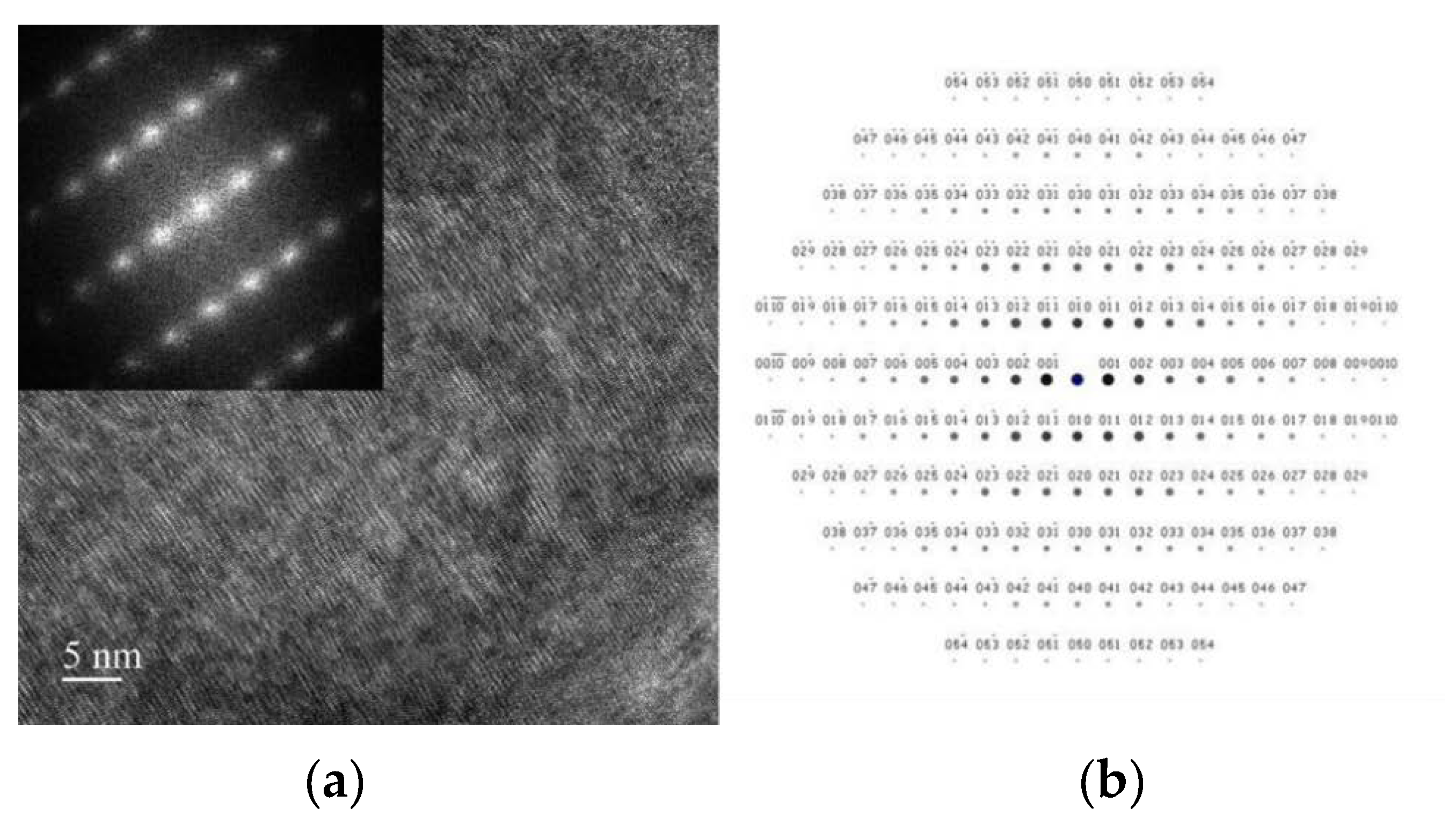
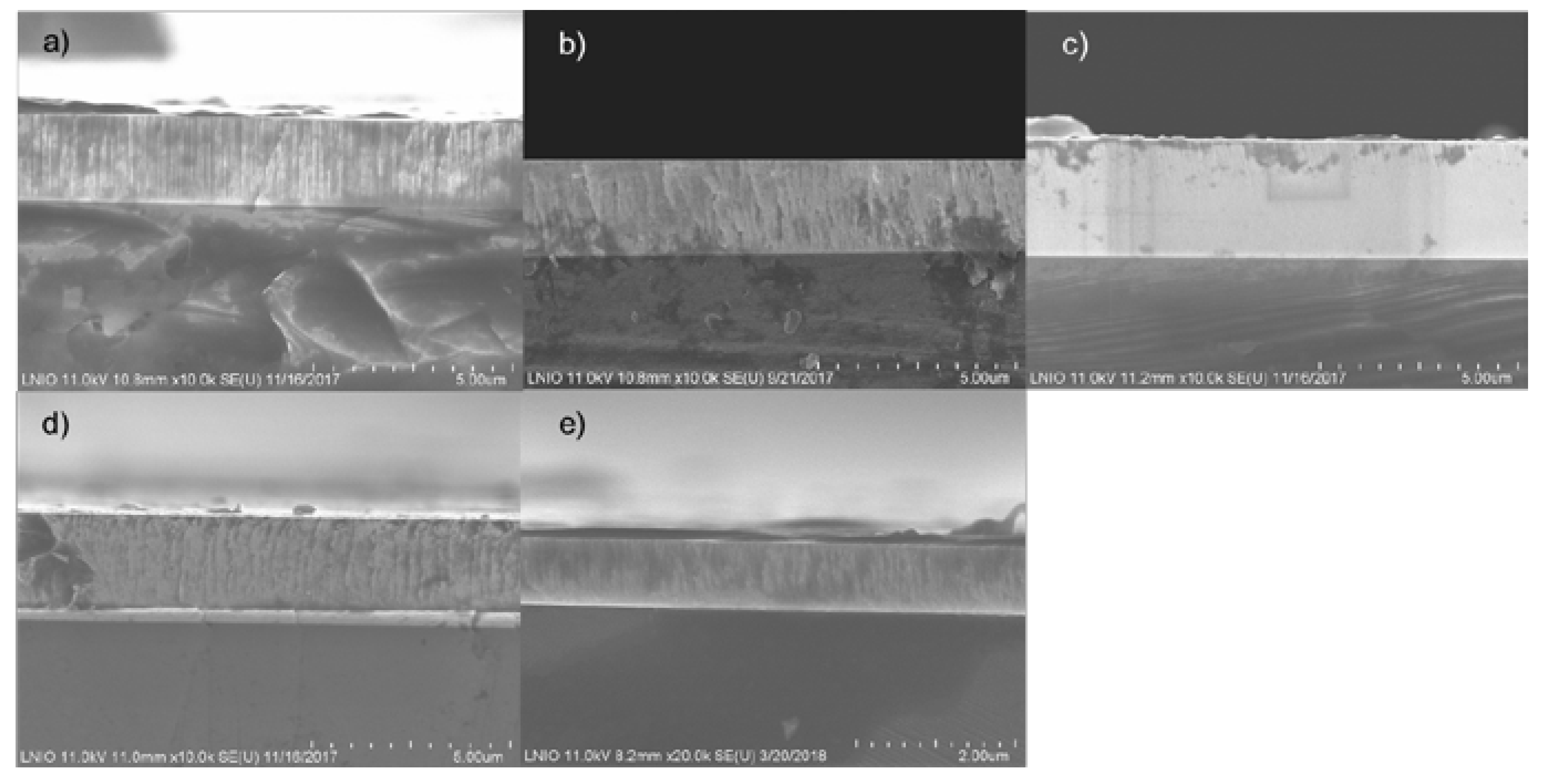
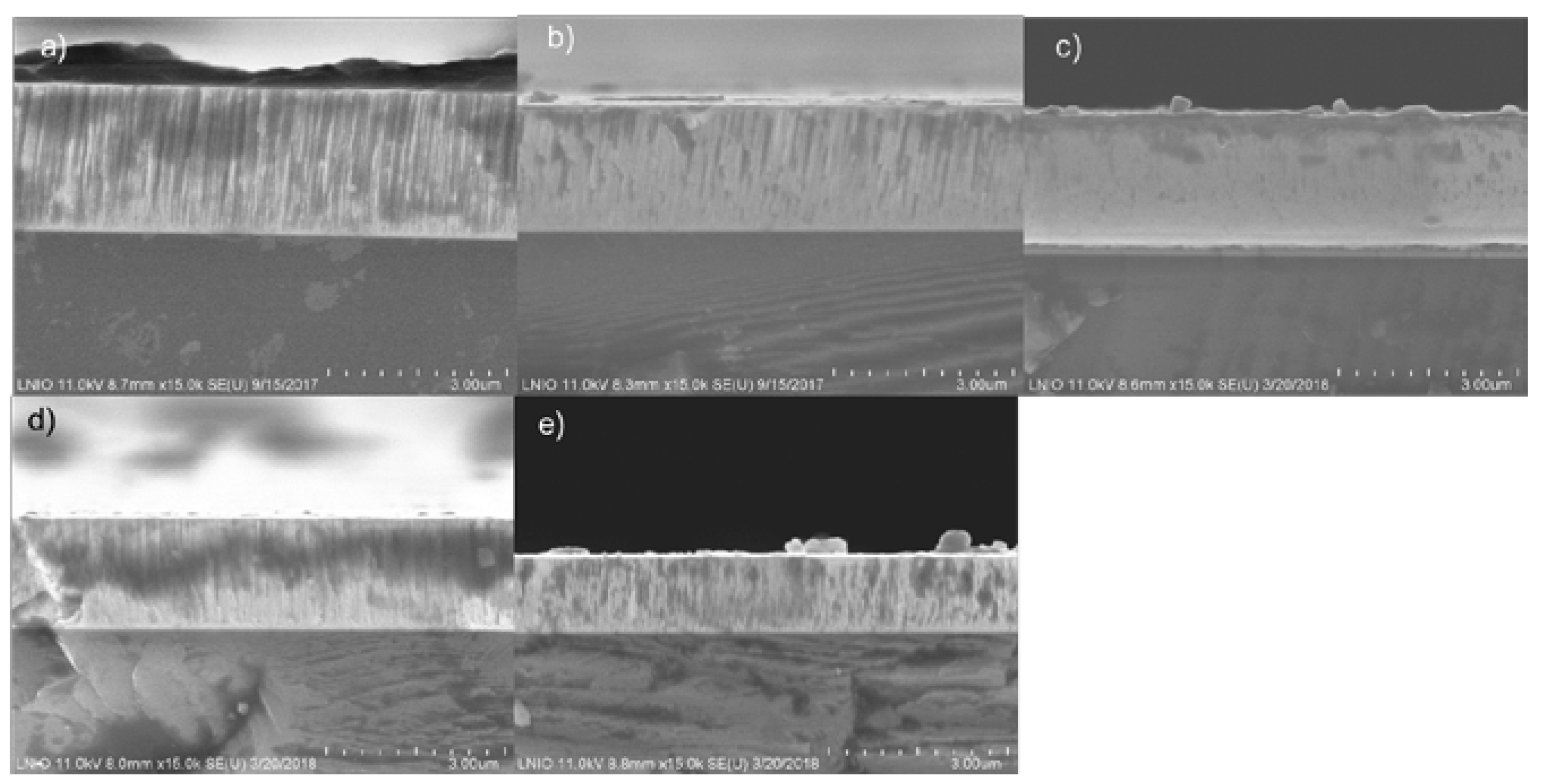
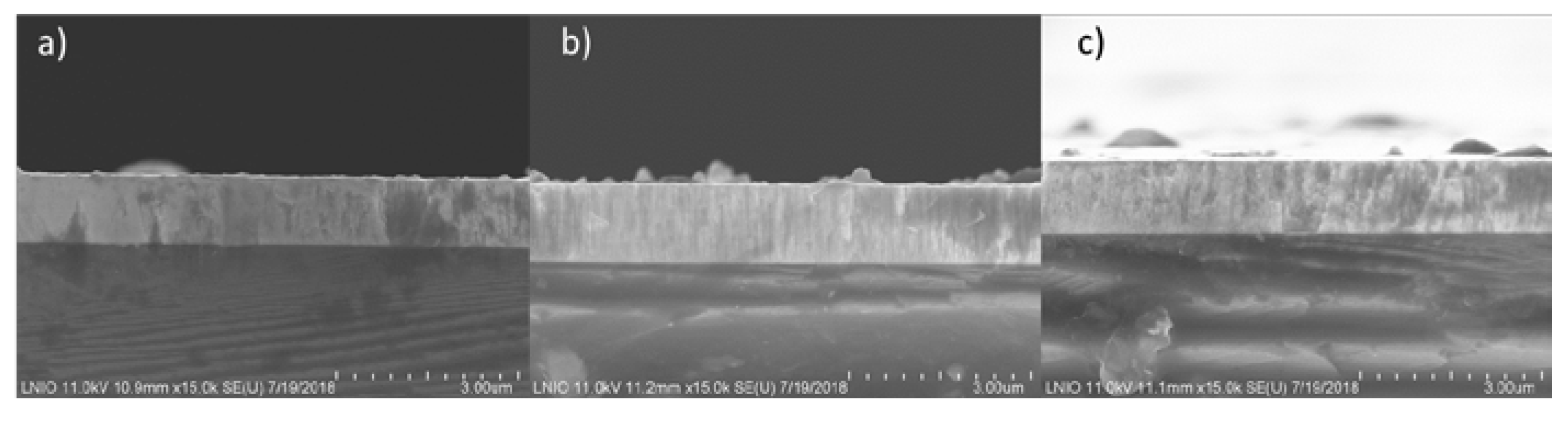
3.3. Morphology
3.4. Mechanical Properties
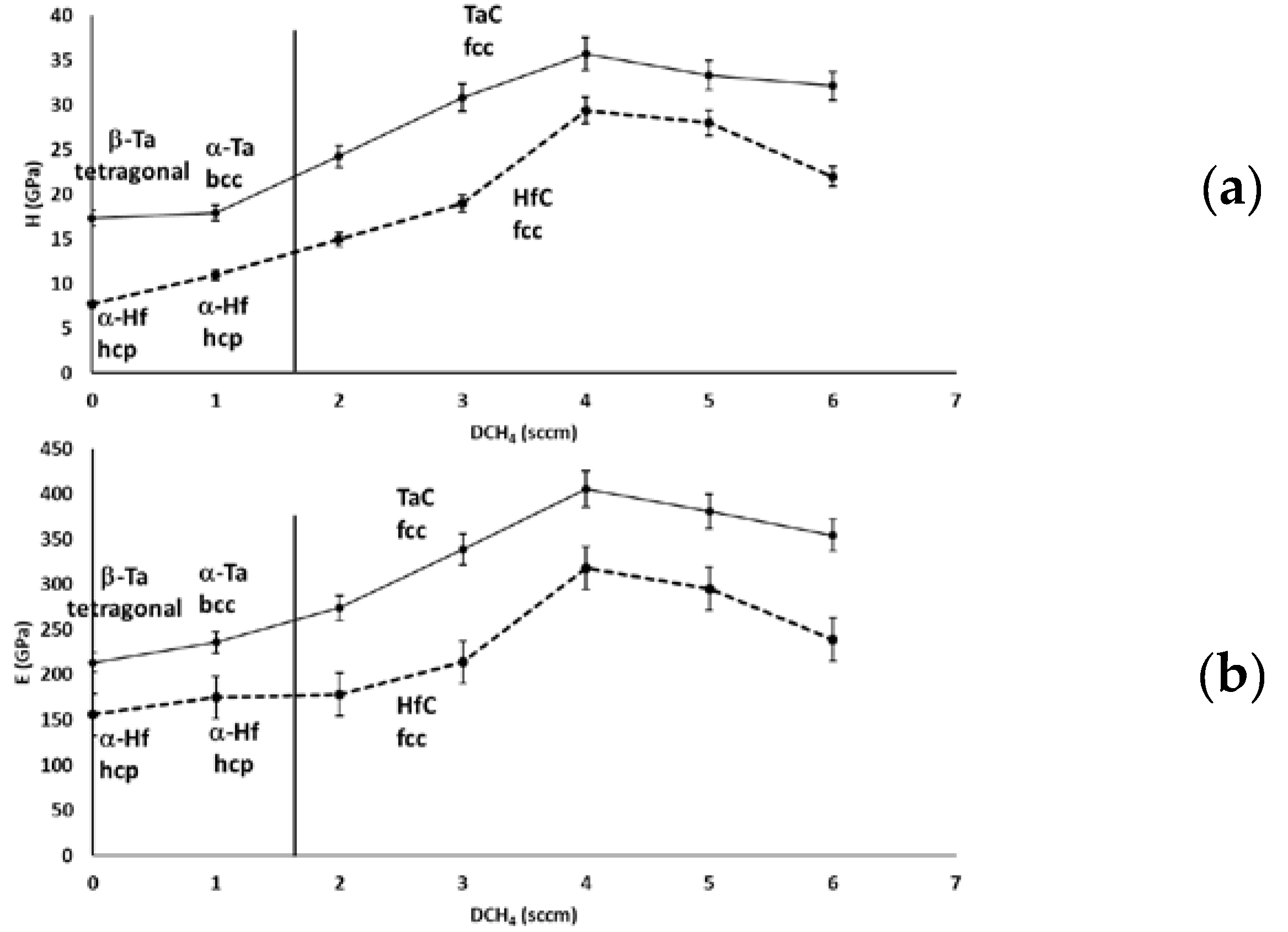
3.4.1. Nanoindentation
3.4.2. Residual Stresses
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Fotovvati, B.; Namdari, N.; Dehghanghadikolaei, A. On Coating Techniques for Surface Protection: A Review. J. Manuf. Mater. Process. 2019, 3, 28. [Google Scholar] [CrossRef] [Green Version]
- Aoudia, K.; Lamri, S.; Achache, S.; Retraint, D.; Verdy, C.; Langlade, C.; Azem, S.; Sanchette, F. Structural and Mechanical Properties of Arc-Sprayed Ni–Cr Coating Post-Treated by Surface Mechanical Attrition Treatment (SMAT). Coatings 2018, 8, 424. [Google Scholar] [CrossRef] [Green Version]
- Dehghanghadikolaei, A.; Fotovvat, B. Coating Techniques for Functional Enhancement of Metal Implants for Bone Replacement: A Review. Materials 2019, 12, 1795. [Google Scholar] [CrossRef] [Green Version]
- Sahith, M.S.; Giridhara, G.; Suresh Kumar, R. Development and analysis of thermal barrier coatings on gas turbine blades–A Review. Mater. Today Proc. 2018, 5, 2746–2751. [Google Scholar] [CrossRef]
- Mehboob, G.; Liua, M.; Xu, T.; Hussain, S.; Mehboob, G.; Tahir, A. A review on failure mechanism of thermal barrier coatings and strategies to extend their lifetime. Ceram. Int. in press. [CrossRef]
- Alhussein, A.; Achache, S.; Deturche, R.; Sanchette, F.; Pulgarin, C.; Kiwi, J.; Rtimi, S. Beneficial effect of Cu on Ti-Nb-Ta-Zr sputtered uniform/adhesive gum films accelerating bacterial inactivation under indoor visible light. Colloids Surf. B Biointerfaces 2017, 152, 152–158. [Google Scholar] [CrossRef]
- Creus, J.; Idrissi, H.; Mazille, H.; Sanchette, F.; Jacquot, P. Corrosion behaviour of AllTi coating elaborated by cathodic arc PVD process onto mild steel substrate. Thin Solid Film. 1999, 346, 150–154. [Google Scholar] [CrossRef]
- Creus, J.; Berziou, C.; Cohendoz, S.; Perez, A.; Rebere, C.; Reffass, M.; Touzain, S.; Allely, C.; Gachon, Y.; Heau, C.; et al. Reactivity classification in saline solution of magnetron sputtered or EBPVD pure metallic, nitride and Al-based alloy coatings. Corros. Sci. 2012, 57, 162–173. [Google Scholar] [CrossRef]
- Lomello, F.; Sanchette, F.; Schuster, F.; Tabarant, M.; Billard, A. Influence of bias voltage on properties of AlCrN coatings prepared by cathodic arc deposition. Surf. Coat. Technol. 2013, 224, 77–81. [Google Scholar] [CrossRef]
- Arab Pour Yazdi, M.; Lomello, F.; Wang, J.; Sanchette, F.; Dong, Z.; White, T.; Wouters, Y.; Schuster, F.; Billard, A. Properties of TiSiN coatings deposited by hybrid HiPIMS and pulsed-DC magnetron co-sputtering. Vacuum 2014, 109, 43–51. [Google Scholar] [CrossRef]
- Cotton, J. Ultra-High-Temperature Ceramics. Adv. Mater. Process. 2010, 168, 26–28. [Google Scholar]
- Paul, A.; Binner, J.; Vaidhyanathan, B. UHTC Composites for Hypersonic Applications. In Ultra-High Temperature Ceramics; Fahrenholtz, W.G., Wuchina, E.J., Lee, W.E., Zhou, Y., Eds.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2014; pp. 144–166. [Google Scholar] [CrossRef] [Green Version]
- Qualification of New UHTC Materials for Solar Receiver at High Temperature –PROMES. Available online: https://www.promes.cnrs.fr/index.php?page=qualification-of-new-uhtc-materials-for-solar-receiver-at-high-temperature (accessed on 19 March 2018).
- Sani, E.; Mercatelli, L.; Fontani, D.; Sans, J.-L.; Sciti, D. Hafnium and tantalum carbides for high temperature solar receivers. J. Renew. Sustain. Energy 2011, 3, 063107. [Google Scholar] [CrossRef] [Green Version]
- Fahrenholtz, W.G.; Wuchina, E.J.; Lee, W.E.; Zhou, Y. (Eds.) Ultra-High Temperature Ceramics: Materials for Extreme Environment Application; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2014. [Google Scholar] [CrossRef] [Green Version]
- Martin, D.S. Advances in Inorganic Chemistry and Radiochemistry; Emeléus, H.J., Sharpe, A.G., Eds.; Elsevier: Amsterdam, The Netherlands, 2002; Volume 4. [Google Scholar] [CrossRef]
- Cedillos, O.; Jayaseelan, D.; Lee, B. Fabrication of TaC-HfC Ceramics for Ultra-High Temperature Applications, Ultra-High Temperature Ceramics: Materials for Extreme Environmental Applications II. 2012. Available online: http://dc.engconfintl.org/uhtc/25 (accessed on 2018).
- Cedillos-Barraza, O.; Manara, D.; Boboridis, K.; Watkins, T.; Grasso, S.; Jayaseelan, D.D.; Konings, R.J.M.; Reece, M.J.; Lee, W.E. Investigating the highest melting temperature materials: A laser melting study of the TaC-HfC system. Sci. Rep. 2016, 6, 37962. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lasfargues, H.; Glechner, T.; Koller, C.M.; Paneta, V.; Primetzhofer, D.; Kolozsvári, S.; Holec, D.; Riedl, H.; Mayrhofer, P.H. Non-reactively sputtered ultra-high temperature Hf-C and Ta-C coatings. Surf. Coat. Technol. 2017, 309, 436–444. [Google Scholar] [CrossRef]
- Evans, R.D.; Doll, G.L.; Glass, J.T. Mechanical property development in reactively sputtered tantalum carbide/amorphous hydrocarbon thin films. J. Mater. Res. 2006, 21, 1500–1511. [Google Scholar] [CrossRef]
- Evans, R.D.; Doll, G.L.; Meng, W.J.; Mei, F.; Glass, J.T. Effects of applied substrate bias during reactive sputter deposition of nanocomposite tantalum carbide/amorphous hydrocarbon thin films. Thin Solid Film. 2007, 515, 5403–5410. [Google Scholar] [CrossRef]
- Evans, R.D.; Howe, J.Y.; Bentley, J.; Doll, G.L.; Glass, J.T. Influence of Deposition Parameters on the Composition and Structure of Reactively Sputtered Nanocomposite TaC/a-C:H Thin Films. J. Mater. Res. 2005, 20, 2583–2596. [Google Scholar] [CrossRef]
- Vargas, M.; Castillo, H.A.; Restrepo-Parra, E.; De La Cruz, W. Stoichiometry behavior of TaN, TaCN and TaC thin films produced by magnetron sputtering. Appl. Surf. Sci. 2013, 279, 7–12. [Google Scholar] [CrossRef]
- Shuo, W.; Kan, Z.; Tao, A.; Chaoquan, H.; Qingnan, M.; Yuanzhi, M.; Mao, W.; Weitao, Z. Structure, mechanical and tribological properties of HfCx films deposited by reactive magnetron sputtering. Appl. Surf. Sci. 2015, 327, 68–76. [Google Scholar] [CrossRef]
- Li, G. Microstructure and mechanical properties of hafnium carbide coatings synthesized by reactive magnetron sputtering. J. Coat. Technol. Res. 2010, 7, 403–407. [Google Scholar] [CrossRef]
- Jansson, U.; Lewin, E. Sputter deposition of transition-metal carbide films—A critical review from a chemical perspective. Thin Solid Film. 2013, 536, 1–24. [Google Scholar] [CrossRef]
- Yate, L.; Coy, L.E.; Aperador, W. Robust tribo-mechanical and hot corrosion resistance of ultra-refractory Ta-Hf-C ternary alloy films. Sci. Rep. 2017, 7, 3080. [Google Scholar] [CrossRef] [PubMed]
- Stoney, G.G. The tension of metallic films deposited by electrolysis. Proc. R. Soc. Lond. A 1909, 82, 172–175. [Google Scholar] [CrossRef] [Green Version]
- Achache, S.; Lamri, S.; Alhussein, A.; Billard, A.; François, M.; Sanchette, F. Gum Metal thin films obtained by magnetron sputtering of a Ti-Nb-Zr-Ta target. Mater. Sci. Eng. A 2016, 673, 492–502. [Google Scholar] [CrossRef]
- Young, D.A. Phase Diagrams of the Elements; University of California Press: Berkeley, CA, USA, 1991. [Google Scholar]
- Read, M.H.; Altman, C. A new structure in tantalum thin films. Appl. Phys. Lett. 1965, 7, 51–52. [Google Scholar] [CrossRef]
- Baker, P.N. Preparation and properties of tantalum thin films. Thin Solid Film. 1972, 14, 3–25. [Google Scholar] [CrossRef]
- Westwood, W.D.; Boynton, R.J.; Wilcox, P.S. The effects of argon pressure on the properties of sputtered tantalum films. Thin Solid Film. 1973, 16, 1–25. [Google Scholar] [CrossRef]
- Gerstenberg, D.; Calbick, C.J. Effects of nitrogen, methane, and oxygen on structure and electrical properties of thin tantalum films. J. Appl. Phys. 1964, 35, 402–407. [Google Scholar] [CrossRef]
- Kwon, K.-W.; Lee, H.-J.; Sinclair, R. Solid-state amorphization at tetragonal-Ta/Cu interfaces. Appl. Phys. Lett. 1999, 75, 935–937. [Google Scholar] [CrossRef]
- Colin, J.J.; Abadias, G.; Michel, A.; Jaouen, C. On the origin of the metastable β-Ta phase stabilization in tantalum sputtered thin films. Acta Mater. 2017, 126, 481–493. [Google Scholar] [CrossRef]
- Bernoulli, D.; Müller, U.; Schwarzenberger, M.; Hauert, R.; Spolenak, R. Magnetron sputter deposited tantalum and tantalum nitride thin films: An analysis of phase, hardness and composition. Thin Solid Film. 2013, 548, 157–161. [Google Scholar] [CrossRef]
- Jiang, A.; Yohannan, A.; Nnolim, N.O.; Tyson, T.A.; Axe, L.; Lee, S.L.; Cote, P. Investigation of the structure of β-tantalum. Thin Solid Film. 2003, 437, 116–122. [Google Scholar] [CrossRef] [Green Version]
- Petrov, I.; Barna, P.B.; Hultman, L.; Greene, J.E. Microstructural evolution during film growth. J. Vac. Sci. Technol. A 2003, 21, S117–S128. [Google Scholar] [CrossRef]
- Smith, C.J.; Yu, X.-X.; Guo, Q.; Weinberger, C.R.; Thompson, G.B. Phase, hardness, and deformation slip behavior in mixed HfxTa1-Xc. Acta Mater. 2018, 145, 142–153. [Google Scholar] [CrossRef]
- Ghaffari, S.A.; Faghihi-Sani, M.A.; Golestani-Fard, F.; Mandal, H. Spark plasma sintering of TaC–HfC UHTC via disilicides sintering aids. J. Eur. Ceram. Soc. 2013, 33, 1479–1484. [Google Scholar] [CrossRef]
- Gaballa, O. Processing development of 4TaC-HfC and related carbides and borides for extreme environments. Grad. Theses Diss. 2012. [Google Scholar] [CrossRef]
- Kurbatkina, V.V.; Patsera, E.I.; Levashov, E.A.; Timofeev, A.N. Self-propagating high-temperature synthesis of single-phase binary tantalum-hafnium carbide (Ta,Hf) C and its consolidation by hot pressing and spark plasma sintering. Ceram. Int. 2018, 44, 4320–4329. [Google Scholar] [CrossRef]
- Windischmann, H. Intrinsic stress in sputter-deposited thin films. Crit. Rev. Solid State Mater. Sci. 1992, 17, 547–596. [Google Scholar] [CrossRef]
- Hoffman, D.W.; Thornton, J.A. The compressive stress transition in Al, V, Zr, Nb and W metal films sputtered at low working pressures. Thin Solid Film. 1977, 45, 387–396. [Google Scholar] [CrossRef]
- Barth, M.; Ensinger, W.; Hoffmann, V.; Wolf, G.K. Stress and adhesion of chromium and boron films deposited under ion bombardment. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. At. 1991, 59, 254–258. [Google Scholar] [CrossRef]
- Doljack, F.A.; Hoffman, R.W. The origins of stress in thin nickel films. Thin Solid Film. 1972, 12, 71–74. [Google Scholar] [CrossRef]






| Deposited Material | DCH4 (sccm) | DAr (sccm) | Discharge Current (A) | Total Pressure (Pa) | |
|---|---|---|---|---|---|
| Ta | 0 | 30 | 0 | 2 | 1 |
| TaCx | 1–6 | 30 | 3.2–16.7 | 2 | 1 |
| Hf | 0 | 30 | 0 | 2 | 1 |
| HfCx | 1–6 | 30 | 3.2–16.7 | 2 | 1 |
| Ta Target | Hf Target | |
|---|---|---|
| Discharge current (A) | 1–3 | 1–3 |
| Frequency (kHz) | 50 | 50 |
| Target–substrate distance (cm) | 13 | 13 |
| Pressure (Pa) | 1 | 1 |
| DCH4 (sccm) | 8 | 8 |
| DAr (sccm) | 30 | 30 |
| CH4 Flow Rate (sccm) | TaCx Coatings Formula | HfCx Coatings Formula | ||
|---|---|---|---|---|
| 1 | 17.6 | TaC0.35 | 10.1 | HfC0.20 |
| 2 | 32.9 | TaC0.66 | 16.8 | HfC0.34 |
| 3 | 37.9 | TaC0.76 | 30.2 | HfC0.60 |
| 4 | 51.7 | TaC1.03 | 40.5 | HfC0.81 |
| 5 | 51.8 | TaC1.04 | 45.1 | HfC0.90 |
| 6 | 50.0 | TaC1.00 | 47.4 | HfC0.95 |
| Authors | Ref. | Deposited Material | Elaboration Method | Hardness (GPa) | Young’s Modulus (GPa) |
|---|---|---|---|---|---|
| D. Bernoulli et al. | [38] | Ta | DC magnetron sputtering | 14 | not measured |
| Lasfargues et al. | [19] | TaCx | Unbalanced magnetron sputtering system | 40 | 400–500 |
| HfCx | 34–36 | 400–500 | |||
| Evans et al. | [20] | TaCx | Reactive unbalanced magnetron sputtering | 9–20 | 109–220 |
| Shuo et al. | [24] | HfCx | DC reactive magnetron sputtering | 10–34 | not measured |
| Ta | Reactive pulsed DC magnetron sputtering | 17 | 213 | ||
| This work | TaCx | 18–36 | 236–405 | ||
| Hf | 8 | 156 | |||
| HfCx | 11–29 | 175–318 |
| Coatings | 3 A on Ta 1 A on Hf | 2 A on Ta 2 A on Hf | 1 A on Ta 3 A on Hf |
|---|---|---|---|
| DCH4 (sccm) | 8 | 8 | 8 |
| H (GPa) | 22.6 | 23.8 | 22.8 |
| E (GPa) | 235 | 249 | 238 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Monteynard, A.; Luo, H.; Chehimi, M.; Ghanbaja, J.; Achache, S.; François, M.; Billard, A.; Sanchette, F. The Structure, Morphology, and Mechanical Properties of Ta-Hf-C Coatings Deposited by Pulsed Direct Current Reactive Magnetron Sputtering. Coatings 2020, 10, 212. https://doi.org/10.3390/coatings10030212
de Monteynard A, Luo H, Chehimi M, Ghanbaja J, Achache S, François M, Billard A, Sanchette F. The Structure, Morphology, and Mechanical Properties of Ta-Hf-C Coatings Deposited by Pulsed Direct Current Reactive Magnetron Sputtering. Coatings. 2020; 10(3):212. https://doi.org/10.3390/coatings10030212
Chicago/Turabian Stylede Monteynard, Alexis, Huan Luo, Mohamed Chehimi, Jaafar Ghanbaja, Sofiane Achache, Manuel François, Alain Billard, and Frédéric Sanchette. 2020. "The Structure, Morphology, and Mechanical Properties of Ta-Hf-C Coatings Deposited by Pulsed Direct Current Reactive Magnetron Sputtering" Coatings 10, no. 3: 212. https://doi.org/10.3390/coatings10030212





